Enhancement of MXene optical properties towards medical applications via metal oxide incorporation
Karolinekersin
Enoch
a,
Aravindkumar
Sundaram
 a,
Stephen Selvamani
Ponraj
a,
Sathya
Palaniyappan
a,
Sahaya Dennis Babu
George
b and
Rajesh Kumar
Manavalan
a,
Stephen Selvamani
Ponraj
a,
Sathya
Palaniyappan
a,
Sahaya Dennis Babu
George
b and
Rajesh Kumar
Manavalan
 *c
*c
aCentre for Advanced Materials, Aaivalayam – Dynamic Integrated Research Academy and Corporations (A-DIRAC), Coimbatore 641046, India
bChettinad College of Engineering and Technology, Karur, 639114, India
cInstitute of Natural Science and Mathematics, Ural Federal University, 620002 Yekaterinburg, Russia. E-mail: rajeshkumar_vgm@yahoo.com
First published on 2nd October 2023
Abstract
MXenes have garnered research attention in the field of biomedical applications due to their unique properties, such as a large surface area, low toxicity, biocompatibility, and stability. Their optical behavior makes them versatile for a wide range of biomedical applications, from diagnostics to therapeutics. Nonetheless, MXenes have some minor limitations, including issues with restacking, susceptibility to oxidation, and a non-semiconducting nature. These limitations have prompted researchers to explore the incorporation of metal oxides into MXene structures. Metal oxides possess advantageous properties such as a high surface area, biocompatibility, intriguing redox behavior, catalytic activity, semiconducting properties, and enhanced stability. Incorporating metal oxides into MXenes can significantly improve their conductivity, surface area, and mechanical strength. In this review, we emphasize the importance of incorporating metal oxides into MXenes for light-influenced biomedical applications. We also provide insights into various preparation methods for incorporating metal oxides into MXene structures. Furthermore, we discuss how the incorporation of metal oxides enhances the optical behavior of MXenes. Finally, we offer a glimpse into the future potential of metal oxide-incorporated MXenes for diverse biomedical applications.
1. Introduction
The use of nanomaterials in biomedical applications is becoming increasingly crucial, offering significant support to the healthcare system and advancements in diagnostics and therapeutics. Many nanomaterials are used in biomedical applications like biosensors, drug delivery, bioimaging, and implants.1,2 Different dimensional nanomaterials are employed in various biomedical applications since nanomaterials show many beneficial properties in different dimensions.3 Two-dimensional nanomaterials have gained more attention in various biomedical applications due to their tunable surface area and electrical and optical properties.4 In particular, graphene,5 antimonene,6 black phosphorus,7 dichalcogenides,8 and oxides9 are widely used 2D nanomaterials in biomedical applications. Nevertheless, MXenes have gained immense attention in the 2D nanomaterials research10 and have an idiosyncratic two-dimensional (2D) structure embedded with transition metal carbides, nitrides, and carbo-nitrides. These structures evolved in 2011 when Prof. Michel W. Barsoum and Prof. Yury Gogotsi from Drexel University, Philadelphia discovered the first-ever MXene–titanium-carbide layers by exfoliating 3D titanium aluminium carbide (Ti3AlC2) via the acid etching technique.3 The geometrical structures of MXenes are symbolized as Mn+1XnTx, where M represents an early transition metal (such as Sc, Ti, Zr, Hf, V, Nb, Ta, Cr, Mo, Mn), X is C and N, T is an exterior windup unit especially hydroxyl, oxygen or fluorine groups, and n = 1, 2, or 3. Akin to graphene, MXenes are generally formed by exfoliating their 3D precursors, represented as a top–down approach.4 The preparation and fabrication modalities of MXenes involve both the top and bottom scaling approaches, more specifically the top scaling methods are vastly applied relative to the bottom–up scaling methods that have seen rare utilization. The top–down scaling methods mainly comprise liquid-based chemical and mechanical exfoliation from the bulk crystal of the MAX phase. There are different types of MAX phases available like Ti2AlC, Ti2AlN, and V2GeC, Ti3SiC2, Ti3GeC2, Ti3AlC2, Ti3SnC2, Ta3AlC2, Ti4AlN3, Ti4SiC3, Ti4GeC3, Ta4AlC3, Nb4AlC3, V4AlC3, and Ti4G2C3.11 The conversion of the MAX phase into MXene is generally performed via wet chemistry methods by means of etching the atomic (A) layers from their corresponding layered precursors (MAX phase) with the use of strong etchants namely HF,12 LiF,13 NH4HF2,14 and NH4F.15 In addition, non-HF etchants such as Lewis acids16 and molten salts17 (LiF, NaF, KF, NaOH) based electrochemical and hydrothermal methods have been introduced.18As depicted in Fig. 1, the 3D precursors for MXenes are termed MAX phases, ternary carbides, or nitrides described with the general formula Mn+1AXn, in which A stands for an A group element (mostly leading group IIIA or IVA). The A layers in MXenes are more active than the M–X layers. Because M–X bonds are much stronger than M–A bonds, A layers can be selectively etched using a strong acid (i.e., hydrofluoric acid, HF) to produce Mn+1Xn layers further detached by sonication.19 The exterior of MXenes is typically winded up with fluorine (–F), hydroxide (–OH), and oxygen (–O) groups by the etching process, which provides high surface energy.3 Hence, the final geometrical symbol of MXenes is displayed as Mn+1XnTx, where Tx is the surface functional group.6 In addition, MXenes have various properties that can be employed in their fundamental prospects, such as structural, chemical, optical,20 electronic, and even biological.10 Many experimental and theoretical studies20 explored the stupendous potential of MXenes for various applications in optoelectronics, photonics, catalysis, and many other areas.19,21 MXenes emerge as new 2D nanoplatforms for advanced optoelectronics to be explored in various biomedical applications like biosensing,22 bioimaging,23 and therapeutic applications.24,25 The essential mechanical and electronic properties can be attained by tailoring the elemental composition and chemical functionalization.26 The exclusive traits of MXenes involve a high surface area, high atomic numbers (some transition metals), the presence of hydrophilic functional groups, and paramagnetic behaviour. The functional groups on MXenes also contribute to their rigidness and flexibility, which is crucial in thin film formation as part of bio-electronic devices.27
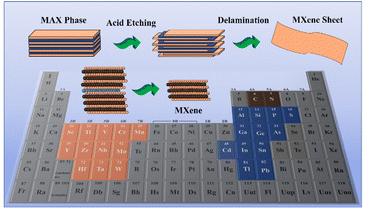 | ||
| Fig. 1 Schematic representation of MAX phase elements in the periodic table and synthesis steps of MXenes from the MAX phase. | ||
Most importantly, the high surface area is beneficial for drug loading and delivery for theranostic applications in various synergistic diseases. Furthermore, hydrophilic functional groups are also crucial for drug loading and targeted drug delivery, as modification or functionalization increases biocompatibility for living cells/tissues.28,29 MXenes, comprising high atomic number and paramagnetic transition metals, are more relevant for biomedical imaging because they show superior X-ray attenuation for computed tomography (CT) scans and can also be employed as magnetic resonance imaging (MRI) contrast agents.29 Distinct types of MXenes could be prepared based on different approaches; therefore, the biocompatibility assessment of MXene-based biomaterials is essential for general biomedical applications.30 Metal oxides are widely employed in various biomedical applications in the diagnostic and therapeutic fields as they possess biocompatibility, a high surface area, good chemical and mechanical stability, and a unique structure.31 Besides, the unique properties of metal oxides, such as enhanced electron transfer kinetics, strong adsorption capability, high sensitivity, and high optical absorption ability, make them more suitable for incorporation into MXenes. This enhances the optical properties for biomedical applications.32,33 Metal oxides like TiO2,34 ZnO,35 ZrO2,36 Fe2O3,37 MnO2,38 MgO,39 CeO2,40 and CuO41 are broadly used in biomedical applications. In the part of the set, various metal oxide-based hybrid and composite formulations of MXenes were reported elsewhere for biomedical applications.42–44 In recent years, the incorporation or coating of metal oxides over MXenes has gained prominence due to the substantial enhancement of their optical properties for various light-influenced medical applications.45–47 This has sparked significant interest in understanding the underlying mechanisms of metal oxide incorporation into MXenes. However, to date, a comprehensive review of how the technology of metal oxide incorporation alone improves MXenes’ optical properties and their applications in medicine has not been published.
This review emphasized the crucial part of tuning the optical properties of MXenes via incorporating metal oxides for different photo-influenced biomedical applications, as presented in Fig. 2. Also, the unique enhancement of the optical properties of MXenes using metal oxides resulting in advanced biomedical applications has been covered with recent tenets and trends. This review discussed the importance of incorporating metal oxides into MXenes to enhance their optical behavior. The different preparation methods for incorporating metal oxides into MXenes were covered, along with their advantages and disadvantages. This review emphasized the scope and challenges of metal oxides masked with MXenes for future research.
2. Complementing MXene with metal oxide composites
The optical properties of the MXene rely on the energy structures, the dispersion of linear and nonlinear dielectric function (ε), or the refractive index (n = √εμ).48,49 MXenes have a linear absorption loss of ∼1% nm−1,50 a nonlinear absorption coefficient of about 10–21 m2 V−2,51 and a negative nonlinear refractive index of about 10–20 m2 W−1.51 MXenes have exclusive characteristics, with enormous electrons gathered close to the Fermi level of MXene to serve as an electron reservoir, supplying decisive transfer of charge carriers. The beneficial properties of MXene include surface-modified functional groups that tune the bandgap of the obtained materials. These functional groups generate a negative charge on the surface of MXene, which electrostatically attracts the cations.52 It also furnishes a platform to enhance interfacial contact between the semiconductor and MXenes, resulting in the transfer or separation of charge carriers in composites to enhance the optical properties.53 Though MXene comprises many unique properties, it shows limitations like lack of semiconducting behaviour, restacking of MXene layers after exfoliation, easy oxidation, and low electrical conductivity.54 It is also worth mentioning that metal oxides lack conductivity and have more significant band gaps. Hence, the heterostructures of MXenes incorporated with metal oxides exhibit favourable improvement in enhancing the optical properties to be employed in the photocatalytic performance for various biomedical applications. Different metal oxides, like SnO2,55 TiO2,56 and MnO2,57 have been incorporated into MXenes to enhance their optical properties. Several studies58 suggested that incorporating NiFe2O4 into MXene heterostructures cogently assisted in averting the restacking of MXene flakes to increase the surface area of the heterostructure, and the surface area of MXene was found to be 19.26 and improved to 49.1812 m2 g−1 after the incorporation of NiFe2O4.3. Different methods to mask metal oxides in MXene composites
MXenes are typically synthesized by wet chemical acidic etching of MAX phases using robust etching solutions with fluoride ions (F−) such as hydrofluoric acid (HF)59 and ammonium bifluoride60 and a mixture of hydrochloric acid and lithium fluoride. They can be prepared in single to few flakes or multiple stacks of MXene sheets. The morphology of MXenes can be tuned by adjusting the concentration of the etching solution, etching time, ultrasonication, and temperature. The literature also highlights exciting methods such as wet chemical reduction, self-oxidation, self-reduction, and pressure-less sintering for incorporating metal oxides into MXenes. Other techniques, including dry mixing/thermal pressing, solution blending, emulsion mixing, in situ polymerization, and lamination stacking, have also been utilized. Metal oxides are also connected with MXenes using simple chemical methods like blending and precipitation.3.1 Co-precipitation method
Co-precipitation is a simple and fastest technique to prepare metal oxide-masked MXenes. This method has advantages like high yield, high product purity, and lack of necessity for organic solvents. As shown in Fig. 3, MXenes and metal oxides are dispersed in a suitable solvent at a particular temperature with a suitable reducing agent and the pH is maintained to form intermediate precipitates resulting from nucleation and growth.61,62 The parameters like temperature, pressure, concentration, pH, stirring speed, and reaction time are of significant concern in controlling nanostructure size and shape distribution. First, the63 MXenes are dispersed in a specified solvent, mostly in distilled water, followed by the addition of the precursor for metal oxide with either a suitable stabilizing agent or targeting moieties depending upon application usage. For instance, for anti-bacterial application, Ti3C2 is masked using cuprous oxide (Cu2O).63As depicted in Fig. 4, aminated MXenes reacted with cupric nitrate trihydrate as a precursor for synthesizing Cu2O and polyvinylpyrrolidone (PVP), and hydrazine hydrate is used as stabilizing and reducing agents, respectively.63 A similar and simple synthesis approach is attempted for preparing the Cu2O/MXene composite with copper(II) sulfate, PVP, and N2H2 as the precursor, stabilizing, and reducing agents, respectively.64 To support the co-precipitation synthesis route, various metal oxide-masked MXenes are synthesized, such as Ti3C2/Al2O3/Ag,65 Ti3C2/SiO2/Ag,66 and Ti3C2/SiO2/Pd,66 Ti3C2F/FeWO4,67 TiO2/Ti3C2/Cu2O, and WO3/MXenes.45
 | ||
| Fig. 4 Schematic illustration of the etching, striping and reduction process of Cu2O/MXene synthesis via the co-precipitation method. (Reproduced with permission from ref. 64 Copyright, 2020 Elsevier.) | ||
3.2 Dispersion or mixing
Another approach for preparing MXene/metal oxide nanocomposite is the dispersion of both MXene/metal oxide in a suitable solvent under ultrasonication, as depicted in Fig. 5. Ultrasonication mixing or mechanical stirring assists in properly distributing MXene/metal oxide to form a composite after removing the solvent and drying. In general, the parameters like ultrasonication power and frequency, temperature, and ultrasonication time play a crucial role in this method. Following a longer sonication time and less ultrasonication power is essential for proper dispersion.68 As evidence, Fig. 6 shows the dispersion or mixing method of CuO-masked MXene preparation via the ultrasonication route for photocatalytic anti-bacterial applications.46 The composites, NiFe2O4/MXene58 and CuFe2O4/Ti3C2,69 were also prepared by dispersion in an ultrasonicated water bath for an hour, followed by vacuum drying.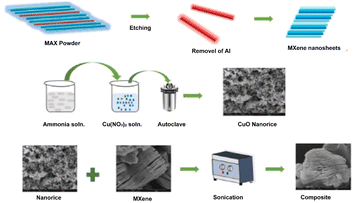 | ||
| Fig. 6 Schematic illustration of CuO/MXene synthesis via the dispersion/mixing method. (Reproduced with permission from ref. 46 Copyright, 2022 Elsevier.) | ||
3.3 Facile in situ growth
Fig. 7 shows the facile in situ technique; redox reactions grow the metal oxide in situ over the MXene exterior. For example, Fig. 8 shows the in situ growth of iron-oxide on the surface of Ta4C3 MXene according to the redox reaction of MXene for photoactivated cancer theranostic applications. The exterior is stabilized or modified with soybean phospholipids for enhanced delivery of nanoparticles at the site of action. Akin to the abovementioned technique, manganese oxide is grown over Ti3C2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70 by a simple redox reaction for cancer therapeutics. Briefly, the KMnO4 aqueous solution was mixed with Ti3C2 aqueous solution under magnetic stirring at room temperature, followed by centrifugation resulting in the composite nanosheet. The challenge is that many MXenes need to be fabricated from the MAX phase before mixing, and sometimes it requires retaining the stratified architecture to supply more cavities for metals or metal compounds to be deposited.
70 by a simple redox reaction for cancer therapeutics. Briefly, the KMnO4 aqueous solution was mixed with Ti3C2 aqueous solution under magnetic stirring at room temperature, followed by centrifugation resulting in the composite nanosheet. The challenge is that many MXenes need to be fabricated from the MAX phase before mixing, and sometimes it requires retaining the stratified architecture to supply more cavities for metals or metal compounds to be deposited.
 | ||
| Fig. 8 Schematic illustration of in situ growth of iron oxide over Ta4C3 MXene. (Reproduced with permission from ref. 91 Copyright, 2018 IVY Spring.) | ||
The kinds of composites reported to date have been synthesized in four ways: (i) ex situ mixing, (ii) self-oxidation mixing, (iii) one-step in situ growth, and (iv) multistep in situ conversion. The choice of an appropriate method varies according to the target material and its precursors. Different synthetic ways result in additional product structures and performances (Table 1).
| Methods | Advantages | Disadvantages | Ref. |
|---|---|---|---|
| Co-precipitation | Purity, scalability, versatility, homogeneous incorporation of metal oxides, easy to functionalize with organic and inorganic materials | Limited choice of precursors, challenge to control the layer thickness | 128 and 129 |
| Dispersion or mixing | Uniform thickness, high surface area, tunability of size, thickness, surface chemistry, and stability | Solvent selection is critical, reproducibility, time consuming | 130 and 131 |
| Facile in situ growth | Controlled morphology, homogeneous distribution of metal oxide in MXene matrix, reduced agglomeration, tailored composition | Undesired materials or by products, longer reaction times | 132 and 133 |
4. Applications of metal oxide masked MXenes
The enhanced surface area, biocompatibility, hydrophilicity, and strong plasmonic effects of MXenes have gained more attention in biomedical applications. MXenes are widely employed in biomedical applications such as drug delivery, biosensors, cancer theranostics, and antimicrobial activity. The incorporation of metal oxides has enhanced the catalytic, photothermal, therapeutic, and diagnostic performance of MXenes. This section discusses the antibacterial, anticancer, and sensing applications of metal oxide-masked MXenes.4.1 Anti-bacterial activity
2D nanosheets of MXenes exhibit anti-bacterial activity owing to their high surface area, hydrophilicity, and negatively charged surface.71 As it has smaller electrons at the Fermi level, incorporating metal oxide can increase the number of electrons. The surface of MXene has the dexterity to accept electrons from the metal oxide resulting in a substantial number of electrons that ease the charge transfer between bacterial cell walls. The formation of MXene-based heterostructures like NiFe2O4/Ti3C2 (NiFe) proved their anti-bacterial activity against Gram-negative E. coli bacteria using the colony count method. It is revealed that NiFe/MXene composite phases showed the best anti-bacterial performance compared to individual NiFe and MXene flakes. The addition of NiFe facilitated a cross-linked network, high surface area, and enhanced magnetic properties.58The same Ti3C2 is also anchored with cuprous oxide (Cu2O) for analyzing photocatalytically triggered anti-bacterial activity.64 The anti-bacterial studies shown in Fig. 9 revealed that the Cu2O/MXene nanosheet exhibits excellent anti-bacterial activity against S. aureus and P. aeruginosa in comparison with MXene, Cu2O, and their mixture (MXene and Cu2O), thus resulting in the admirable increase in the bacteriostasis efficiencies to around 97.04% and 95.59% against both the bacteria, respectively.
 | ||
| Fig. 9 Schematic representation of light induced antibacterial activity mechanisms and therapeutic effects of Cu2O/Ti3C2 (A–C). (a) Mechanism of optical antibacterial action; (b) profiling of antibacterial activity on S. aureus and P. aeruginosa; and (c) quantified therapeutic efficiency; (d) bacteriostasis percentage. (Reproduced with permission from ref. 64 Copyright, 2020 Elsevier.) | ||
The abundant electrons on the surface of MXene accepted from Cu2O facilitated better charge transfer between bacteria and Cu2O/MXene nanosheets, revealing the synergistic effect of Cu2O and the bacteriostatic action of MXene. The production of reactive oxygen species responsible for the damage of bacterial cell membranes and cell death was analyzed by photocatalytically triggered anti-bacterial activity. The Cu2O/MXene induced by sunlight decreases the number of bacterial colonies by generating reactive oxygen species (ROS), which can ruin cell membranes and eventually cause cell death by oxidative stress. It is proved that Cu2O/MXene produced greater ROS than Cu2O. Hence, the separation efficiency of photoinduced charges of Cu2O was significantly ameliorated by combining with MXene nanosheets as a heterojunction structure. It is due to the transfer of a photoinduced electron from Cu2O to MXene, which suppressed the recombination of electron–hole pairs because the Fermi potential level of the MXene (0.71 V vs. NHE, pH = 7) was substantially lesser than the conduction band of Cu2O (−0.703 V vs. NHE, pH = 7). Moreover, the MXene grabbed more electrons on the surface from Cu2O, facilitating the charge transfer between bacteria and Cu2O/MXene nanosheets. The accumulated charges of intracellular ROS, including hydrogen peroxide (H2O2), superoxide anions (O2−), or hydroxyl radicals (–OH), resulted in cell membrane damage and cell death.
Excitingly, studies have shown that the incorporation of different structures of metal oxides on MXenes can alter their anti-bacterial activity.72 For that, the anti-bacterial action of randomly oriented (i.e., dispersed flower-like MnO2 and MoS2) versus vertically aligned MnO2 and MoS2 nanomaterials grown individually on GO, rGO, and Ti3C2 MXene substrates (i.e., MnO2/GO, MoS2/rGO, and MoS2/MXene) against both Gram-positive and Gram-negative bacteria was studied. It has been proven that the sharp edges of 2D nanosheets play a role in targeting and ruining the bacterial cell wall, resulting in the loss of membrane integrity and, eventually, bacteria death.73 This study was intended to evaluate the impact of 2D nanosheets on the peptidoglycan mesh (PM) in the bacterial cell wall. It was revealed that vertically aligned 2D nanosheet motifs show a higher anti-bacterial activity against both bacteria classes than 2D nanomaterials.72 To uphold the structural importance of metal oxide masked MXenes in optical-based anti-bacterial applications, a multifunctional hydrogel scaffold is developed using polyethyleneimine grafted Pluronic F127 (F127-PEI) and oxidized sodium alginate and incorporated MXene@CeO2 nanocomposites and the anti-bacterial activity against both Gram-positive and Gram-negative bacteria is evaluated. The incorporation of MXene@CeO2 nanocomposites contributed to exquisite antimicrobial efficiency in the multifunctional hydrogen scaffold (FOM) group, revealing a 100% reduction of the bacterial colonies. It is mentioned that the cationic PEI in the FOM attracted the negatively charged bacterial cell membrane, disrupting transmembrane potential, and inducing cell death. Furthermore, incorporating “nano-knife” like MXene@CeO2 nanocomposites broke the integrity of the cell membrane and resulted in synergistic inhibition of bacterial growth.74
Alternatively, ceramic oxide and noble metal nanoparticles are also incorporated over MXene to enhance the optical characteristics of MXene in killing bacteria.75 In advance, Ti3C2 MXene was modified with Al2O3/Ag, SiO2/Ag, and SiO2/Pd nanoparticles. The bioactivities of Ti3C2 MXene, Ti3C2/Al2O3/Ag, Ti3C2/SiO2/Ag, and Ti3C2/SiO2/Pd nanocomposites were analyzed qualitatively by an agar diffusion method against both Gram-negative (Escherichia coli) and Gram-positive bacteria (Bacillus sp., Staphylococcus aureus, and Sarcina lutea). The anti-bacterial studies in Fig. 8 revealed that the modification of Ti3C2 MXene with Al2O3 + Ag, SiO2 + Ag, and SiO2 + Pd resulted in significantly increased anti-bacterial properties; the results of the experiment with Staphylococcus aureus with the application of the dilution method revealed that all the tested samples were able to inhibit the growth of potentially pathogenic bacterial strains. The considered mechanism of action for Ti3C2 MXene is related to the direct physical interactions between the edges of the nanosheets and the bacterial cell wall or the membrane surface. As a result, cells lose their integrity, and the internal cytoplasm is released together with DNA.
Cong Liu et al.63 integrated a covalent organic framework with MXene structures, anchored Cu2O nanoparticles on the surface of Ti3C2. The prepared Ti3C2/TpPa−1/Cu2O systems exhibited anti-bacterial activity against S. aureus and P. aeruginosa. It is reported that the enhancement of anti-bacterial activity is because the synergistic effect of Ti3C2/TpPa−1/Cu2O; tppa−1 increased the contact range with bacteria and killed bacteria by generating ROS as it has a vast surface area. Ti3C2 assisted the flow of carriers between layers, which improved the separation efficiency of electron–hole pairs and caused more ROS generation. Besides, copper ions released by Cu2O denatured the bacterial DNA and exhibited toxicity to bacteria. Rather than Cu2O metal oxide, recently, CuO was masked over Ti3C2 by the ultrasonication route for photocatalytic anti-bacterial applications.46 It is revealed that the anti-bacterial activity of CuO/MXene is due to the vital force of attraction generated between the negatively charged bacterial cell membrane and positively charged heavy metal ions present on the sample surface. After penetrating the cell membrane, heavy metal ions react with proteins containing thiol (–SH) groups and inactivate the protein resulting in the death of bacteria. Under the metal oxide section, some rare-earth metal-based oxides are also masked on the surface of MXene. As a promising metal oxide gadolinium (Gd3+) doped V2O5 (GVO), nanostructures with MXenes are demonstrated to have an anti-bacterial effect on both Gram-positive (S. aureus) and Gram-negative (P. vulgaris) strains.76 GVO/MXene creates reactive oxygen species (ROS) in the presence of light and causes the eradication of RNA, resulting in the death of bacterial strains. It is demonstrated that the efficiency of the prepared nanocomposites was higher than that of the individual nanoparticle. Akin to this, WO3/MXene nanocomposites also presented as photoinduced anti-bacterial agents against the Gram-positive strain S. aureus and Gram-negative strains E. coli, Pneumonia, and P. vulgaris and demonstrated better activity against the Gram-positive strain S. aureus. The WO3/MXene composite showed good activity at a low concentration against pneumonia in Gram-negative strains. It is stated that the WO3/MXene composite does not exhibit anti-bacterial activity against E. coli and P. vulgaris, whereas WO3 and MXene showed good anti-bacterial activity. It is reported that the zero anti-bacterial activity against E. coli bacteria and P. vulgaris is due to an extra outer membrane that increased the resistance to the WO3/MXene composite. The increased concentration of composites also decreased the anti-bacterial activity owing to the size and agglomeration. CuFe2O4/MXene nanohybrids are also employed for evaluating the anti-bacterial activity against E. coli, Pseudomonas aeruginosa, Klebsiella pneumonia, Proteus vulgaris, and Staphylococcus aureus. The anti-bacterial studies revealed that composites developed better anti-bacterial performance than individual nanoparticles. It is reported that the anti-bacterial activity is due to the interaction of the positively charged metal particles with the negatively charged bacterial cells.69 Unlike metal oxide alone, carbon-based graphite is also used in a hybrid metal oxide–carbon–MXene structure against photocatalytic and bacteriostatic applications. For example, TiO2 is supported with graphite over Ti3C2 MXene, and the hybrid nanostructures revealed exquisite anti-bacterial efficiency against E. coli. The nanostructures expressed anti-bacterial activity under light exposure owing to the excessive radicals that cause oxidative stress in bacterial cells. It is reported that controlled oxidation of delaminated MXene structures contributes to anti-bacterial activity and assembly of hybrid nanostructures (TiO2–Ti3C2) with graphitic carbon persuaded the generation of oxidative stress in bacteria to promote cell lysis.77 List of various metal oxide-masked MXene in anti-bacterial applications is detailed in Table 2.
| S. no. | Metal oxide | MXene | Bacteria | Remarks | Ref. |
|---|---|---|---|---|---|
| 1 | NiFe2O4 | Ti3C2 | Escherichia coli (E. coli) | NiFe/MXene exhibited excellent antibacterial activity against Gram negative E. coli bacteria | 58 |
| 2 | Cu2O | Ti3C2 | Staphylococcus aureus (S. aureus) and P. aeruginosa | Ti3C2/TpPa−1/Cu2O nanocomposites demonstrated good antibacterial activity of 98.90% and 99.62%, and enhanced by 50% and 33% when compared to pure TpPa−1-COF | 63 |
| 3 | MnO2 | Ti3C2 | Bacillus subtilis and E. coli | Vertically aligned 2D MnO2 nanosheets on grapheme oxide and Ti3C2 Mxene revealed maximal antimicrobial activity, signifying that the edges of the nanosheets weakens the bacterial cell | 72 |
| 4 | CeO2 | Ti3C2 | E. coli (Gram-negative bacteria), S. aureus (Gram-positive bacteria) | MXene@CeO2 nanocomposite based multifunctional hydrogel exhibited distinct antibacterial behaviour against E. coli, S. aureus, MRSA and showed 100% reduction in bacterial colonies | 74 |
| 5 | SiO2 | Ti3C2 | E. coli, Sarcina lutea, S. aureus, and Bacillus sp. | NiFe/MXene exhibited excellent antibacterial activity against Gram negative E. coli bacteria | 75 |
| 6 | TiO2 | Ti3C2 | E. coli | TiO2–Ti3C2Tx killed 97% E. coli bacteria under light exposure by producing the excessive radicals that cause oxidative stress on bacterial cells | 77 |
| 7 | Cu2O | Ti3C2 | S. aureus and P. aeruginosa | Cu2O/MXene nanocomposite displayed good antibacterial activity against S. aureus and P. aeruginosa with the bacteriostasis efficiency of 97.04% and 95.59%, respectively | 63 |
| 8 | WO3 | Ti3C2 | S. aureus, E. coli, K. pneumonia, P. vulgaris | WO3/MXene nanocomposite expressed good antibacterial activity due to the electrostatic attraction between the nanocomposite and the bacterial cell membrane | 45 |
| 9 | CuFe2O4 | Ti3C2 | E. coli, Pseudomonas aeruginosa, Klebsiella pneumonia, Proteus vulgaris and S. aureus | CuFe2O4/Ti3C2 composites showed good antibacterial activity due to the addition of CuFe2O4 | 69 |
4.2 Anti-cancer activity
Cancer is a necrotic epidemic disease with no specific treatment. Massive research has been in progress, working on cancer therapy for decades, with some improvement, yet many drawbacks persist. Conventional treatments like surgery,78 chemotherapy,79 and radiation therapy80 are the most used treatments;81 they exhibit several limitations, like incomplete removal of tumors, damage to healthy tissue, hair loss, nausea, and bowel issues. To conquer these barriers, nanomaterials were predominantly studied as a targeted drug delivery method for cancer therapy.82,83 One of these materials is Ti3C2, also known as MXene, a 2D material studied for many biomedical applications, tumor detection (i.e., as contrast agents), cancer therapy, and drug delivery.84 MXenes are endowed with the following beneficial properties suitable for cancer therapy. (i) In the presence of functional groups, such as –OH, –O, or –F, MXenes are hydrophilic in nature,24,26 which is favourable for surface modification. (ii) The large surface area makes the MXene host massive molecules for synergistic therapy, including immune adjuvants,85 photosensitizers (PS),86 and chemotherapeutic drugs.87 (iii) Several compositions of MXenes, including Ti2C, Ti3C2, Nb2C, and Mo2C, have been tested to be non-toxic and biocompatible with living organisms. Excellent biocompatibility might be due to the metal in the “M” layer, like Ti, Mo, and Nb, being relatively inert to living organisms. Other significant elements, such as nitride and carbon, are essential in the structure of biological organisms. (iv) They exhibit substantial plasmonic effects in the NIR region, making them more favourable for both in vivo photothermal (PTT) and photoacoustic (PA) imaging in the first or the second biological window. Therefore, MXenes were studied by many researchers for cancer photothermal therapy by killing cancer cells by heat, leading to protein denaturation and cell death.88 It was reported that MXenes with a size of around 180 nm could explicitly target and reach the cancerous microenvironment by enhanced permeability and retention of EPR.89 Although MXenes showed good photothermal efficiency that makes them a potential candidate for photothermal therapy, the internalization of MXenes into cancer cells was not studied extensively.Lin et al.90 applied MXenes to treat tumors using PTT for the first time. Typical representatives of MXenes, such as Ti3C2, Nb2C, Ta4C3, and Ti2C, are endorsed to have excellent photothermal properties and are used in PTT. Using Ti3AlC2 powder as the raw material, the 2D nanosheets of Ti3C2 were functionalized with soybean phospholipid (SP) for high-efficiency PTT of tumors. It is revealed that introducing SP enhanced the stability of Ti3C2 nanosheets in various dispersions and improved their applicability in tumor diagnosis and treatment. Furthermore, the composite nanosheet Ti3C2–SP had excellent PTT performance, as Ti3C2–SP at a low concentration of 72 μg ml−1 could be heated to above 55 °C in 6 min, which fulfilled the temperature requirements of PTT for the ablation of tumor tissue. Zhuang Liu et al.91 prepared (Ta4C3)-based MXene nanosheets anchored with iron oxide nanoparticles modified using soybean phospholipid (SP) to increase the stability. The prepared Ta4C3–IONP–SPs were evaluated for MR/CT dual-modality imaging and photothermal hyperthermia of breast cancer. Without NIR radiation, the prepared Ta4C3–IONP–SP nanosheets failed to exhibit cytotoxicity on breast 4T1 cancer cells at different concentrations (0, 12.5, 25, 50, 100, 200 ppm). Upon NIR radiation for 5 min at the power density of 1.5 W cm−2, the photothermal-killing effect was observed with increased Ta4C3–IONP–SP concentrations, where more cancer cells were ablated and then killed under NIR irradiation. The therapeutic efficacy of the Ta4C3–IONP–SP MXene in vivo was determined in 4T1 tumor-bearing mice and is represented in Fig. 10. It is observed that the temperature of the tumors from mice in post-injection of Ta4C3–IONP–SPs increases from about 34 °C to 48 °C within 10 min, which is adequately high to ablate cancer cells and tumor tissues. Two days after photothermal hyperthermia, the initial tumors disappeared in the group injected with Ta4C3–IONP–SPs under laser irradiation, leaving black scars at tumor sites. It was found that around 1.02% Ta4C3–IONP–SPs accumulated in the tumor tissue passively due to the EPR effect. Retention of Ta4C3–IONP–SPs in the liver and spleen may be associated with the significant macrophage uptake of nanoparticles in reticuloendothelial systems. Primarily, the high photothermal-conversion efficiency of Ta4C3–IONP–SP composite nanosheets (η: 32.5%) has accomplished complete tumor eradication without reoccurrence, demonstrating the highly efficient breast-tumor hyperthermia performance.
 | ||
| Fig. 10 (A) Photothermal and therapeutic evaluation profiles of Ta4C3–IONP–SPs under a laser, (B) the corresponding infrared thermal images. (C) Heating/cooling, (D) temperature-changing profile, (E) UV-vis spectra and (F) 3D-schematic illustration of Ta4C3–IONP–SPs as PTAs for 4T1 cells. (G) Relative viability and (I) confocal fluorescence images. (Reproduced with permission from ref. 91 Copyright 2018, IVY Spring.) | ||
2D niobium carbide (Nb2C) MXene was first reported by Huijing Xiang et al.92via a two-step chemical exfoliation strategy for photothermal conversion in the NIR-II bio window, which demonstrated excellent in vivo photothermal ablation capability in the NIR-II bio window. The Nb2C@mSiO2 nanosystem reported by the same group was loaded with AIPH molecules as the free-radical source. Upon 1064 nm NIR-II laser irradiation, the heating of nanoparticles (NPs) triggered the rapid release and decomposition of encapsulated initiators to generate free radicals independent of oxygen levels, resulting in significant in vitro cell apoptosis and in vivo tumor eradication, which is herein regarded as the cancer-therapeutic modality of “thermodynamic therapy”. The synergistic therapeutic efficiency of the photothermal effect was exhibited. Dong Yang Zhang et al.93 prepared TiO2−x decorated titanium carbide MXene for photoacoustic/photothermal bimodal imaging guided near-infrared II (NIR-II) photothermal enhanced SDT of the tumor.
Upon ultrasound/NIR-II radiation, Ti3C2@TiO2−x nanoplatform provoked substantial cellular killing in vitro and complete tumor eradication in vivo without recurrence of tumors and systemic toxicity. MXene-based multifunctional composites, Fe3O4/MnOx–Nb2C, were employed for the tumor-responsive T1 and T2 MRI-guided photothermal ablation of breast cancer in the NIR-II biowindow. The Fe3O4 composites act as T2 contrast agents for T2-weighted MR imaging. This composite nanosheet was modified with soybean phospholipid to enhance the stability, biosafety in physiological environments, and long circulation time. It is reported that the high photothermal-conversion efficiency of Fe3O4/MnOx–Nb2C composite MXenes (η = 30.9%) dexterously ablated tumor cells without further reoccurrence both in vitro and in vivo after laser irradiation in the second biological window (NIR-II, 1064 nm).94 Two-dimensional tantalum carbide was employed for the photothermal cancer ablation. Manganese oxide nanoparticles were grown on the surface of Ta4C3 by the redox reaction between the MXene surface and strongly oxidative MnO4, and the surface of the MXene composites was modified with soybean phospholipid to enhance biosafety. The composite exhibited good photothermal conversion efficiency for ablating the tumor cells and acted as a contrast agent. It is indicated that tantalum-based and MnOx components in the prepared composites acted as high-performance contrast agents for simultaneous computed tomography and T1-weighted magnetic resonance imaging, respectively. It is revealed that the composites showed significant tumor growth suppression by photothermal hyperthermia.70 MXene composites and metal oxide composites were utilized for magnetic hyperthermia. Using the redox reaction-induced growth, MnOx was grown on titanium carbide MXene sheets. The incorporation of MnOx on the surface of nanosheets acts as the pH-responsive contrast agent for T1-weighted MR imaging. Simultaneously the ultrathin Ti3C2 nanosheets revealed high photothermal-conversion performance for efficient thermal ablation of tumors via PTT.90 A summary of various metal oxide-masked MXenes in anti-cancer activities is listed in Table 3.
| S. no. | Metal oxide | MXene | Light | Power | Summary | Ref. |
|---|---|---|---|---|---|---|
| 1 | Iron oxide | Ta4C3 | NIR I | 1.5 W cm−2 | Ta4C3–IONP–SPs composite MXenes achieved complete tumor eradication with high photothermal-conversion efficiency (η: 32.5%) | 124 |
| 2 | SiO2 | Nb2C | NIR II | 1 W cm−2 | SiO2/Nb2C MXene showed that a better photothermal-conversion effect provoked cancer cell apoptosis | 92 |
| 3 | TiO2 | Ti3C2 | NIR II | 1 W cm−2 | Titanium carbide, Ti3C2 showed good photothermal efficiency and demonstrated complete tumor elimination in 4T1 tumor bearing mice | 93 |
| 4 | Fe3O4 | Nb2C | NIR II | 1.5 W cm−2 | Fe3O4/MnOx–Nb2C composite nanosheets expressed high photothermal-conversion efficiency in the NIR-II biowindow (1064 nm, η 30.9%) | 94 |
| 5 | MnOx | Ti3C2 | NIR I | 1 W cm−2 | MnOx/Ti3C2 composite MXenes exhibited excellent contrast-enhanced PA-imaging properties and demonstrated efficient tumor ablation and tumor-growth suppression | 90 |
4.3 Sensing applications
The unique physical, chemical, and ion transport properties of MXenes make them an excellent choice in many applications, exclusively in sensing95,96 and biosensing.97–99 MXenes feature excellent biocompatibility and allow secure immobilization of enzymes and proteins on their surface.100–102 They possess a large specific surface area, anisotropic charge carrier conductivity, environmental friendliness, and excellent chemical stability.103 Surface dangling bonds enable MXenes to attract various functional groups, making them unique for sensing applications. Metal oxides are used to fabricate a heterostructure or composite with MXenes to enhance sensing quality. Metal oxides have various beneficial properties like high thermal and chemical stability,33 substantial mechanical strength,104 sizeable piezoelectric coefficient,105 high exciton binding energy, and optical gain,106 making them an ideal material for the sensor.107As depicted in Fig. 11,108 hybrid composites as heterojunctions using partially-oxidized Ti3C2 sheets and photo-active NiWO4 nanoparticles (NPs) are prepared for selective sensing of prostate-specific antigens (PSA). Electrocatalytic and photoelectrochemical (PEC) studies revealed a remarkable electrochemical response, with a redox couple shift to 0.27 V/0.15 V and a two-fold rise in the measured current output (1.2 μA). It is mentioned that the predominant performance of MX–NiWO4 is due to the increased surface area, high conductivity of Ti3C2, and exposed surface-active sites of MX–NiWO4. The ideal interfacial arrangement of MX–NiWO4 assisted in the electrocatalytic mechanism-based PEC immuno-sensing of PSA. The developed PEC biosensor's detection range was from 1.2 fg mL−1 to 0.18 mg mL−1 with a detection limit of 0.15 fg mL−1. In another study,109 a TiO2/Ti3C2/Cu2O heterostructure was fabricated by in situ generations of TiO2 NPs on Ti3C2 MXenes and the introduction of Cu2O via a one-step hydrothermal reaction. The impact of Ti3C2, as a mediator between TiO2 and Cu2O, adequately accelerated the charge transfer and prolonged photoelectrons lifetime, which made the fabricated sensor extremely sensitive and conductive. It is proven that heterojunction demonstrated good PEC activity with dissolved O2 as electron scavengers, during which H2O2 is produced as an intermediate to further trap the photogenerated electrons. Glucose detection was observed in the change in the photocurrent of TiO2/Ti3C2/Cu2O, decreasing photocurrent with the increased glucose concentration in a wide range from 100 nM to 10 μM with a low detection limit of 33.75 nM (S/N = 3).
 | ||
| Fig. 11 Schematic illustration of mechanisms of surface adsorption of NiWO4 NPs over ultra-thin Ti3C2Tx sheets. (Reproduced with permission from ref. 108 Copyright 2022, Elsevier.) | ||
To uphold the superior sensing characteristics of MXenes, metal oxide-based MXene heterojunctions67 are fabricated by electrospinning FeWO4 bimetallic nanofibers tagged on delaminated single-layered Ti3C2 MXenes for the detection of rutin. The heterostructure revealed favourable electrocatalytic properties for rutin oxidation due to its electroactive interconnecting network of single-layered MXene with metal-oxide fibers having high defective edge/plane sites. Compared to bare glassy carbon electrodes (GCE) and composites, the prepared structures exhibited superior performance. Volumetric studies of the fabricated heterostructure displayed an ultralow detection limit of 0.42 nM, an ultrawide linear determination range from 4 to 147 nM, and a high sensitivity of 0.3799 μA nM−1 cm−2. The electrochemical studies of the MXene–FeWO4 nanocomposite showed favourable stability and persistent anti-interference ability. The prepared nanocomposite revealed excellent activity in detecting RT in human serum, orange juice, and black tea samples.
Interestingly, hybrid combinations of110 titanium dioxide/MXene and polyvinyl alcohol/graphene oxide (TiO2/MXene–PVA/GO) composites are prepared to modify a screen-printed carbon electrode (SPCE) for urinary norepinephrine (NE) detection. The as-prepared composite substantially upgraded the sensor performances due to the favourable electrocatalytic activity of TiO2, high conductivity of MXene, and auto-sample preconcentration via the PVA/GO hydrogel. Cyclic voltammetry and amperometry studies revealed that the composites expressed their electrochemical response at +0.4 V, and oxidation of NE is proportional to NE. The contribution of the PVA/GO hydrogel is shown in the increased surface area and sample volume absorption, increasing sensor electrochemical signals and sensitivity.
Similarly,111 TiO2 nanoparticle-modified organ-like Ti3C2 MXene (TiO2–Ti3C2) nanocomposite is used to immobilize hemoglobin (Hb). Spectroscopic and electrochemical studies demonstrated that the TiO2–Ti3C2 nanocomposite is an excellent immobilization matrix biocompatible with redox proteins, affording good protein bioactivity and stability. The direct electron transfer of Hb due to the unique organ-like hybrid structure of TiO2–Ti3C2 improved the detection of H2O2 with a wide linear range of 0.1–380 μM for H2O2 (sensitivity of 447.3 μA mM−1 cm−2), and a meager detection limit of 14 nM for H2O2. It is reported that incorporating TiO2 nanoparticles enhanced biocompatibility, bioactivity, and prolonged stability. In addition to TiO2, masked MXene sensors,112 TiO2/Ti3C2 nanocomposite, along with covalent organic polymers (NUF), are prepared for the detection of dopamine (DA) and uric acid (UA). The differential pulse voltammetry studies revealed that TiO2/TiCT/NUF expressed high sensing activity with low detection limits of 0.2 and 0.18 nM (S/N = 3) in the concentration ranges from 0.002 to 100 μM and 0.001 to 60 μM for simultaneous determination of DA and UA, respectively. The simultaneous detection is achieved due to the abundant redox sites, large electroactive area, and heterostructure's enhanced electron capacity. Different combinations of metal oxide masked MXenes for sensor applications are listed in Table 4.
| S. no. | Metal oxide | MXene | Analyte | Remarks | Ref. |
|---|---|---|---|---|---|
| 1 | NiWO4 & TiO2 | Ti3C2 | Prostate-specific antigens | Ti3C2Tx–TiO2/NiWO4 hybrid composite showed photoelectrochemical sensing of prostate specific antigen, over a wide detection range from 1.2 fg mL−1 to 0.18 mg mL−1, with a detection limit of 0.15 fg mL−1 | 108 |
| 2 | TiO2 | Ti3C2 | H2O2 | TiO2 nanoparticle modified Ti3C2 MXene nanocomposite used for the detection of H2O2 with a low detection limit of 14 nM, a wide linear range of 0.1–380 μM for H2O2, and especially, an excellent long-term stability | 111 |
| 3 | TiO2/Cu2O | Ti3C2 | Glucose | The TiO2/Ti3C2Tx/Cu2O composite was probed as a photoelectrochemical sensing system for glucose detection in a linear range from 100 nM to 10 μM with a low detection limit of 33.75 nM | 109 |
| 4 | FeWO4 | Ti3C2 | Rutin | The MXene/FeWO4 composite showed excellent selectivity for Rutin detection among a series of interfering ions and biomolecules | 67 |
| 5 | TiO2 | Ti3C2 | Norepinephrine | The TiO2/MXene–PVA/GO based hydrogel used for the detection of norepinephrine with a detection limit (3σ) of 6 nM | 110 |
| 6 | TiO2 | Ti3C2 | Glucose | TiO2–Ti3C2 was employed as an electrogenerated chemiluminescence biosensor for the detection of glucose in the wide concentration range of 20 nM–12 mM with a low detection limit of 1.2 nM | 125 |
| 7 | TiO2 | Ti3C2 | Dopamine & uric acid | TiO2/Ti3C2 demonstrated good selectivity and reproducibility for dopamine and uric acid detection in urine and serum samples with recoveries of 98.4 to 100.9% | 112 |
| 8 | TiO2 | Ti3C2 | Glutathione | The TiO2/Ti3C2 heterostructure film exhibited high stability, sensitivity, and selective detection of glutathione | 126 |
| 9 | ZnO | Ti3C2 | Glucose | ZnO/MXene was employed to prepare a skin-attachable and stretchable electrochemical enzymatic sensor for the detection of glucose and exhibited enhanced sensitivity in sweat samples (29 μA mM−1 cm−2), low limit of detection (LOD ≈ 17 μM), and a broad linear detection range (LDR = 0.05–0.7 mM) that satisfies glucose detection application in human sweat | 127 |
4.4 Other biomedical applications
Like other fascinating applications, tissue engineering and regenerative medicine also extended the fields of MXenes due to their remarkable features like conductivity, large surface area, hydrophilicity, two-dimensional geometry, and particle size regulation. MXenes combined with polymers and hydrogels are widely explored in tissue engineering, regenerative medicine and wound dressing because of their immunomodulatory and anti-inflammatory properties.113,114 Jacek Wychowaniec et al. developed an rGO–MXene hydrogel as a cellular network for tissue engineering applications. It is reported that the rGO-based hydrogel without MXene had a hydrophobic surface with an elastic modulus of 40k Pa and rGO–MXene-based hydrogel expressed a hydrophilic surface, softer with an elastic modulus of 20 kPa. MXene Ti2C, along with cryogen, was reported for cardiac tissue engineering, i.e. Ti2C proportion provided enough mechanical properties and an appropriate conductivity to match the natural myocardium and promoted the repair of myocardial infarction. MXenes are used in wound healing along with hydrogels under electric stimulation as they have the ability to enhance the electrophysiological properties of wound dressing materials.115,116 Besides, MXene coupled with CeO2-based nanozyme was reported to have a synergistic effect in hyperthermia-enhanced tumour nano catalytic therapy.117 Though MXenes were employed in all these applications, metal oxide-masked MXenes have not been explored much.As described above, various metal oxides were utilized for effectively improving the optical properties of MXenes for various biomedical applications. However, depending on the metal oxide type, the applications vary. For example, TiO2 is a known photocatalyst and is effectively utilized for various ROS-generating applications, such as anti-bacterial and anti-cancer, since its Ti2+ ionic form exhibits ROS-generation activities. Similarly, copper and cobalt-masked MXenes are also used since they help generate ROS in the presence of external light. On the other hand, metal oxides such as cerium and manganese exhibit concentration-dependent ROS scavenging activities; these nanoformulations were used in anti-oxidant applications. So, the type of application was determined by the type of metal oxide incorporation.
5. Scope and perspectives
MXenes have attractive and tunable properties, but limitations like easy oxidation and restacking are encountered. In the context of MXenes and metal oxide incorporated MXenes, stability issues often arise due to their sensitivity to oxidation. The terminal surface functional groups of MXenes (–OH, –F) are particularly susceptible to oxidation. The high reactivity of MXenes, coupled with the presence of defects and impurities, may contribute to this lack of stability. Additionally, improper mixing of metal oxides with MXenes can result in a lack of structural stability. To enhance and improve the stability of MXenes and metal oxide incorporated MXene, various approaches can be employed, including surface modifications, protective coatings, and suitable composites.Overcoming these limitations is crucial to exploring the optical properties of MXenes for biomedical applications. Metal oxides have a high surface area, biocompatibility, interesting redox, catalytic, and semiconducting activity, and good mechanical stability. The use of metal oxide masked MXenes for clinical applications is still growing. Some studies and factors must be explored for improved diagnostic and therapeutic applications. (i) Enhancing biocompatibility, including biodegradability, biodistribution, and toxicity, using the incorporation of metal oxides based on surface modification, concentration, and structure. (ii) The interaction between metal oxide masked and biological organisms, i.e., the immunological effect of MXenes and the immune system, in vivo studies need to be conducted to optimize the size, shape, surface, and physical and chemical properties of metal-oxide masked MXenes. (iii) The incorporation or encapsulation of the drug in metal oxide masked MXenes, and their performance must be studied for theranostic applications. (iv) Optimizing the optical properties and catalytic reactions of MXenes using metal oxides must be explored widely for enhancing the sensing applications. (v) The impact of incorporating different structures of metal oxides like rods, spheres, sheets, and quantum dots towards clinical applications can be prioritized in future research. More research will develop a different combination of metal oxide-masked MXenes to enhance diagnostic and therapeutic performances. With the exceptional properties of MXenes and the potential of metal oxides, enormous improvement can be implemented in developing cancer therapies, drug delivery platforms, biosensors, and bioelectronics.
Like most inorganic nanosystems, the poor biodegradability of 2D MXene nanosheets is the pivotal issue, which may hinder their further oncological research and clinical translation. Therefore, it is essential to systematically evaluate the biodegradation performance of MXene nanosheets in a complex physiological environment. To date, several MXenes have undergone individual studies and case-by-case phenomenon evaluations, including Ti3C2,118 Ta4C3,119 Nb2C,120 MoC2,121 V2C,122 and TiN.123 However, the biological effects of MXenes are only preliminarily assessed at the cellular and animal level. Currently, reliable data on the long-term biological effects and biosafety are still highly lacking, which requires further systematic and in-depth evaluation and assessment. Although many studies have demonstrated the great potential of MXenes in biomedical applications, their clinical translation is still in its infancy. Therefore, the systematic investigation of the interaction between MXenes and biological microenvironments should be systematically investigated in the following fundamental research.
Additionally, the effect of surface functionalization on the biological behavior of MXenes requires further investigation. Significantly, the scale-up fabrication of 2D MXenes is a significant aspect for their design and engineering. A further and deeper exploration of materials science and clinical translation requires cooperation among researchers of interdisciplinary and industrial sectors. It is highly expected that the development of nanotechnology and bioscience will achieve more fundamental and technological breakthroughs to afford the limitless application of MXenes in varied biomedical areas shortly, provided the challenges facing and critical issues are adequately solved.
6. Conclusion
This review has emphasized the significance of incorporating metal oxides into the MXene framework for biomedical applications. According to the literature survey, metal oxides have played a crucial role in enhancing the high surface area, electrical conductivity, stability, and sensitivity of MXenes. It is evident that the incorporation of metal oxides into the MXene structure holds great promise for future biomedical applications. However, the mechanism of interaction between metal oxides and MXenes is still in its infancy, requiring further investigation. This compilation underscores the need for more research on utilizing metal oxide/MXene nanocomposites for various biomedical applications. The biomedical applications of 2D MXenes face limitations such as easy aggregation, complex surface engineering, inadequate stability, and potential toxicity. Factors such as lateral size, the number of layers, and the degree of oxidation significantly influence degradation and stability during in vivo circulation.The interaction between MXene nanosheets and the physiological environment, the analysis of metabolic pathways, and the potential side effects of MXene nanosheets remain unclear. There exists a notable paradox where rapid degradation may affect our desired therapeutic outcomes while meeting biological safety requirements. Therefore, the appropriate degradation rate should be considered in the design and synthesis of MXenes to simultaneously pursue ideal therapeutic outcomes and low toxicity.
This review lays a solid foundation for further research by providing technical details and in-depth insights that warrant focused attention. A thorough investigation of these aspects will provide excellent solutions to the challenges faced in biomedical applications. Additionally, further research in the rapid assessment of nanomaterials for biomedical applications within a microfluidic platform is anticipated.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The Russian Federation's Ministry of Science and Higher Education provided support for the research, which Dr Rajesh Kumar Manavalan sincerely acknowledges (Ural Federal University project within the Priority 2030 Program) and contract number 40/is2. The authors acknowledge the support and funding from Aaivalayam.References
- A. P. Ramos, et al., Biomedical applications of nanotechnology, Biophys. Rev., 2017, 9(2), 79–89 CrossRef PubMed.
- M. Mabrouk, et al., Nanomaterials for biomedical applications: Production, characterisations, recent trends and difficulties, Molecules, 2021, 26(4), 1077 CrossRef PubMed.
- Y. Gogotsi and B. Anasori, The rise of MXenes, ACS Publications, 2019, pp. 8491–8494 Search PubMed.
- K. A. Papadopoulou, et al., A perspective on MXenes: Their synthesis, properties, and recent applications, J. Appl. Phys., 2020, 128(17), 170902 CrossRef.
- H. Kaur, et al., Progress and challenges of graphene and its congeners for biomedical applications: Drug delivery, gene delivery, biosensing, bioimaging, and tissue engineering, J. Mol. Liq., 2022, 120703 CrossRef.
- M. Garg and A. Thakur, A review: Biomedical applications of phosphorene, antimonene, and germanene-based 2D material/hydrogel complexes, J. Mater. Sci., 2022, 1–12 Search PubMed.
- N. Sultana, et al., Synthesis, Modification, and Application of Black Phosphorus, Few-Layer Black Phosphorus (FLBP), and Phosphorene: A Detailed Review, Mater. Adv., 2022, 3, 5557–5574 RSC.
- D. Presutti, et al., Transition metal dichalcogenides (TMDC)-based nanozymes for biosensing and therapeutic applications, Materials, 2022, 15(1), 337 CrossRef PubMed.
- M. D. Prakash, et al., Performance analysis of ion-sensitive field effect transistor with various oxide materials for biomedical applications, Silicon, 2022, 14(11), 6329–6339 CrossRef.
- J.-C. Lei, X. Zhang and Z. Zhou, Recent advances in MXene: Preparation, properties, and applications, Front. Phys., 2015, 10(3), 276–286 CrossRef.
- S. Panda, et al., MXene based emerging materials for supercapacitor applications: Recent advances, challenges, and future perspectives, Coord. Chem. Rev., 2022, 462, 214518 CrossRef.
- V. Natu, et al., Effect of Base/Nucleophile Treatment on Interlayer Ion Intercalation, Surface Terminations, and Osmotic Swelling of Ti3C2Tz MXene Multilayers, Chem. Mater., 2022, 34(2), 678–693 CrossRef.
- X. Zhang, W. Zhang and H. Zhao, Electrochemical performance of Ti3C2Tx MXenes obtained via ultrasound assisted LiF-HCl method, Mater. Today Commun., 2022, 33, 104384 CrossRef.
- U. Khan, et al., Synthesis of fluorine free MXene through lewis acidic etching for application as electrode of proton supercapacitors, J. Alloys Compd., 2022, 926, 166903 CrossRef.
- H. Chen, H. Wang and C. Li, Mechanically Induced Nanoscale Architecture Endows a Titanium Carbide MXene Electrode with Integrated High Areal and Volumetric Capacitance, Adv. Mater., 2022, 34(43), 2205723 CrossRef PubMed.
- Q. Tang, et al., Boosted CO2 photoreduction performance on Ru-Ti3CN MXene-TiO2 photocatalyst synthesized by non-HF Lewis acidic etching method, J. Colloid Interface Sci., 2022, 619, 179–187 CrossRef PubMed.
- J. Chen, et al., Molten Salt–Shielded Synthesis (MS3) of MXenes in Air, Energy Environ. Mater., 2022, 1–6 Search PubMed.
- I. Ashraf, et al., Hydrothermal synthesis and water splitting application of d-Ti3C2 MXene/V2O5 hybrid nanostructures as an efficient bifunctional catalyst, Int. J. Hydrogen Energy, 2022, 47(64), 27383–27396 CrossRef.
- F. Cao, et al., Mixed-Dimensional MXene-Based Composite Electrodes Enable Mechanically Stable and Efficient Flexible Perovskite Light-Emitting Diodes, Nano Lett., 2022, 4246–4252 CrossRef PubMed.
- L. Gao, et al., Optical Properties of Few-Layer Ti3CN MXene: From Experimental Observations to Theoretical Calculations, ACS Nano, 2022, 16(2), 3059–3069 CrossRef PubMed.
- B. Zhu, et al., Two-Dimensional Nitrogen-Doped Ti3C2 Promoted Catalysis Performance of Silver Nanozyme for Ultrasensitive Detection of Hydrogen Peroxide, ChemElectroChem, 2022, 9(10), e202200050 CrossRef.
- R. Khan and S. Andreescu, MXenes-based bioanalytical sensors: Design, characterization, and applications, Sensors, 2020, 20(18), 5434 CrossRef PubMed.
- M. Huang, et al., MXene and black phosphorus based 2D nanomaterials in bioimaging and biosensing: Progress and perspectives, J. Mater. Chem. B, 2021, 9(26), 5195–5220 RSC.
- J. Huang, et al., Progress and biomedical applications of MXenes, Nano Sel., 2021, 2(8), 1480–1508 CrossRef.
- Y. Xu, et al., 2D-ultrathin MXene/DOXjade platform for iron chelation chemo-photothermal therapy, Bioact. Mater., 2022, 14, 76–85 CrossRef PubMed.
- Y. Wang, et al., MXenes: Focus on optical and electronic properties and corresponding applications, Nanophotonics, 2020, 9(7), 1601–1620 CrossRef.
- S. Li, et al., New opportunities for emerging 2D materials in bioelectronics and biosensors, Curr. Opin. Biomed. Eng., 2020, 13, 32–41 CrossRef.
- G. Liu, et al., Surface modified Ti3C2 MXene nanosheets for tumor targeting photothermal/photodynamic/chemo synergistic therapy, ACS Appl. Mater. Interfaces, 2017, 9(46), 40077–40086 CrossRef PubMed.
- L. Bai, et al., Surface modification engineering of two-dimensional titanium carbide for efficient synergistic multitherapy of breast cancer, J. Mater. Chem. B, 2020, 8(30), 6402–6417 RSC.
- A. Sundaram, et al., Engineering of 2D transition metal carbides and nitrides MXenes for cancer therapeutics and diagnostics, J. Mater. Chem. B, 2020, 8(23), 4990–5013 RSC.
- A. Sundaram, et al., Transition metal carbide—MXene, in Handbook of Carbon-Based Nanomaterials, Elsevier, 2021, pp. 671–709 Search PubMed.
- A. B. Sengul and E. Asmatulu, Toxicity of metal and metal oxide nanoparticles: a review, Environ. Chem. Lett., 2020, 18(5), 1659–1683 CrossRef.
- M. S. Chavali and M. P. Nikolova, Metal oxide nanoparticles and their applications in nanotechnology, SN Appl. Sci., 2019, 1(6), 1–30 Search PubMed.
- K. ur Rehman, et al., A Coronopus didymus based eco-benign synthesis of Titanium dioxide nanoparticles (TiO2 NPs) with enhanced photocatalytic and biomedical applications, Inorg. Chem. Commun., 2022, 137, 109179 CrossRef.
- J. Jiang, J. Pi and J. Cai, The advancing of zinc oxide nanoparticles for biomedical applications, Bioinorg. Chem. Appl., 2018, 2018, 1062562 Search PubMed.
- T. V. Tran, et al., Green synthesis of ZrO2 nanoparticles and nanocomposites for biomedical and environmental applications: a review, Environ. Chem. Lett., 2022, 1–23 Search PubMed.
- M. Mahdavi, et al., Synthesis, surface modification and characterisation of biocompatible magnetic iron oxide nanoparticles for biomedical applications, Molecules, 2013, 18(7), 7533–7548 CrossRef PubMed.
- M. Wu, et al., Manganese dioxide nanosheets: from preparation to biomedical applications, Int. J. Nanomed., 2019, 14, 4781 CrossRef PubMed.
- F. L. Rashid, et al., Novel phase change materials, MgO nanoparticles, and water based nanofluids for thermal energy storage and biomedical applications, Int. J. Pharm. Phytopharm. Res., 2018, 8(1), 46–56 Search PubMed.
- A. B. Shcherbakov, et al., CeO2 nanoparticle-containing polymers for biomedical applications: A review, Polymers, 2021, 13(6), 924 CrossRef PubMed.
- M. E. Grigore, et al., Methods of synthesis, properties and biomedical applications of CuO nanoparticles, Pharmaceuticals, 2016, 9(4), 75 CrossRef PubMed.
- A. Maleki, et al., Biomedical Applications of MXene-Integrated Composites: Regenerative Medicine, Infection Therapy, Cancer Treatment, and Biosensing, Adv. Funct. Mater., 2022, 32(34), 2203430 CrossRef.
- P. Iravani, S. Iravani and R. S. Varma, MXene-chitosan composites and their biomedical potentials, Micromachines, 2022, 13(9), 1383 CrossRef PubMed.
- L. Chen, et al., Biomedical Applications of MXenes: From Nanomedicine to Biomaterials, Acc. Mater. Res., 2022, 3(8), 785–798 CrossRef.
- A.-Z. Warsi, et al., Synthesis, Characterization, Photocatalysis, and Anti-bacterial Study of WO3, MXene and WO3/MXene Nanocomposite, Nanomaterials, 2022, 12(4), 713 CrossRef PubMed.
- I. A. Alsafari, Synthesis of CuO/MXene nanocomposite to study its photocatalytic and anti-bacterial properties, Ceram. Int., 2022, 48(8), 10960–10968 CrossRef.
- G. Liu, et al., Magnetically separable MXene@ Fe3O4/Au/PDA nanosheets with photothermal-magnetolytic coupling anti-bacterial performance, Appl. Surf. Sci., 2022, 590, 153125 CrossRef.
- M. Ding, et al., Novel α-Fe2O3/MXene nanocomposite as heterogeneous activator of peroxymonosulfate for the degradation of salicylic acid, J. Hazard. Mater., 2020, 382, 121064 CrossRef PubMed.
- K. Chaudhuri, et al., Optical properties of MXenes, in 2D Metal Carbides and Nitrides (MXenes), Springer, 2019, pp. 327–346 Search PubMed.
- K. Hantanasirisakul, et al., Fabrication of Ti3C2Tx MXene transparent thin films with tunable optoelectronic properties, Adv. Electron. Mater., 2016, 2(6), 1600050 CrossRef.
- X. Jiang, et al., Broadband nonlinear photonics in few–layer MXene Ti3C2Tx (T = F, O, or OH), Laser Photonics Rev., 2018, 12(2), 1700229 CrossRef.
- B. Fu, et al., MXenes: Synthesis, optical properties, and applications in ultrafast photonics, Small, 2021, 17(11), 2006054 CrossRef PubMed.
- B. Onel, et al., A new G-quadruplex with hairpin loop immediately upstream of the human BCL2 P1 promoter modulates transcription, J. Am. Chem. Soc., 2016, 138(8), 2563–2570 CrossRef PubMed.
- X. Jiang, et al., Two-dimensional MXenes: From morphological to optical, electric, and magnetic properties and applications, Phys. Rep., 2020, 848, 1–58 CrossRef.
- C. Wang, et al., The SnO2/MXene Composite Ethanol Sensor Based on MEMS Platform, Chemosensors, 2022, 10(3), 109 CrossRef.
- J. Low, et al., TiO2/MXene Ti3C2 composite with excellent photocatalytic CO2 reduction activity, J. Catal., 2018, 361, 255–266 CrossRef.
- Y. Zhu, et al., MnO2-MXene Composite as Electrode for Supercapacitor, J. Electrochem. Soc., 2022, 169(3), 030524 CrossRef.
- T. Rasheed, et al., A cost-effective approach to synthesize NiFe2O4/MXene heterostructures for enhanced photodegradation performance and anti-bacterial activity, Adv. Powder Technol., 2021, 32(7), 2248–2257 CrossRef.
- A. Iqbal, et al., Improving oxidation stability of 2D MXenes: synthesis, storage media, and conditions, Nano Convergence, 2021, 8(1), 1–22 CrossRef PubMed.
- X. Zhao, M. Radovic and M. J. Green, Synthesizing MXene nanosheets by water-free etching, Chem, 2020, 6(3), 544–546 Search PubMed.
- N. Akhtar, et al., Synthesis and characterization of MXene/BiCr2O4 nanocomposite with excellent electrochemical properties, J. Mater. Res. Technol., 2021, 15, 2007–2015 CrossRef.
- N. Ottman, et al., Soil exposure modifies the gut microbiota and supports immune tolerance in a mouse model, J. Allergy Clin. Immunol., 2019, 143(3), 1198–1206 CrossRef PubMed.
- C. Liu, et al., Synthesis of MXene/COF/Cu2O heterojunction for photocatalytic bactericidal activity and mechanism evaluation, Chem. Eng. J., 2022, 430, 132663 CrossRef.
- W. Wang, et al., A photo catalyst of cuprous oxide anchored MXene nanosheet for dramatic enhancement of synergistic anti-bacterial ability, Chem. Eng. J., 2020, 386, 124116 CrossRef.
- M. Z. Jakubczak, Evaluation of the effectiveness of anti-bacterial filtration materials modified with nanocomponents based on Ti3C2, Zakład Biologii, 2020 Search PubMed.
- X. Lian, et al., Electrical Properties and Biological Synaptic Simulation of Ag/MXene/SiO2/Pt RRAM Devices, Electronics, 2020, 9(12), 2098 CrossRef.
- K. S. Ranjith, et al., An ultrasensitive electrochemical sensing platform for rapid detection of rutin with a hybridized 2D–1D MXene-FeWO4 nanocomposite, Sens. Actuators, B, 2021, 344, 130202 CrossRef.
- M. Malaki, A. Maleki and R. S. Varma, MXenes and ultrasonication, J. Mater. Chem. A, 2019, 7(18), 10843–10857 RSC.
- I. A. Alsafari, et al., Synthesis, characterization, photocatalytic and anti-bacterial properties of copper Ferrite/MXene (CuFe2O4/Ti3C2) nanohybrids, Ceram. Int., 2021, 47(20), 28874–28883 CrossRef.
- H. B. Na, et al., Development of a T1 contrast agent for magnetic resonance imaging using MnO nanoparticles, Angew. Chem., 2007, 119(28), 5493–5497 CrossRef.
- K. Rasool, et al., Anti-bacterial activity of Ti3C2Tx MXene, ACS Nano, 2016, 10(3), 3674–3684 CrossRef PubMed.
- L. Liu, et al., A reactive copper-organophosphate-MXene heterostructure enabled anti-bacterial, self-extinguishing and mechanically robust polymer nanocomposites, Chem. Eng. J., 2022, 430, 132712 CrossRef.
- S. Begum, et al., 2D and heterostructure nanomaterial based strategies for combating drug-resistant bacteria, ACS Omega, 2020, 5(7), 3116–3130 CrossRef PubMed.
- H. Zheng, et al., Bioactive anti-inflammatory, anti-bacterial, conductive multifunctional scaffold based on MXene@ CeO2 nanocomposites for infection-impaired skin multimodal therapy, Chem. Eng. J., 2021, 424, 130148 CrossRef.
- A. Rozmysłowska-Wojciechowska, et al., Influence of modification of Ti3C2 MXene with ceramic oxide and noble metal nanoparticles on its antimicrobial properties and ecotoxicity towards selected algae and higher plants, RSC Adv., 2019, 9(8), 4092–4105 RSC.
- T. Tahir, et al., Synthesis of sponge like Gd3+ doped vanadium oxide/2D MXene composites for improved degradation of industrial effluents and pathogens, Ceram. Int., 2022, 48(2), 1969–1980 CrossRef.
- K. Rajavel, et al., Photocatalytic and bactericidal properties of MXene-derived graphitic carbon-supported TiO2 nanoparticles, Appl. Surf. Sci., 2021, 538, 148083 CrossRef.
- S. Tohme, R. L. Simmons and A. Tsung, Surgery for cancer: a trigger for metastases, Cancer Res., 2017, 77(7), 1548–1552 CrossRef PubMed.
- Y. S. Birhan and H.-C. Tsai, Recent developments in selenium-containing polymeric micelles: prospective stimuli, drug-release behaviors, and intrinsic anticancer activity, J. Mater. Chem. B, 2021, 9(34), 6770–6801 RSC.
- R. Baskar, et al., Cancer and radiation therapy: current advances and future directions, Int. J. Med. Sci., 2012, 9(3), 193 CrossRef PubMed.
- I. dos Santos Guimarães, et al., Conventional cancer treatment, in Cancer Treatment-Conventional and Innovative Approaches, IntechOpen, 2013 Search PubMed.
- T. A. Baudino, Targeted cancer therapy: the next generation of cancer treatment, Curr. Drug Discovery Technol., 2015, 12(1), 3–20 CrossRef PubMed.
- A. Sundaram, et al., Advanced nanomaterials for hypoxia tumor therapy: challenges and solutions, Nanoscale, 2020, 12(42), 21497–21518 RSC.
- X. Han, et al., 2D ultrathin MXene–based drug–delivery nanoplatform for synergistic photothermal ablation and chemotherapy of cancer, Adv. Healthcare Mater., 2018, 7(9), 1701394 CrossRef PubMed.
- L. M. Dong, et al., Two-dimensional metal carbides and nitrides (MXenes): preparation, property, and applications in cancer therapy, Nanophotonics, 2020, 9(8), 2125–2145 CrossRef.
- A. Gazzi, et al., Photodynamic therapy based on graphene and MXene in cancer theranostics, Front. Bioeng. Biotechnol., 2019, 7, 295 CrossRef PubMed.
- M. Abbas, et al., Nanotechnology for cancer drug design, delivery, and theranostics applications, in Biogenic Nanoparticles for Cancer Theranostics, Elsevier, 2021, pp. 1–26 Search PubMed.
- Z. Huang, et al., Two-dimensional MXene-based materials for photothermal therapy, Nanophotonics, 2020, 9(8), 2233–2249 CrossRef.
- S. Shurbaji, et al., Characterization of MXene as a cancer photothermal agent under physiological conditions, Front. Nanotechnol., 2021, 63 Search PubMed.
- C. Dai, H. Lin, G. Xu, Z. Liu, R. Wu and Y. Chen, Biocompatible 2D titanium carbide (MXenes) composite nanosheets for pH-responsive MRI-guided tumor hyperthermia, Chem. Mater., 2017, 29(20), 8637–8652 CrossRef.
- Z. Liu, et al., 2D superparamagnetic tantalum carbide composite MXenes for efficient breast-cancer theranostics, Theranostics, 2018, 8(6), 1648 CrossRef PubMed.
- H. Xiang, et al., Hypoxia-irrelevant photonic thermodynamic cancer nanomedicine, ACS Nano, 2019, 13(2), 2223–2235 Search PubMed.
- D.-Y. Zhang, et al., In situ TiO2−x decoration of titanium carbide MXene for photo/sono-responsive antitumor theranostics, J. Nanobiotechnol., 2022, 20(1), 1–14 CrossRef PubMed.
- Z. Liu, et al., Redox chemistry-enabled stepwise surface dual nanoparticle engineering of 2D MXenes for tumor-sensitive T1 and T2 MRI-guided photonic breast-cancer hyperthermia in the NIR-II biowindow, Biomater. Sci., 2022, 10(6), 1562–1574 RSC.
- X. Zhang, et al., Ti3C2-MXene@N-doped carbon heterostructure-based electrochemical sensor for simultaneous detection of heavy metals, J. Electroanal. Chem., 2022, 911, 116239 CrossRef.
- D. Yi, et al., Ti3CN MXene-based ultra-sensitive optical fiber salinity sensor, Opt. Lett., 2022, 47(1), 138–141 CrossRef PubMed.
- Y. Pei, et al., Ti3C2TX MXene for sensing applications: recent progress, design principles, and future perspectives, ACS Nano, 2021, 15(3), 3996–4017 CrossRef PubMed.
- M. Xin, et al., MXenes and their applications in wearable sensors, Front. Chem., 2020, 8, 297 CrossRef PubMed.
- K. Grabowski, et al., Recent advances in MXene-based sensors for Structural Health Monitoring applications: a review, Measurement, 2021, 110575 Search PubMed.
- B. Xu, C. Zhi and P. Shi, Latest advances in MXene biosensors, J. Phys.: Mater., 2020, 3(3), 031001 Search PubMed.
- A. Sinha, et al., MXene-based sensors and biosensors: next-generation detection platforms, in Handbook of Nanomaterials in Analytical Chemistry, Elsevier, 2020, pp. 361–372 Search PubMed.
- R. Thenmozhi, et al., MXene Based Transducer for Biosensor Applications, J. Electrochem. Soc., 2021, 168, 117507 CrossRef.
- C. Verma and K. K. Thakur, Recent advances in MXene-based electrochemical sensors, Eur. J. Mol. Clin. Med., 2020, 7, 4429–4450 Search PubMed.
- D. Guo, G. Xie and J. Luo, Mechanical properties of nanoparticles: basics and applications, J. Phys. D: Appl. Phys., 2013, 47(1), 013001 CrossRef.
- T. Sebastian and F. Clemens, Piezoelectric application of metal oxide nanofibers, in Metal Oxide-Based Nanofibers and Their Applications, Elsevier, 2022, pp. 215–246 Search PubMed.
- R. Parra and H. Farrell, Binding energy of metal oxide nanoparticles, J. Phys. Chem. C, 2009, 113(12), 4786–4791 CrossRef.
- M. A. Carpenter, S. Mathur and A. Kolmakov, Metal oxide nanomaterials for chemical sensors, Springer Science & Business Media, 2012 Search PubMed.
- R. A. Soomro, et al., NiWO4-induced partial oxidation of MXene for photo-electrochemical detection of prostate-specific antigen, Sens. Actuators, B, 2021, 328, 129074 CrossRef.
- G. Chen, et al., Efficient Z-Scheme heterostructure based on TiO2/Ti3C2Tx/Cu2O to boost photoelectrochemical response for ultrasensitive biosensing, Sens. Actuators, B, 2020, 312, 127951 CrossRef.
- S. Boobphahom, et al., TiO2/MXene-PVA/GO hydrogel-based electrochemical sensor for neurological disorder screening via urinary norepinephrine detection, Microchim. Acta, 2021, 188(11), 1–12 CrossRef PubMed.
- F. Wang, et al., TiO2 nanoparticle modified organ-like Ti3C2 MXene nanocomposite encapsulating hemoglobin for a mediator-free biosensor with excellent performances, Biosens. Bioelectron., 2015, 74, 1022–1028 CrossRef PubMed.
- X. Lu, et al., A covalent organic polymer–TiO2/Ti3C2 heterostructure as nonenzymatic biosensor for voltammetric detection of dopamine and uric acid, Microchim. Acta, 2021, 188(3), 1–11 CrossRef PubMed.
- C. He, et al., Combinatorial Photothermal 3D-Printing Scaffold and Checkpoint Blockade Inhibits Growth/Metastasis of Breast Cancer to Bone and Accelerates Osteogenesis, Adv. Funct. Mater., 2021, 31(10), 2006214 CrossRef.
- J. K. Wychowaniec, et al., Unique cellular network formation guided by heterostructures based on reduced graphene oxide - Ti3C2Tx MXene hydrogels, Acta Biomater., 2020, 115, 104–115 CrossRef PubMed.
- B. Guo and P. X. Ma, Conducting Polymers for Tissue Engineering, Biomacromolecules, 2018, 19(6), 1764–1782 CrossRef PubMed.
- K. Dzobo, et al., Advances in Regenerative Medicine and Tissue Engineering: Innovation and Transformation of Medicine, Stem Cells Int., 2018, 2018, 2495848 Search PubMed.
- M. Tang, et al., Dual active nanozyme-loaded MXene enables hyperthermia-enhanced tumor nanocatalytic therapy, Chem. Eng. J., 2022, 449, 137847 CrossRef.
- H. Lin, et al., Two-Dimensional Ultrathin MXene Ceramic Nanosheets for Photothermal Conversion, Nano Lett., 2017, 17(1), 384–391 CrossRef PubMed.
- H. Lin, Y. Chen and J. Shi, Insights into 2D MXenes for Versatile Biomedical Applications: Current Advances and Challenges Ahead, Adv. Sci., 2018, 5(10), 1800518 CrossRef PubMed.
- H. Lin, et al., A Two-Dimensional Biodegradable Niobium Carbide (MXene) for Photothermal Tumor Eradication in NIR-I and NIR-II Biowindows, J. Am. Chem. Soc., 2017, 139(45), 16235–16247 CrossRef PubMed.
- W. Feng, et al., Ultrathin Molybdenum Carbide MXene with Fast Biodegradability for Highly Efficient Theory-Oriented Photonic Tumor Hyperthermia, Adv. Funct. Mater., 2019, 29(22), 1901942 CrossRef.
- H. Cao , et al., A Genetic Algorithm Based Motor Controller System Automatic Layout Method, in 2019 10th International Conference on Power Electronics and ECCE Asia (ICPE 2019 - ECCE Asia), 2019.
- Z. Xie, et al., Biocompatible Two-Dimensional Titanium Nanosheets for Multimodal Imaging-Guided Cancer Theranostics, ACS Appl. Mater. Interfaces, 2019, 11(25), 22129–22140 CrossRef PubMed.
- C. Dai, et al., Two-dimensional tantalum carbide (MXenes) composite nanosheets for multiple imaging-guided photothermal tumor ablation, ACS Nano, 2017, 11(12), 12696–12712 CrossRef PubMed.
- Y. Sun, et al., In situ growth of TiO2 nanowires on Ti3C2 MXenes nanosheets as highly sensitive luminol electrochemiluminescent nanoplatform for glucose detection in fruits, sweat and serum samples, Biosens. Bioelectron., 2021, 194, 113600 CrossRef PubMed.
- J. Csavina, et al., Effect of wind speed and relative humidity on atmospheric dust concentrations in semi-arid climates, Sci. Total Environ., 2014, 487, 82–90 CrossRef PubMed.
- V. Myndrul, et al., MXene nanoflakes decorating ZnO tetrapods for enhanced performance of skin-attachable stretchable enzymatic electrochemical glucose sensor, Biosens. Bioelectron., 2022, 207, 114141 CrossRef PubMed.
- M. P. Browne, D. Tyndall and V. Nicolosi, Curr. Opin. Electrochem., 2022, 34, 101021 CrossRef.
- L. Tan, J. Lv, X. Xu, H. Zhao, C. He, H. Wang and W. Zheng, Ceram. Int., 2019, 45, 6597–6600 CrossRef.
- G. Murali, J. K. Reddy Modigunta, Y. H. Park, J. H. Lee, J. Rawal, S. Y. Lee, I. In and S. J. Park, ACS Nano, 2022, 16, 13370–13429 CrossRef PubMed.
- R. B. Rakhi, B. Ahmed, D. H. Anjum and H. N. Alshareef, ACS Appl. Mater. Interfaces, 2016, 8, 18806–18814 CrossRef PubMed.
- W. Xi, J. Jin, Y. Zhang, R. Wang, Y. Gong, B. He and H. Wang, Nanoscale, 2022, 14, 11923–11944 RSC.
- C. Li, G. Jiang, M. Demir, Y. Sun, R. Wang and T. Liu, J. Energy Storage, 2022, 56, 105986 CrossRef.
| This journal is © The Royal Society of Chemistry 2023 |




