Tomoyuki Ikai and Takumu Yoshida
Org. Biomol. Chem., 2019,17, 8537-8540
DOI:
10.1039/C9OB01828J,
Communication
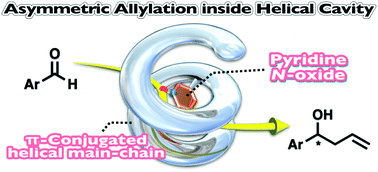
Catalytically active chiral π-conjugated polymers (poly-1(NO)r) bearing pyridine N-oxide pendants were synthesized by ternary copolymerization of a D-glucose-bound diethynyl compound with two types of thieno[3,4-b]thiophene comonomer, one of which contained a pyridine N-oxide group. When the pyridine N-oxide content in the copolymer was 10 mol% (poly-1(NO)0.10), the polymer backbone formed a one-handed helical structure in acetonitrile. Pyridine N-oxide pendants arranged inside the helical cavity of poly-1(NO)0.10 exhibited catalytic activity for the asymmetric allylation of benzaldehydes, producing the corresponding allyl alcohols with up to 43% ee.