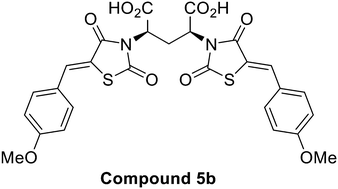
The synthesis of the first dimeric inhibitor of E. coli dihydrodipicolinate synthase (DHDPS) is reported herein. Inspired by 2,4-thiazolidinedione based ligands previously shown to inhibit DHDPS, a series of dimeric inhibitors were designed and synthesised, incorporating various alkyl chain bridges between two 2,4-thiazolidinedione moieties. Aiming to exploit the multimeric nature of this enzyme and enhance potency, a dimeric compound with a single methylene bridge achieved the desired outcome with low micromolar inhibition of E. coli DHDPS observed. This work highlights the continued importance of investigation into DHDPS as an antibacterial target. Furthermore, we demonstrate the design of dimeric ligands can provide a promising strategy to improve potency in the search for novel bioactive compounds.