Kellie L. Weeks, Jack D. Williams and Gregory R. Boyce
Org. Biomol. Chem., 2021,19, 8018-8020
DOI:
10.1039/D1OB01505B,
Communication
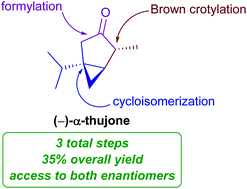
The stereocontrolled three-step synthesis of either enantiomer of α-thujone from commercially available 3-methyl-1-butyne is described. The enantioselectivity originates from a Brown crotylation which is then conferred to the all-carbon quaternary center via chirality transfer in a gold-catalyzed cycloisomerization. The route is highly atom economical and requires no protecting groups or redox manipulations.