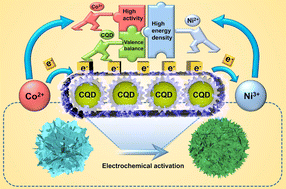
The valence and coordination structure of transition metals in electrode materials play a crucial role in the electrochemical energy storage process. However, it is still challenging to modulate the chemical environment of transition metals in multi-metal-based electrode materials because of the presence of charge exchange between the different metal ions. Here, a bimetallic-based electrode material, Co0.03Ni0.97LDH, with low electrochemical activity is transformed into a highly active one through a simple and efficient electrochemical activation process with the assistance of carbon quantum dots (CQDs). It reveals that CQDs can provide a fast charge transfer channel for the unique valence regulation between Co and Ni, resulting in the generation of high concentrations of Co3+ and Ni2+, which is beneficial for upgrading the energy density of the electrode material and mitigating the Jahn–Teller distortion during the conversion of Ni2+/Ni3+. Moreover, the distinctive 5-coordination structures of Co can effectively stabilize the active sites of both Ni2+ and Co3+. The activated CQD-modified Co0.03Ni0.97LDH composites (A-CQD/Co0.03Ni0.97LDH) deliver a high specific capacity of 2408 F g−1 at 1 A g−1 and maintain a high capacity retention of 90% after 2000 cycles at 10 A g−1. The assembled asymmetric supercapacitor and the aqueous Ni–Zn battery show a high energy storage density of 0.25 mW h cm−2 at a power density of 2.25 mW cm−2 and 1.44 mW h cm−2 at 0.72 mW cm−2, respectively. The impressive results provide a feasible strategy for the rational design of multi-metal-based electrode materials.