 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Carbon dot–phthalocyanine hybrids: synergistic effects that boost their multifaceted applications
Carla I. M.
Santos
 *ab,
Ana Catarina
Almeida
*ab,
Ana Catarina
Almeida
 c,
Ana L. F.
Martins
c,
Ana R.
Araújo
b,
Leandro M. O.
Lourenço
c,
Ana L. F.
Martins
c,
Ana R.
Araújo
b,
Leandro M. O.
Lourenço
 *b,
Gil
Gonçalves
*b,
Gil
Gonçalves
 *cd and
M. Graça P. M. S.
Neves
*cd and
M. Graça P. M. S.
Neves
 *b
*b
aCentro de Química Estrutural (CQE), Instituto Superior Técnico, Av. Rovisco Pais, 1049-001 Lisboa, Portugal. E-mail: carla.santos@tecnico.ulisboa.pt
bLAQV-REQUIMTE, Department of Chemistry, University of Aveiro, Campus Universitário de Santiago, 3810-193 Aveiro, Portugal. E-mail: leandrolourenco@ua.pt; gneves@ua.pt
cTEMA-Nanotechnology Research Group, Mechanical Engineering Department, University of Aveiro, Campus Universitario de Santiago, 3810-193 Aveiro, Portugal. E-mail: ggoncalves@ua.pt
dIntelligent Systems Associate Laboratory (LASI), 4800-058 Guimarães, Portugal
First published on 12th April 2025
Abstract
Since their discovery, carbon dots (CDs) have been extensively studied for their potential in diverse applications owing to their unique properties such as high biocompatibility, excellent water solubility, low toxicity, minimal photobleaching, and exceptional chemical versatility. These characteristics position CDs as promising candidates for overcoming the limitations of various molecular compounds. This review provides a comprehensive analysis of the synergistic effects arising from the integration of CDs and phthalocyanines (Pcs) to form hybrids with distinct photophysical and photochemical properties. This study explores recent advances in the development of Pc@CD hybrids, focusing on their synthesis, conjugation strategies, and synergistic effects that impact their performance in several areas, including optical sensing, electrocatalysis, photodynamic processes and photocatalysis. Emphasis is given to chemical methods that enable efficient conjugation and the role of the generation of reactive oxygen species in driving these applications. Additionally, the discussion also addresses key challenges, highlighting innovative solutions and proposing future research directions to fully harness the potential of Pc@CD hybrids in diverse scientific and technological breakthroughs.
1. Introduction
1.1. Carbon dots’ structure and properties
Carbon dots (CDs) are considered a new emerging class of zero-dimensional (0D) carbon-based luminescent nanomaterials. Although CDs have just recently gained popularity, it is postulated that they should be one of the oldest and most abundant nanoparticles on the Earth, resulting from natural phenomena such as combustion.1,2 Their denomination arises from the fact that they are mainly composed of carbon in different types of hybridization states. Typically, CDs present a broad photoluminescence (PL) profile where the emission is extremely dependent on the excitation wavelength. The optical properties of the CDs are usually controlled by intrinsic structural features including the size and morphology, functional groups, and heteroatom doping. CDs are defined by a size below 10 nm with a quasi-spherical morphology, which are composed of an sp2/sp3 carbon core that can be easily doped with heteroatoms like boron, nitrogen, and/or sulfur.3 Their surface usually presents abundant functional groups such as carboxyl, hydroxyl, amine, and/or polymer chains, depending on the raw material and synthesis method applied for their fabrication.4 Although important pieces of the puzzle about the chemical structure of CDs have been uncovered, some critical knowledge remains unknown, owing to the complexity of the reaction pathways involved in their manufacture.5 Consequently, the molecular origin and other key factors that control CDs’ photophysical properties are still, in most cases, an enigma that has been slowly revealed.6 So far, it is well known that some properties of CDs can be mainly attributed to their core, such as toxicity, while other properties such as dispersibility and optical properties can be mainly ascribed to their surface functional groups.71H and 13C nuclear magnetic resonance (NMR) has been recently explored as an essential tool for the identification of the functional groups of CDs, thanks to its accuracy in disclosing molecular species.8 The impact of CDs on scientific research has been mostly associated with their extraordinary optical properties, including tuneable photoluminescence,9 excitation wavelength-dependent emission,10 and multiphoton excitation.11–13The intense research on light-emitting CDs was sparked in 2006 with the discovery of room-temperature light emission.14 The pioneer CDs presented emissions mostly located in the blue- or green-light region; however, researchers recently developed new synthetic strategies to shift their emission to red- and near-infrared regions (therapeutic window) to improve their relevance for biomedical applications.15–18 Nowadays, CDs can present a broad range of emission bands covering almost the full spectrum, including UV-Vis and infrared regions.19–21
The extensive versatility of chemical modifications in CDs renders them highly desirable for overcoming diverse technological challenges. Several strategies have been employed, including heteroatom22 or metal23 surface passivation,24 edge group modification,25 or organic functionalization.6 Compared to heteroatom doping, surface functionalization, based on well-established organic reactions, is more controllable. Various approaches have been explored for the chemical modification of CDs including surface chemistry interactions (e.g., hydrophobic, electrostatic, and π–π interactions) or through the establishment of strong molecular bonds (e.g., covalent bonding and coordination). The chemical surface modification of CDs can contribute to tuning solvents’ dispersibility, induce desired biological targeting, regulate catalytic performance, improve the photoluminescence (PL) emission, and regulate the band gaps of CDs. These extraordinary features of CDs allowed their fast proliferation for the development of advanced novel materials that found relevant applications in several fields, including biomedicine, sensing, (photo)catalysis, electronics, energy, and environmental remediation. In addition, CDs present lower toxicity, minor production costs, and excellent biocompatibility when compared with some traditional semiconductor inorganic quantum dots containing cadmium or lead.26–29
The extensive diversity of methodologies explored thus far, coupled with the wide array of available carbon-based raw materials, has led to an exponential growth in the preparation of various types of CDs. The categorization of CDs is closely linked to the extent of carbonization and graphitization within their carbon core. Therefore, the synthetic parameters of CDs can be customized to achieve specific properties (Fig. 1A).
Effective control of the experimental parameters is critical for controlling the structure, morphology, and final properties of CDs. The first step in the design of CDs is to properly define the starting materials that can originate from organic or inorganic precursors. Organic precursors, such as citric acid, glucose, or amino acids, tend to produce amorphous or quasi-graphitic CDs, whereas inorganic precursors might result in more graphitic CDs. The temperature at which synthesis occurs can significantly affect the degree of carbonization and graphitization. Higher temperatures often lead to more ordered graphitic structures, whereas lower temperatures induce the formation of amorphous carbon structures. The reaction duration is also an important parameter, with longer reaction times favouring more extensive carbonization and graphitization, resulting in CDs with a higher degree of crystallinity. The type of atmosphere (air, nitrogen, or argon) can also affect the carbonization process, where inert atmospheres such as nitrogen or argon can promote graphitization by minimizing oxidation. The concentration of the precursors, the pH of the reaction solution, and the choice of solvent can influence the reaction kinetics, and consequently, the structure of the CDs. The carbonization process can also be affected by the solvent type. The introduction of dopants or surface functional groups during the synthetic process can further modify the properties of CDs. Post-synthesis treatments, such as annealing or surface passivation, can be applied to modify the properties of CDs after synthesis. The diverse range of CDs reported in recent years has sparked vigorous debates regarding their classification and nomenclature. Consequently, the absence of well-defined parameters for standardization has resulted in similar CDs being referred to by different designations. As a result of this imprecise terminology, distinguishing between different types of CDs in the literature has become a considerable challenge. To address this classification challenge, we will adopt the categorization proposed by Prato et al.,5 which is based on three fundamental criteria: morphology, quantum confinement effects (QCEs), and crystallinity (Fig. 1A). According to this classification, CDs can be divided into four primary subtypes. Graphene quantum dots (GQDs) feature a graphitic planar structure modified with chemical functional groups either at the edges or within interlayer defects. These functional groups are responsible for their intriguing quantum confinement effects and edge effects. GQDs typically have a higher ratio of sp2 carbon atoms, resembling a few layers of graphene in their core, with the surface covered by sp3 carbon atoms. Their QCEs are linked to the size of the emissive π domains. The carbon quantum dots (CQDs) exhibit a spherical core–shell morphology, with a crystalline lattice containing a high density of sp2 hybridized carbon atoms in the core and a shell intercalated typically with oxygen and nitrogen functional groups. These structural features result in quantum confinement effects within the emissive π domains. Carbon nanodots (CNDs) are quasi-spherical nanoparticles characterized by a carbonized core structure and surface functional groups. Carbonized polymer dots (CPDs), typically possessing quasi-spherical morphologies, have hybrid structures comprised of sp2, sp3, or both sp2 and sp3 hybridized carbon atoms in their core. The surfaces are densely covered with polymer chains, and their photoluminescence properties are primarily attributed to the crosslink-enhanced emission effect. Two major approaches can be found in the literature regarding the preparation of CDs: bottom-up and top-down approaches (Fig. 1A).5,30–33
The bottom-up approach typically explores molecular organic precursors, carbohydrates, and polymers as raw materials for the preparation of CDs. Most of the precursors, after being subjected to hydrothermal treatment, microwave (MW)-assisted synthesis, or pyrolysis, undergo partial dehydration and dehydrogenation before forming the CDs’ backbone structure.34–37 Bottom-up methods are typically used to afford CPDs but have also been extensively used to prepare GQDs and CQDs. Usually, the preparation of CDs using this approach requires harsh reaction conditions, such as high temperature and pressure. In addition, it was reported that the addition of other chemical agents to the reaction medium, such as acids, can favor the formation of CDs.38 The hydrothermal synthesis of CDs using organic precursors in a Teflon-lined autoclave has been one of the most explored bottom-up strategies owing to the simplicity of the experimental protocol. The basic premise of CD formation consists of the reaction between the carbon precursors to form small graphitic structures upon heating. As mentioned above, the size and degree of carbonization of CDs can be effectively controlled by optimizing different reaction parameters, such as solvents, temperature, and reaction time.39 The most explored precursor for the synthesis of CDs is citric acid (CA), which, in conjugation with different nitrogen-containing precursors, allows obtaining CDs with different optical features in a controlled manner.40–42
The top-down approach is characterized by the extensive fragmentation of carbon-based materials, whether they are in macro-, micro-, or nano-form, into tiny nanosized particles. This strategy typically employs carbon allotropes as raw materials, including graphite, carbon black, carbon fibers, carbon nanotubes (CNTs), carbon nanohorns (CNHs), fullerenes, and various graphene-based materials. The primary objective is to convert the large sp2 carbon domains within these materials into smaller sp2 domains, ultimately forming GQDs.13 Consequently, following this conversion process, the inherent zero bandgap of the graphene structure transforms into a nonzero bandgap due to quantum confinement. This transformation was accompanied by an increase in edge defects, which played a crucial role in the properties of the resulting GQDs. The controlled process of fragmenting graphene can be achieved through several methods, including arc discharge, ball milling, laser ablation, chemical oxidation, and electrochemical etching.43 In particular, the chemical oxidation of carbon nanostructures using MW or hydrothermal treatment has garnered significant attention for the preparation of GQDs. Among these methods, graphene oxide (GO) derivatives have emerged as a popular choice as precursor materials for the top-down synthesis of GQDs.44–46 The high oxidation state of GO nanosheets and their derivatives, characterized by randomly dispersed small sp2 domains within an sp3 matrix, facilitates efficient chemical nanocutting. This process ultimately results in the formation of oxygen-doped GQD fragments with tunable optical properties.
The chemical structure of CDs plays a crucial role in mediating their functionalization, whether through covalent or non-covalent approaches. As mentioned above, CDs possess various functional groups on their surface, such as amino, hydroxyl, and carboxyl, which improve their water stability and provide ideal sites for functionalization with ions, molecules, biomolecules, and polymers. Among these, amine and carboxyl groups are most commonly used for covalent conjugation with PCs through amide bond formation. The concentration of these functional groups on the CD surface typically determines the success and yield of the reaction. CDs can also undergo additional chemical modifications to increase the density of these functional groups. A common approach involves converting hydroxyl (–OH) groups to carboxyl (–COOH) groups, which is easily implemented through the reaction with chloroacetic acid under basic conditions.46 Non-covalent conjugation of CDs with PCs is another widely explored approach for the synthesis of novel hybrid materials. The surface functional groups can facilitate electrostatic interactions with positively or negatively charged PCs, depending on the pH of the reaction medium. Another approach relies on π–π stacking or establishing hydrophobic interactions. In contrast to covalent conjugation, where a higher density of surface functional groups is beneficial, non-covalent conjugation is more effective when CDs have lower surface functionalization and larger aromatic domains.32 Although the aromatic structure of CDs is highly dependent on the synthesis method, various reduction post-treatments have been explored to enhance their graphitic domains, thereby improving their conjugation efficiency with PCs.
However, it is important to note that the chemical structure of PCs can be tuned with complementary functionalization to enhance their covalent or non-covalent interactions with CDs.
1.2. Phthalocyanines’ structural features and synthetic approaches
Pcs are a class of macrocycles of synthetic origin that are structurally similar to analogous porphyrin derivatives, but with a core composed of four isoindole units linked through aza-bridges (Fig. 1B). The term “phthalocyanine” (Pc) was suggested by Linstead in 1933 to describe these blue-green pigments and resulted from the combination of the prefix phthalo, highlighting their synthetic origin from phthalic anhydride and derivatives, and cyanine due to their dominating blue color. However, the first reference to these blue-green derivatives appeared in 1907, when Tcherniac et al. noticed the formation of a small amount of a blue compound after heating o-cyanobenzamide.47 In 1927, Von der Weid et al. of the University de Fribourg described the formation of a highly stable blue material during the chemical reaction of 1,2-dibromobenzene with copper cyanide in pyridine reflux.48–50 However, the compound was not correctly identified (molecular formula C26H18N6Cu),48 and only six years later (1933), Linstead reported the correct structure as the free-base phthalocyanine H2Pc and its Cu(II) complex CuPc (Fig. 1B). In the same period, Scottish Dyes Ltd also detected a highly stable and insoluble blue impurity during their attempts to produce phthalimide from phthalic anhydride and ammonia, which was patented due to its economic interest, and was posteriorly identified, namely by X-ray crystallography, as the Fe(II) complex of phthalocyanine.51–56 With this progress, synthetic routes were reported57–60 and, in 1935, Cu(II) phthalocyanine was synthesized at an industrial scale and used as a blue pigment in the paint industry. Linstead's investigations were encouraging, but in the case of other scientific areas, the insolubility of these pigments limited their use. In the 60s, the synthesis of highly soluble Pc derivatives allowed the diversification of the applications of these chromophores to other fields.61–63 In the synthesis of this type of macrocycle, a great variety of precursors can be used, such as o-cyanobenzamide, phthalic acid, phthalic anhydride, phthalimide, diiminoisoindoline, and phthalonitrile (Fig. 1B).61,64 Among the most common precursors are phthalonitriles, which, depending on the reaction conditions (e.g., reaction time, temperature, solvent, type of atmosphere, base, and catalyst selected),65 are initially transformed into diiminoisoindoline derivatives via reaction with ammonia. This is followed by cyclotetramerization, which usually occurs in alcoholic solutions at high temperatures. It is noteworthy that the reaction is favoured by the use of a catalytic amount of organic base, such as 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) or 1,5-diazabicyclo[4.3.0]non-5-ene (DBN) in pentanol.66–68 However, reactions with other precursors, such as phthalimide, phthalic acid, and phthalic anhydride, require urea as a nitrogen donor. To prepare free-base Pcs, lithium or magnesium is commonly used as a template, followed by demetallation of the obtained Pc complexes under dilute acidic conditions. On the other hand, the synthesis of metallophthalocyanines (MPc, M = metal ion) can involve two different approaches: (i) the metal is inserted directly into the macrocycle during the tetramerization process using a phthalonitrile and an adequate metal salt69 and (ii) the metal is introduced in the previously prepared free-base H2Pc using an adequate metal salt carrier.70 In the first approach, access to the metal complexes of Pcs requires the heating of the phthalonitrile derivative with the adequate metallic salt in a solvent with a high boiling point, such as dimethylaminoethanol (DMAE), N,N′-dimethylformamide (DMF) or 1-chloronaphthalene.66 However, some procedures can be carried out at lower temperatures by using a basic catalyst namely DBU in pentan-1-ol66 or even at room temperature by using lithium in DMAE.71 These conditions are also relevant for converting H2Pcs adequately substituted at peripheral positions into different MPcs (M = Zn2+, Mg2+, In3+, Ga3+, Ge3+, and Si4+, among others). Most Pcs’ applications are related to the delocalization of 18 π-electrons, leading to relevant physico-chemical and photophysical/photochemical features. In general, their electronic spectra present a broad band at around 300–400 nm called the Soret band (S-band) and an intense Q-band in the range of 600–750 nm. Moreover, the excitation of Pcs at a specific wavelength reveals fluorescence emission between 600 and 800 nm. It is well established that the required features of a Pc for a special target application can be modulated through structural alterations at the peripheral α- and/or β-positions, leading to new systems with distinct characteristics, such as (photo)stability, water solubility, electron transfer abilities, suitable singlet oxygen (1O2) quantum yields (ΦΔ), and fluorescence quantum yields (ΦF). This is responsible for the unequivocal success of Pcs in several scientific areas, namely: (i) biomimetic models of photosynthetic primary processes;72 (ii) catalysts73,74 or photocatalysts;73,75 (iii) sensors;70,76 (iv) photosensitizers (PSs) for antimicrobial photodynamic therapy (aPDT) under different contexts;77–80 (v) PSs for photodynamic therapy (PDT) of malignant diseases,81–83 (vi) G-quadruplex aptamer binding,84–86 and (vii) markers in photomedicine.87 More recently, the possibility of combining Pcs with different carbon-based nanostructures (e.g., CNTs, GO, or CDs)88–90 to develop new conjugates with improved features for different applications has been an important focus of several research projects.In this review, we will delve into a comprehensive discussion of various synthetic strategies employed to conjugate CDs with phthalocyanine (Pc) dyes through covalent and non-covalent interactions to obtain high-performance hybrids. The combination of these two entities has demonstrated remarkable synergistic effects and improved water solubility and biocompatibility with promising outcomes in their applications as nanoprobes for sensing, electrocatalysts, and phototherapeutic agents.91–95
2. Functionalization of carbon dots with phthalocyanines
CDs feature various functional groups on their surface, including amino, hydroxyl, and carboxyl units, which enhance their water stability and provide ideal anchoring sites for further functionalization. The chemical versatility of CDs enables them to establish a wide range of interactions with various types of ionic, molecular, and macromolecular entities via covalent and non-covalent interactions.32 Interactions through covalent bonds require the presence of complementary functionalities in the Pcs and CDs. Since CDs can contain abundant carboxyl or amino groups on their surface, coupling mediated by amide bonds must be performed with Pcs bearing amino and carboxyl functionalities, respectively. In general, the esterification approach involves the carboxyl groups of the CDs and hydroxyl groups present on the Pcs, whereas the sulfonylations require CDs functionalized with amino groups and Pcs with sulfonyl chloride functionalities. Most reported covalent strategies are based on amidation and esterification reactions (Fig. 1C). In the development of assemblies of Pc@CDs based on non-covalent bonds, their formation can occur via electrostatic, complexation, or π–π interactions (Fig. 1C). These interactions are mainly governed by the inverse surface charges between Pcs and CDs or by the strong quenching promoted by the aromatic nature of Pcs and CDs.In this review, we delve into a comprehensive discussion of the various strategies employed to covalently and non-covalently conjugate CDs with Pc dyes. The combination of these two entities has demonstrated remarkable synergistic effects, including alterations in fluorescence quantum yield values (φf), generation of oxidative species, and improved water solubility and biocompatibility. In recent years, these intriguing Pc@CD nanoconjugates have primarily been applied as nanoprobes for biosensing, electrocatalysts, and antibacterial and phototherapeutic agents.91,93,96,97 The current section is organized according to the type of interaction involving both components in the hybrid, non-covalent or covalent, which is preceded by a brief reference concerning their impact on the final features of Pc@CDs. In each section, and when possible, the studies are organized based on their application or potential envisaged. Next, we present a detailed discussion of the properties and applications of Pc@CD conjugates based on their non-covalent or covalent nature. Importantly, the inconsistent nomenclature used for CDs in the literature poses a significant challenge in distinguishing between the different types of CDs. To avoid potential misunderstandings, we will use the generic term “CDs” to refer to this class of carbon-based nanomaterials.
2.1. Non-covalent functionalization: electrostatic and/or π–π interaction
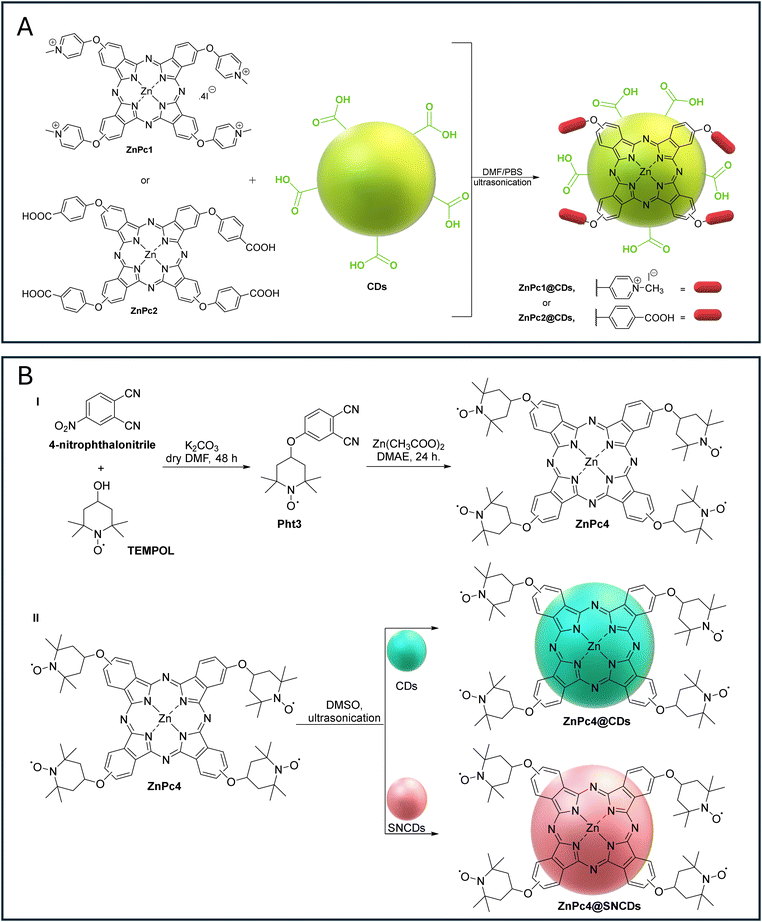 | ||
| Fig. 2 (A) ZnPc1@CDs and ZnPc2@CDs obtained via ionic and π–π stacking interactions with CDs.100 (B) (I) Synthesis of ZnPc4 and hybrids (II) ZnPc4@CDs and ZnPc4@SNCDs. (III) Graphical representation of selective screening of different biomolecules and metal ions and their effects on the fluorescence response of 0.5 mg mL−1 of the ZnPc4@CD and ZnPc4@SNCD nanoprobes in DMSO. | ||
The ZnPc1@CDs were obtained by the addition of CDs in phosphate-buffered saline (PBS) solution at pH = 9 to the cationic ZnPc1 in a DMF/PBS solution, followed by ultrasonication; the alkaline conditions were selected to favor electrostatic interactions between the negatively charged CDs and the positively charged ZnPc1 (Fig. 2A). The hybrid ZnPc2@CDs were obtained under similar conditions, but at pH = 7, to minimize electrostatic repulsion and favor π–π interactions. The extensive evaluation of the photophysical properties of the resulting hybrid materials allowed us to conclude that the fluorescence quenching of the CDs in the hybrids when compared with that of CDs alone (ΦF = 0.22 versus ca. 0.18 for ZnPc1,2@CDs) is due to FRET; this conclusion was also supported by an improvement in the emission of the nearby ZnPc acceptors after excitation at 340 nm where the CDs absorb. The low FRET efficiency obtained for both hybrids (ZnPc1 = 0.18 and ZnPc2 = 0.14) was justified by the low spectral overlap between the emission of the donors (CDs) and the absorption of the acceptors (ZnPc derivatives). In the same work, the authors found that the covalent attachment of Zn(II) tetraaminophthalocyanine (ZnTAPc = ZnPc3) to the CDs resulted in a reduction in the fluorescence (ΦF = 0.12), although the FRET efficiency was higher (ΦF = 0.45). This behavior was attributed to the closeness of both components and their stronger interactions. Since other mechanisms such as electron transfer or exciton annihilation could also lead to fluorescence quenching, further experimental investigations, such as time-resolved fluorescence spectroscopy or a detailed energy level analysis, would help clarify other contributions to the fluorescence quenching of the CDs in the presence of ZnPcs.
Photophysical studies revealed that the ΦF and lifetime (τf) of CDs decreased significantly upon conjugation with ZnPc4 (Table 1) due to Förster resonance energy transfer (FRET). The higher FRET efficiency observed for ZnPc4@SNCDs (0.92) compared to ZnPc4@CDs (0.66) was attributed to a better spectral overlap (J). The donor–acceptor distances (8.1 and 9.2 nm) confirmed effective energy transfer within the 1–10 nm range. Titration assays with ascorbic acid (AA) restored the fluorescence of CDs and SNCDs (“turned ON”), suggesting that AA acted as a spacer or radical scavenger (Fig. 2B). The absence of an enhanced permeation retention (EPR) signal in the paramagnetic nanoassembly further supported this last mechanism. The nanoprobes exhibited high selectivity for AA, with detection limits of 0.2 nM (ZnPc4@SNCDs) and 0.8 nM (ZnPc4@CDs), and excellent specificity against biomolecules and metal ions (e.g., citric acid, glucose, dopamine, folic acid, Fe3+, or Cl−) (Fig. 2B). Their application in real sample quantification yielded near-quantitative recoveries, while the spectra of free CDs and SNCDs remained unchanged in the presence of AA.
| CDs type | Chemical conjugation | Phthalocyanine | Φ f CDs | Φ f Pc | Φ f hybrid | Application | Ref. |
|---|---|---|---|---|---|---|---|
a DMSO.
b PBS.
c DMSO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PBS mixture.
d H2O. PBS mixture.
d H2O.
|
|||||||
| SNCDs | Non-covalent (π–π stacking) | 2,9(10),16(17),23(24)-Tetrakis[(4-methylpiperidin-1-yl)oxyl]phthalocyaninato zinc(II) (ZnPc4) | SNCDs (0.81)a | * | ZnPc4@SNCDs (0.15)a | Ascorbic acid detection | 103 |
| CDs | CDs (0.22)a | * | ZnPc4@CDs (0.09)a | ||||
| CDs | Non-covalent (π–π stacking) | 2,9(10),16(17),23(24)-Tetrakis(1-pyrrolidine-2,5-dione)phthalocyaninato zinc(II) (ZnPc5) | CDs (0.31)b | ZnPc5 (0.2)a | ZnPc5@CDs (0.10)b | Biothiol detection (cysteine, homocysteine, or glutathione) | 106 |
| PEI-CDs | PEI-CDs (0.88)c | * | |||||
| M-CDs | MCDs (0.06)b | * | * | ||||
| PEI-CDs | Non-covalent (π–π stacking) | 2,9(10),16(17),23(24)-Tetrakis(2-pyridiniumsulphanyl)phthalocyaninato zinc(II) tetraiodide (ZnPc6) | PEI-CDs (0.68)b | * | ZnPc6-Au@Ag@PEI-CDs (0.10)b | Biothiol and Hg2+ detection | 107 |
| PEI-CDs-Au@Ag | PEI-CDs-Au@Ag (0.25)b | ||||||
| NCDs | Non-covalent (electrostatic interactions) | 2,9(10),16(17),23(24)-Tetrakis(4-pyridiniumsulphanyl)phthalocyaninato zinc(II) tetraiodide (ZnPc 12) | NCDs (0.10)d | ZnPc12 (0.18)a | * | ds-DNA detection | 108 |
| 2,3,9,10,16,17,23,24-Octakis(4-pyridiniumsulphanyl)phthalocyaninato zinc(II) octaiodide (ZnPc 13) | ZnPc13 (0.21)a | ||||||
| CDs | Non-covalent (π–π stacking) | 2,9(10),16(17),23(24)-Tetrakis(2,6-di-tert-butyl-4-methylphenoxy)phthalocyaninato zinc(II) (CoPc8) | CDs (0.31)a | * | * | Cyanide ion detection | 109 |
The ability of maleimide units to react specifically with thiols via Michael addition110 prompted Achadu et al. to develop ZnPc5@CDs nanoassemblies (Fig. 3A) to evaluate their potential as nanoprobes of biothiols (e.g., cysteine, homocysteine, or glutathione);106 these derivatives, which are involved in several biological processes, are increasingly recognized as potential biomarkers for certain diseases. The required ZnPc5 was synthesized by reacting Zn(II) tetraaminophthalocyanine (ZnPc3) with maleic anhydride (Fig. 3A). Pristine CDs were obtained by oxidizing GO in H2SO4 and HNO3. The fluorescence quenching observed in the ZnPc5@CDs nanoassemblies was attributed to maleimide attachment. For comparison, maleimide carbon dots (M@CDs) were prepared by reacting polyethyleneimine-grafted CDs (PEI-CDs) with 4-maleimidobenzoic acid, resulting in a “turn-OFF” state. However, the photoluminescence (PL) intensity of both nanoassemblies was gradually restored upon addition of cysteine, homocysteine, or glutathione but was not affected by the presence of several α-amino acids and proteins. M@CDs showed higher sensitivity than ZnPc5@CDs due to the direct covalent attachment of maleimide units. No significant fluorescence increase occurred with non-immobilized ZnPc5 upon adding cysteine, highlighting the key role of CDs in the “turn ON” behavior. However, to complement these studies, a comparison with ZnPc3-based nanoassemblies could provide a more realistic idea of the role of maleimide in these hybrids.
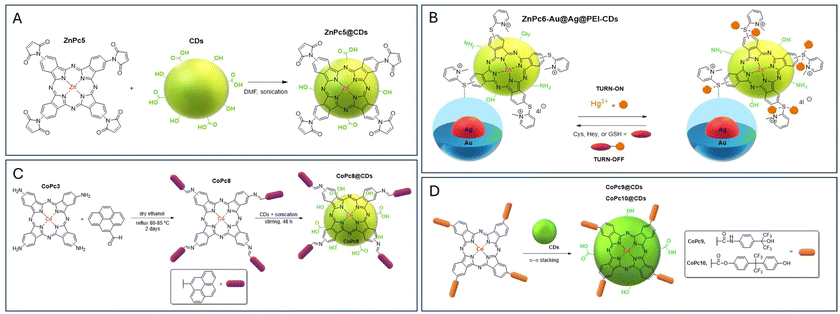 | ||
| Fig. 3 (A) Representation of the ZnPc5@CD nanoassemblies.106 (B) Proposed mechanistic actuation of the “off–on–off” nanoprobe ZnPc6-Au@Ag@PEI-CDs for the detection of Hg2+ and biothiols in aqueous solutions.107 (C) Synthetic route for pyrene-derivatized CoPc8 and CoPc8@CD hybrids.111 (D) Structures of CoPc9 and CoPc10 used for the preparation of CoPc9,10@CD hybrids.112,113 | ||
In the same period, Nyokong et al. developed a dual fluorescence nanoprobe, ZnPc6-Au@Ag@PEI-CDs (Fig. 3B), for detecting Hg2+ and biothiols in aqueous solutions.107 The sensing platform was assembled by electrostatically combining cationic ZnPc6 bearing four thiopyridinium units with a nano-alloy Au@Ag, followed by embedding the conjugate in PEI-CDs obtained from a hydrothermal treatment of GO with hyperbranched polyethyleneimine. The nanoprobe's fluorescence emission was restored in the presence of Hg2+ and “turned OFF” upon the addition of biothiols due to their ability to form strong Hg–S bonds (Fig. 3B). These “off–on–off” nanoprobes were stable and selective, with high resistance to interference from other amino acids (glycine, lysine, histidine, tryptophan, or DL-lactic acid) and metal ions (e.g., Fe2+, Co2+, Mn2+, Cd2+, Cu2+, Ni2+, Pb2+, Mg2+, Ag+, and Zn2+). Their effectiveness was confirmed in real samples spiked with test analytes.
A similar concept was considered in the design of “turn-ON” nanoprobes for detecting Hg2+, obtained through the conjugation of CDs with ZnPc6, the corresponding Al(III) complex AlPc6, ZnPc7 with eight thiopyridinium moieties, and neutral ZnPc6a.107 The fluorescence of the CDs, derived from GO via a hydrothermal method, was initially quenched upon coordination with the Pcs. However, in the presence of Hg2+, the fluorescence was restored due to Hg2+'s strong affinity for mercapto bridges on the Pcs, which disrupted the π–π stacking interaction between the CDs and Pcs. These nanoprobes exhibited high selectivity for Hg2+ over other metal ions, with the best limit of detection (LOD) achieved by octacationic ZnPc7 (0.12 nM), followed by tetracationic ZnPc6 (LOD = 0.52 nM) and AlPc6 (LOD = 0.60 nM). The performance was less effective for neutral ZnPc6a, confirming the relevance of electrostatic interactions in the development of these nanoassemblies.
Achadu et al. described synthetic access to the functional hybrid CoPc8@CDs (Fig. 3C) and demonstrated its use as a selective “turn ON” nanoprobe for CN−.111 The CDs were synthesized by a top-down hydrothermal process, and the Schiff complex CoPc8 was obtained by coupling tetraamino cobalt(II) phthalocyanine CoPc3 with 1-pyrene-1-carboxyaldehyde. The nanoassemblies obtained upon its conjugation with the CDs showed a typical “turn-OFF” behaviour.
The sensing studies showed that in the presence of the anion cyanide, the CDs’ emission was restored and increased linearly with the concentration of CN− (ranging between 1 nM and 50 nM) with a LOD of 0.5 nM. The probe's selectivity for CN− was confirmed through fluorescence recovery assessments in water samples containing various anions (e.g., F−, Cl−, Br−, I−, H2PO4−, HSO4−, NO3−, ClO4−, SCN−, AcO−, HSO3−, SO42−, and CO32−). The inefficacy of the analogous ZnPc8 complex in anion detection supports the hypothesis that axial coordination between the Co metal ion in the Pc inner core and CN− is responsible for disrupting the initial donor–acceptor “turn-OFF” behavior. Considering this fact, to evaluate the real role of the pyrene units in Pc, it would be interesting to compare the sensing results obtained with the nanoassemblies obtained from tetraamino cobalt(II) phthalocyanine CoPc3 or even with non-substituted CoPc. The efficiency of this probe in evaluating trace levels of CN− ions in a real context was confirmed using tap water spiked with known concentrations of CN− between 0.5 and 50 nM with percentage recoveries higher than 97%.
The efficiency of Pc@CD hybrids for gas detection, namely at room temperature, is also attracting some attention. In 2021, Jiang et al. developed nanoassemblies from CDs and CoPc dyes containing hexafluoroisopropanol (HFIP, CoPc9) and hexafluorobisphenol A (6FBPA, CoPc10) substituents (Fig. 3D) and explored their efficiency as sensors towards the chemical warfare agent simulant dimethyl methylphosphonate (DMMP).112 The CoPc9,10@CD hybrids showed strong hydrogen bonds between the two functional units (HFIP and 6FBPA) and the DMMP molecule, leading to an exceptional response behaviour to the analyte. Moreover, it has been reported that the CDs improved the electrical conductivity of the hybrids by π–π interactions with CoPcs and can be considered promising raw materials for use in sarin gas sensors.
In 2021, Zhi Yang et al. developed hybrid materials for NO2 gas sensing based on the anchorage of CDs onto the surface of MPc11 (where Pc is 2,9,16,23-tetracarboxylicphthalocyanine and M = Co, Ni, Cu, or Zn) nanofibers via π–π stacking interactions to form a charge transfer conjugate.113 The addition of CDs greatly increased the conductivity of the Pc fibers, leading to a faster response of the hybrid materials up to 14.5 nA. The sensing mechanism is based on the typical p-type semiconductor behaviour of MPc11@CDs, which have a strong affinity for the electron acceptor molecule NO2. Consequently, after the reaction with the complex, the electrons are trapped by NO2, producing NO2− ions and holes on the nanofiber surface, significantly changing the conductivity of the sensors. The gas-sensitive response (I–V curves) of the CoPc@CD hybrid material to 50 ppm NO2 gas was nearly 16 times higher compared with the individual components. Moreover, the reproducibility, selectivity, and stability of the hybrids are significantly enhanced. The response value was achieved within 100 s, and the recovery curve reached the baseline 125 s after laser exposure.
In the context of biosensing applications, Santiago et al. reported in 2022 that the ZnPc12@NCD and ZnPc13@NCD nanoprobes (Fig. 4A) can induce an “off–on” fluorescence behaviour on nitrogen doped carbon dots (NCDs) after interacting with double-stranded DNA (ds-DNA). These nanoprobes were obtained from positively charged ZnPc12 and ZnPc13 and NCDs prepared by the hydrothermal treatment of GO in the presence of NH4OH and H2O2.108 The ZnPcs were synthesized using the conventional tetramerization of suitable phthalonitriles bearing one or two thiopyridyl units in DMAE and in the presence of Zn(OAc)2, followed by quaternization of the pyridine units with CH3I. Supramolecular Pc@NCD hybrids were prepared in water using solutions of NCDs and Pcs in a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5. This conjugation promoted quenching of the emission of NCDs, but in the presence of ds-DNA, their photoluminescence was restored. This behaviour was justified by the preferential interaction between the positively charged Pcs and the negatively charged dsDNA, with the consequent restoration of the NCDs’ photoluminescence. The limits of detection obtained for ds-DNA with ZnPc13 and ZnPc12 were 66 nM bp and 1.1 mM bp (bp = molar concentration of pairs), respectively. The concentration difference used for each Pc dye is most likely associated with their solubility, depending on the number of peripheral substituents (four versus eight). Both nanosensors exhibit good selectivity for ds-DNA when tested against biothiols and inorganic anions as potential interferents (Fig. 4B).
5. This conjugation promoted quenching of the emission of NCDs, but in the presence of ds-DNA, their photoluminescence was restored. This behaviour was justified by the preferential interaction between the positively charged Pcs and the negatively charged dsDNA, with the consequent restoration of the NCDs’ photoluminescence. The limits of detection obtained for ds-DNA with ZnPc13 and ZnPc12 were 66 nM bp and 1.1 mM bp (bp = molar concentration of pairs), respectively. The concentration difference used for each Pc dye is most likely associated with their solubility, depending on the number of peripheral substituents (four versus eight). Both nanosensors exhibit good selectivity for ds-DNA when tested against biothiols and inorganic anions as potential interferents (Fig. 4B).
 | ||
Fig. 4 (A) Structures of ZnPc12,13 used in the formation of ZnPc12,13@NCD nanohybrids. (B) Selectivity of the nanosensors (ZnPc12@NCDs and ZnPc13@NCDs with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 ratio of Pc 5 ratio of Pc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NCDs and [NCDs] = 2 μM) towards ds-DNA, showing photoluminescence enhancement in the presence of an excess (100 μM) of various relevant analytes. Reproduced under terms of the CC BY 4.0 license.108 Copyright 2022, MDPI. NCDs and [NCDs] = 2 μM) towards ds-DNA, showing photoluminescence enhancement in the presence of an excess (100 μM) of various relevant analytes. Reproduced under terms of the CC BY 4.0 license.108 Copyright 2022, MDPI. | ||
Despite these advantageous properties, the low solubility of Pcs in aqueous media and their lack of specificity pose significant challenges for their biological applications. To overcome these limitations, researchers have investigated the conjugation of Pcs with carbon-based nanomaterials. Among these, CDs have emerged as an appealing choice because of their high biocompatibility, water solubility, and efficient cellular internalization.114,115 The following sections discuss various approaches reported in the literature for synthesizing Pc-based CDs and assessing their performance in terms of emission quantum yield and ROS generation. Such insights are crucial for advancing solutions involving PSs, particularly Pcs. These applications include tumour cell eradication, microorganism inactivation and photocatalysis, underscoring the diverse potential of Pc@CDs in photosensitizing processes.
2.1.3.1. Cancer therapy. To identify efficient Pc@CD hybrids for phototherapeutic applications, Matshitse et al. evaluated how the physicochemical features of the obtained nanoassemblies through electrostatic and/or π–π interactions were affected by the Pc charge. This systematic study was performed using unsubstituted ZnPc, ZnPc1 bearing N-methylpyridyloxy units, and tetrasulfonated ZnPc14 as prototypes, respectively, of neutral, positively, and negatively charged derivatives to construct the corresponding CD nanoassemblies (Fig. 5A).116 CDs with a size near 2.2 nm were obtained through the chemical oxidation of GO using KMnO4 and H2SO4 as oxidative agents.117 The successful conjugation was confirmed by different characterization techniques, and it was concluded that the largest loading was observed for ZnPc1@CDs (14 Pc molecules per CD versus 4 for ZnPc and 3 for ZnPc14). These results were attributed to the establishment of both π–π and electrostatic interactions between the negatively charged CDs and the positively charged ZnPc1.
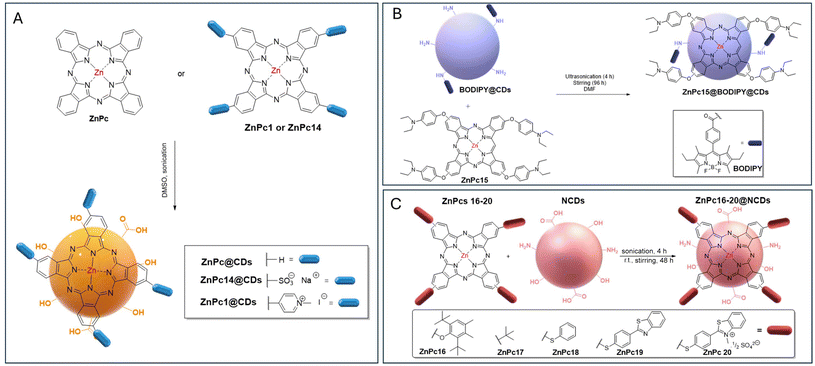 | ||
| Fig. 5 (A) The structures of neutral, negatively charged, and positively charged Pc dyes used to evaluate the impact of charge type on the physicochemical features of ZnPc@CDs.116 (B) Formation of ZnPc15@BODIPY@NCD conjugates via covalent conjugation of NCDs with BODIPY and subsequent π–π interactions with ZnPc15.118 (C) Structures of ZnPc16–20 for the preparation of ZnPc16–20@NCDsvia π–π interactions.119 | ||
The results showed that the ΦF of CDs in DMSO decreased from 11% to 2% upon conjugation to ZnPc1 and ZnPc and to ∼8% for ZnPc14. This quenching was accompanied by a decrease in τf. The lower quenching observed for ZnPc14@CDs was attributed to its lower loading, which reduces aggregation. FRET efficiency analysis indicated a strong energy transfer for ZnPc@CDs (0.81) and ZnPc1@CDs (0.80), while ZnPc14@CDs had a lower efficiency (0.28) due to electrostatic repulsion increasing the donor–acceptor distance (Table 2). ZnPc1@CDs showed the highest FRET efficiency due to the attractive interactions between oppositely charged surfaces. Despite increased triplet quantum yields (ΦT) upon conjugation, this increase was not reflected in the ΦΔ values of the hybrids that remained lower than that of ZnPc alone (ΦΔ = 0.67) or similar to those of free ZnPc1 (ΦΔ = 0.41) and ZnPc14 (ΦΔ = 0.32). This fact was attributed to a screening effect that prevented efficient energy transfer to the ground state of molecular oxygen. Additionally, the presence of water further quenched 1O2, contributing to their lower ΦΔ values, when measured in this solvent.110
| CDs type | Chemical conjugation | Phthalocyanine | Φ CD | Φ Pc | Φ hybrid | Application | Ref. |
|---|---|---|---|---|---|---|---|
| a Φ f in DMSO. b Φ T in DMSO. c Φ Δ in DMSO. d Φ f in H2O. e Φ Δ in H2O. f Φ Δ in DMSO using ultrasound at 35 kHz and light exposure at 7.05 × 1015 photons s−1 cm−2. | |||||||
| CDs | Non-covalent (π–π stacking) | Zinc(II) phthalocyanine (ZnPc) | CDs (0.11)a | * | ZnPc@CDs (0.021)a | Determination of ΦΔ values | 119 |
| 2,9(10),16(17),23(24)-Tetrakis[4-(N-methylpyridyloxy)]phthalocyaninato zinc(II) (ZnPc1) | * | ZnPc1@CDs (0.022)a | |||||
| 2,9(10),16(17),23(24)-Tetrakis(4-sulfonatophenyl)phthalocyaninato zinc(II) (ZnPc14) | * | ZnPc14@CDs (0.079)a | |||||
| NCDs | Non-covalent (π–π stacking) | 2,9(10),16(17),23(24)-Tetrakis(2,6-di-tert-butyl-4-methylphenoxy)phthalocyaninato zinc(II) (ZnPc16) | NCDs (0.17)a | ZnPc16 (0.16)a | ZnPc16@NCDs (0.09)a | PDT against MCF7 breast cancer cells | 119 |
| ZnPc16 (0.78)b | ZnPc16@NCDs (0.77)b | ||||||
| ZnPc16 (0.50)c | ZnPc16@NCDs (0.30)c | ||||||
| NCDs | Non-covalent (π–π stacking) | 2,9(10),16(17),23(24)-Tetrakis(tert-butyl)phthalocyaninato zinc(II) (ZnPc17) | NCDs (0.17)a | ZnPc17 (0.18)a | ZnPc17@NCDs (0.17)a | PDT against MCF7 breast cancer cells | 119 |
| ZnPc17 (0.77)b | ZnPc17@NCDs (0.83)b | ||||||
| ZnPc17 (0.49)c | ZnPc17@NCDs (0.40)c | ||||||
| NCDs | Non-covalent (π–π stacking) | 2,9(10),16(17),23(24)-Tetrakis(phenylsulphanyl)phthalocyaninato zinc(II) (ZnPc18) | NCDs (0.17)a | ZnPc18 (0.14)a | ZnPc18@NCDs (0.01)a | PDT against MCF7 breast cancer cells | 119 |
| ZnPc18 (0.65)b | ZnPc18@NCDs (0.67)b | ||||||
| ZnPc18 (0.50)c | ZnPc18@NCDs (0.45)c | ||||||
| NCDs | Non-covalent (π–π stacking) | 2,9(10),16(17),23(24)-Tetrakis[(thiophenyl)benzo[d]thiazole]phthalocyaninato zinc(II) (ZnPc19) | NCDs (0.17)a | ZnPc19 (0.18)a | ZnPc19@NCDs (0.11)a | PDT against MCF7 breast cancer cells | 119 |
| ZnPc19 (0.67)b | ZnPc19@NCDs (0.70)b | ||||||
| ZnPc19 (0.54)c | ZnPc19@NCDs (0.50)c | ||||||
| NCDs | Non-covalent (π–π stacking) | 2,9(10),16(17),23(24)-Tetrakis[(thiophenyl)benzo[d]thiazolium]phthalocyaninato zinc(II) sulfate (ZnPc20) | NCDs (0.17)a | ZnPc20 (0.10)a | ZnPc20@NCDs (0.09)a | PDT against MCF7 breast cancer cells | 119 |
| ZnPc20 (0.75)b | ZnPc20@NCDs (0.77)b | ||||||
| ZnPc20 (0.35)c | ZnPc20@NCDs (0.30)c | ||||||
| NCDs-biotin | Non-covalent (π–π stacking) | 2,9(10),16(17),23(24)-Tetrakis[2,5-dimethyl-4-(methylmorpholino)-phenoxy]phthalocyaninato zinc(II) (ZnPc21) | NCDs-biotin (0.24)a | ZnPc21 (0.20)a | ZnPc21@NCDs-biotin (0.16)a | PDT against MCF7 breast cancer cells | 120 |
| ZnPc21 (0.68)b | ZnPc21@NCDs-biotin (0.72)b | ||||||
| NCDs-biotin (0.25)d | ZnPc21 (0.67)c | ZnPc21@NCDsbiotin (0.47)c | |||||
| NCDs | Non-covalent (π–π stacking) | 2,9(10),16(17),23(24)-Tetrakis[2,5-dimethyl-4-(methylmorpholin-4-ium)phenoxy]phthalocyaninato zinc(II) disulfate (ZnPc22) | NCDs (0.17)a | ZnPc22 (0.20)a | ZnPc22@NCDs (0.15)a | PDT against MCF7 breast cancer cells | 120 |
| ZnPc22 (0.69)b | ZnPc22@NCDs (0.74)b | ||||||
| ZnPc22 (0.53)c | ZnPc22@NCDs (0.58)c | ||||||
| NCDs (0.20)d | ZnPc22 (0.14)d | ZnPc22@NCDs (0.10)d | |||||
| ZnPc22 (0.11)e | ZnPc22@NCDs (0.08)e | ||||||
| NCDs-biotin | Non-covalent (π–π stacking) | 2,9(10),16(17),23(24)-Tetrakis[2,5-dimethyl-4-(methylmorpholin-4-ium)-phenoxy]phthalocyaninato zinc(II) disulfate (ZnPc22) | NCDs-biotin (0.24)a | ZnPc22 (0.20)a | ZnPc22@NCDs-biotin (0.14)a | PDT against MCF7 breast cancer cells | 120 |
| ZnPc22 (0.69)b | |||||||
| ZnPc22 (0.53)c | |||||||
| NCDs-biotin (0.25)d | ZnPc22 (0.14)d | ||||||
| ZnPc22 (0.11)e | |||||||
| NCDs | Non-covalent (π–π stacking) | 2,9(10),16(17),23(24)-Tetrakis(N-methyl-morpholin-4-ium)phthalocyaninato zinc(II) disulfate (ZnPc23) | NCDs (0.70)a | ZnPc23 (0.16)a | ZnPc23@NCDs (0.14)a | PSDT against MCF-7 breast cancer cells | 121 |
| SNCDs | Non-covalent (electrostatic interactions) | SNCDs (0.80)a | ZnPc23 (0.16)a | ZnPc23@SNCDs (0.14)a | |||
| GQDs@cysteine | Non-covalent (electrostatic interactions | 4-Tetra-(2-bromo-4-methylphenol)phthalocyaninato indium(III) chloride | GQDs@cysteine (0.41)a | InPcBr | InPcBr@ GQDs@cysteine | Determination of ΦΔ values using sono-photochemical methodologies | 122 |
| (0.02)a | (0.81)c | ||||||
| (0.75)c | (1.31)f | ||||||
| (0.96)f | |||||||
| GQDs@ cysteine | Non-covalent (electrostatic interactions) | Tetrakis(2-bromo-4-methylphenoxy)phthalocyaninato zinc(II) | GQDs@cysteine (0.41)a | ZnPcBr | ZnPcBr@ GQDs@cysteine | Evaluation of the photochemical properties using sono-photodynamic procedures | 123 |
| (0.20)a | (0.68)c | ||||||
| (0.63)c | (0.95)f | ||||||
| (0.94)f | ZnPcCl@ GQDs@cysteine | ||||||
| Tetrakis(2-chloro-4-methylphenoxy)phthalocyaninato zinc(II) | ZnPcCl | (0.57)c | |||||
| [0.24)a | |||||||
| (0.55)c | (0.93)f | ||||||
| (0.91)f | |||||||
| GQDs@ cysteine | Non-covalent (electrostatic interactions) | 4-Tetrakis(2-chloro-4-methylphenoxy)phthalocyaninato indium(III) chloride | GQDs@cysteine (0.41)a | InPcCl | InPcCl@GQDs@cysteine | Assessment of singlet oxygen yield, ΦΔ, using ultrasound and light | 124 |
| (0.02)a | |||||||
| (0.65)c | (0.79)c | ||||||
| (0.94)f | (1.17)f | ||||||
To evaluate how the size of CDs could affect the ability of Pcs to generate 1O2, Matshitse et al. considered the development and photophysical/photochemical characterization of assemblies based on cationic ZnPc1 and CDs of different sizes denoted as CD2 (2.6 nm), CD6 (3.3 nm), and CD10 (5.1 nm).125 These CDs were prepared via the chemical cutting of GO using KMnO4 and H2SO4 as oxidative agents and varying the reaction time from 2 to 6 h.117 The results showed that the atomic concentrations of oxygen decreased with increasing sizes of CDs leading to a blue shift in their spectra and can justify the lower Pc loading found for this assembly (3 versus 10 for ZnPc1@CDs10 and ZnPc1@CDs2 and 5 for ZnPc1@CD6). Compared with the initial ZnPc1 (ΦΔ = 0.03), the presence of CDs improved the ΦΔ values in water, with the best performance observed for ZnPc1@CD6 (ΦΔ = 0.27), followed by ZnPc1@CD2 (ΦΔ = 0.17) and ZnPc1@CD10 (ΦΔ = 0.11). The low performance of ZnPc1@CD2 with the highest number of Pcs was attributed to the aggregation effects. This study showed that the structural features of CDs, namely, size, the amount of oxygen, and the presence of Pc, can have an important impact on their phototherapeutic or photocatalytic applications.
In 2018, Nyokong et al. reported the synthesis of supramolecular hybrids constituted of ZnPc15 bearing diethylaminophenoxy substituents embedded in nitrogen CDs covalently linked through an amide bond to carboxylic acid borondipyrromethane (BODIPY@NCDs) (Fig. 5B).118 The photophysical characterization of the ZnPc15@BODIPY@NCDs hybrids showed a slight decrease in both the ΦF and τF values when compared with the CDs alone (0.23 and 2.84 ns versus 0.27 and 3.41 ns). The decrease in ΦF values could be due to the FRET behaviour. Regarding 1O2 production, ZnPc15@BODIPY@NCDs (ΦΔ = 0.70) displayed an improved 1O2 generation ability compared to ZnPc15 (ΦΔ = 0.50) in DMSO.
In 2019, Nene et al. evaluated for the first time the phototherapeutic efficiency of conjugates obtained from a series of ZnPcs (ZnPc16–20) with CDs in human breast cancer MCF-7 cell lines (Fig. 5C).119 The nitrogen-doped CDs used in the synthesis of ZnPc16–20@NCDs assemblies were obtained from GO and ammonia. The results showed that the photophysical/photochemical features of ZnPcs were affected upon conjugation with CDs by π–π interactions, showing a decrease in the ΦF value of the Pcs, which was accompanied by an increase in the ΦT value. All conjugates were able to generate 1O2 species, although with slightly less efficiency than the non-conjugated ZnPc, probably because the CDs’ screening effect limits the facile energy transfer from the triplet state to dioxygen. The ZnPc@NCDs conjugates demonstrated PDT activity in the human breast cancer MCF-7 cell line upon irradiation, with the best results obtained for the ZnPc19@NCDs and ZnPc20@NCDs conjugates when compared with the corresponding non-immobilized ZnPc. At the highest conjugate concentration (100 μg mL−1), cytotoxicity was approximately 50%.
Soon after, the authors reported the development and photodynamic efficiency towards MCF-7 cell lines of the novel hybrid materials ZnPc21,22@NCDs and ZnPc21,22@biotin-NCDs containing neutral and cationic morpholine-substituted Pcs ZnPc21 and ZnPc22 and NCDs without or with tumour-targeting biomolecules such as biotin (Fig. 6).120 Access to neutral ZnPc21 and cationic ZnPc22 required the synthesis of the precursor 4-(2,5-dimethyl-4-(morpholinomethyl)phenoxy)phthalonitrile (Pht4) by the reaction of 2,5-dimethyl-4-(morpholinomethyl)-phenol hydrochloride monohydrate with commercially available 4-nitrophthalonitrile followed by their tetramerization in the presence of Zn(OAc)2, affording ZnPc21. The cationic ZnPc22 was obtained by quaternization of the morpholino units in the presence of dimethylsulfate (DMS) or DMF (Fig. 6A). NCDs functionalized with biotin were prepared by reacting NCDs with biotin in the presence of the carboxyl-activating reagents N,N’-dicyclohexylcarbodiimide (DCC) and 4-dimethylaminopyridine (DMAP) in DMF.120 The synthesis of the nanoplatforms required sonication followed by stirring (Fig. 6B).
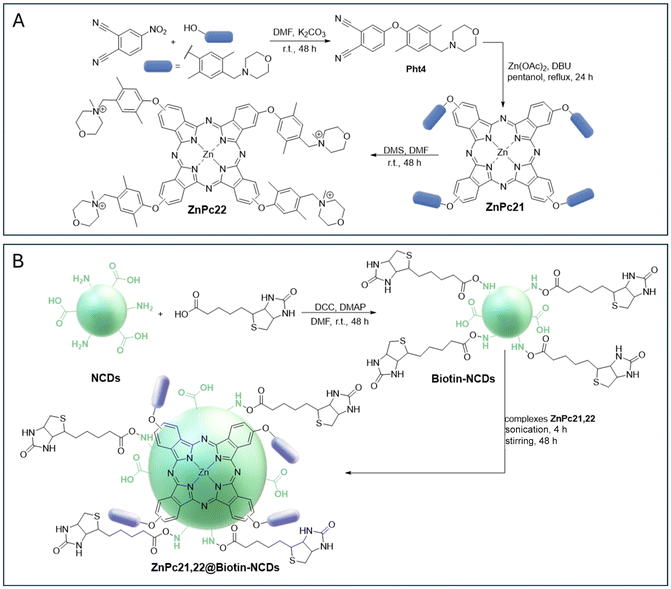 | ||
| Fig. 6 (A) Synthesis of ZnPc21 and ZnPc22. (B) Synthesis of biotin-NCDs and ZnPc21, 22@biotin-NCD conjugates.120 | ||
The photophysical characterization of the ZnPc21,22@NCD and ZnPc21,22@biotin-NCD nanoassemblies showed that the conjugation caused quenching of the emission and also a drop in the ΦΔ values owing to screening effects. However, an increase in PDT activity was observed for the cationic dye in combination with biotin-functionalized NCDs. The PDT activity of the complexes (less cell viability) of ZnPc21 and ZnPc22 was higher than that of their non-functionalized CD conjugates. For ZnPc21@CDs, the low PDT activity may be related to its low ΦΔ values when compared to the non-immobilized ZnPc21 (Table 2). Indeed, cationic complexes generally perform better than non-charged complexes. This has previously been reported for Pcs126 and other charged complexes in cancers,127,128 where the net anionic membrane charge of cancer cells is believed to be susceptible to cationic drug complexes through electrostatic attraction. This may result in improved drug retention, and hence, more available Pcs for therapy. The combination of charge and biotin ligands improved the therapeutic efficacy of the complex.
In a related study, Nyokong et al.121 reported the preparation of ZnPc23@NCD and ZnPc23@NSCD nanoassemblies by conjugating cationic ZnPc23 bearing morpholino moieties with NCDs and NSCDs (Fig. 7A). The NCDs and NSCDs were synthesized from citric acid under hydrothermal conditions (180 °C, 5 h) using urea and thiourea, respectively. The efficiency of the obtained assemblies was evaluated for the treatment of MCF7-cells under PDT, sonodynamic therapy (SDT), and combined PDT plus SDT (PSDT). Pc, CDs, and hybrids demonstrated cytotoxicity for PDT, SDT, and PSDT treatments in a concentration-dependent manner due to cellular oxidative stress generated by the formation of ROS (Fig. 7B). The determined IC50 values showed that Pc presented a relatively lower value than the conjugates for all three explored therapies. The results showed that 1O2 was generated under all therapeutic conditions and its presence increased upon conjugation. However, the generation of ˙OH radicals, which played a significant role in the eradication of cancer cells, only generated in the SDT and PSDT assays, showing a decrease in signal intensity after the conjugation of Pc to the CDs (Table 2).
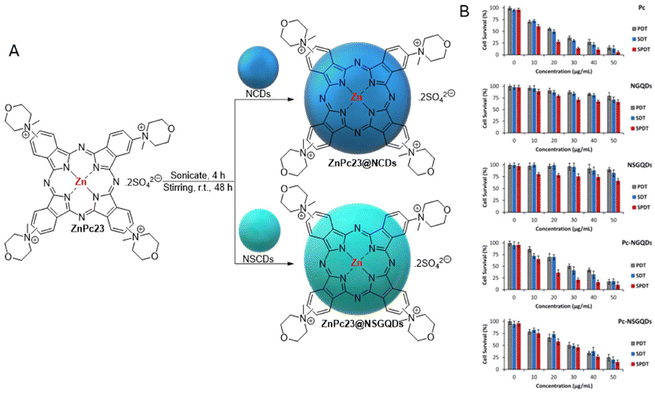 | ||
| Fig. 7 (A) Structures of ZnPc23@NCD and ZnPc23@NSCD conjugates. (B) WST-assay post-PDT, SDT, and PSDT treatments at varied concentrations in water at 10 min irradiation time, n = 3. Parameters: PDT = irradiation dose of 170 J cm−2, power 2.3 W at 680 nm. SDT: frequency = 1 MHz, power = 1 W cm−2, 100% duty cycles. PSDT = combined PDT and SDT parameters with PDT applied first. Reproduced with permission.121 Copyright 2021, Elsevier B.V. | ||
Knowing that current PDT agents often suffer from low singlet oxygen quantum yields, photobleaching, and poor biocompatibility, Erdoğmus et al.122–124 developed new hybrid nanomaterials to address these issues, based on the conjugation of indium(III) and zinc(II) phthalocyanines with halogen and phenoxy substituents using GQDs obtained from citric acid and cysteine (GQDs@cysteine) under hydrothermal conditions. Specifically, tetrakis(2-bromo-4-methylphenoxy)phthalocyaninato zinc(II), tetrakis(2-chloro-4-methylphenoxy)phthalocyaninato zinc(II), 4-tetrakis(2-chloro-4-methylphenoxy)phthalocyaninato indium(III) chloride, and 4-tetrakis(2-bromo-4-methylphenoxy)phthalocyaninato indium(III) chloride as regioisomeric mixtures were immobilized onto GQDs@cysteine through π–π stacking interactions. The photochemical and sonophotochemical properties of the resulting nanomaterials and their precursors were evaluated for potential applications in advanced cancer treatments with enhanced 1O2. Briefly, all derivatives were exposed to light, sound, or a combination of both to measure their singlet oxygen production yield. The nanoconjugates of InPc and ZnPc exhibited improved ΦΔ, compared to non-conjugated Pcs. In addition, it was observed that indium, being heavier than zinc, enhanced intersystem crossing and gave a higher singlet oxygen production yield (Table 2). These three studies confirmed that the sono-photodynamic approach (SPDT) enhances therapeutic efficacy by combining the advantages of light and ultrasound.
2.1.3.2. Antibacterial applications. The conjugation of the CDs and Pcs was also explored for antibacterial applications (Table 3), considering previous reports that showed improvements in the required photophysicochemical features after the formation of the hybrids. Nyokong et al. conducted the first study in the context of aPDT using MPc24@CD nanoassemblies (M = 2H, Zn, or In) against the multidrug-resistant Staphylococcus (Fig. 8).129 The photoactive platforms were prepared from the free-base H2Pc24 with acetylphenoxy units and the corresponding ZnPc24 and InPc24 complexes (Fig. 8A). In the selection of these Pcs, the authors took into account the antibacterial features associated with the reactive carbonyl group.130,131 The synthetic strategy involved the reaction of 4-hydroxyacetophenone with 4-nitrophthalonitrile to afford the required phthalonitrile Pht5, followed by its tetramerization in DMAE and DBU with LiCl or ZnCl2 to obtain H2Pc24 or ZnPc24, respectively (Fig. 8A).129InPc14 was obtained by the reaction of H2Pc24 with anhydrous InCl in dry DMF and DBU. The results showed that the efficiency of the conjugates at producing 1O2 parallels that observed by the non-conjugated Pcs, with the indium complex being the most efficient, followed by the zinc complex, and then the free base (values ranging from ΦΔ = 0.20 to 0.79 in DMSO). The aPDT activity of both phthalocyanines and nanoconjugates was tested against multidrug-resistant Staphylococcus aureus (S. aureus), where InPc24@CDs were highly effective causing a 9.68 log reduction in the bacterial viability at 10 μM (based on Pc) when compared to the 3.77 log reduction of ZnPc24@CDs (Fig. 8C and D).
 | ||
| Fig. 8 (A) Synthesis of H2Pc24, ZnPc24, and InPc24 and (B) conjugation of Pcs with CDs. (C) Survival graphs of S. aureus incubated with 10 μM of photosensitizer for Pcs alone and for the conjugates (D) with irradiation at 670 nm. Reproduced with permission.129 Copyright 2019, Elsevier B.V. | ||
| CDs type | Chemical conjugation | Phthalocyanine | Φ CD | Φ Pc | Φ hybrid | Application | Ref. |
|---|---|---|---|---|---|---|---|
| a Φ f in DMSO. b Φ T in DMSO. c Φ Δ in DMSO. d Φ f in H2O. | |||||||
| CDs | Non-covalent (π–π stacking) | 2,9(10),16(17),23(24)-Tetrakis[(phenoxy)ethan-1-one]phthalocyanine (H2Pc24) | CDs (0.35)a | H2Pc24 (0.20)a | H2Pc24@CDs (0.0.39)a | PDT against S. aureus | 129 |
| H2Pc24 (0.31)b | H2Pc24@CDs (0.27)b | ||||||
| H2Pc24 (0.22)c | H2Pc24@CDs (0.20)c | ||||||
| CDs | Non-covalent (π–π stacking) | 2,9(10),16(17),23(24)-Tetrakis[(phenoxy)ethan-1-one]phthalocyaninato zinc(II) (ZnPc24) | CDs (0.35)a | ZnPc24 (0.13)a | ZnPc24@CDs (0.059)a | PDT against S. aureus | 129 |
| ZnPc24 (0.78)b | ZnPc24@CDs (0.85)b | ||||||
| ZnPc24 (0.72)c | ZnPc24@CDs (0.77)c | ||||||
| CDs | Non-covalent (π–π stacking) | 2,9(10),16(17),23(24)-Tetrakis[(phenoxy)ethan-1-one]phthalocyaninato indium(III) chloride (InPc24) | CDs (0.35)a | InPc24 (0.046)a | InPc24@CDs (0.054)b | PDT against S. aureus | 129 |
| InPc4 (0.84)b | InPc24@CDs (0.90)b | ||||||
| InPc4 (0.75)c | InPc24@CDs (0.79)c | ||||||
| NCDs | Non-covalent (π–π stacking) | 2,9(10),16(17),23(24)-Tetrakis[4-(4-(5-chloro-1H-benzo[d]imidazol-2-yl)phenoxy)]phthalocyaninato zinc(II) (ZnPc25) | NCDs (0.19)d | ZnPc25 (0.17)a | ZnPc25@NCDs (0.12)a | PDT against S. aureus | 132 |
| CBDs | Non-covalent (π–π stacking) | 1, 8(11),15(18),22(25)-Tetrakis[(2,2-dimethyl-1,3-dioxolan-4-yl)methoxy]phthalocyaninato copper(II) | — | — | — | PDT against Gram-positive bacteria (E. hirae and E. faecalis), Gram-negative bacteria (E. coli, P. aeruginosa and B. subtilis) | 133 |
The photodynamic action of ZnPc25@NCD nanoassemblies, obtained from nitrogen-doped CDs (NCDs) and ZnPc25 bearing benzoimidazolylphenoxy units, was evaluated towards Gram-positive bacteria S. aureus.132 The hybrid constituents were obtained following well-established approaches, and the photophysical/photochemical characterization showed improved singlet oxygen (ΦΔ = 0.45) and triplet state quantum yields (ΦT = 0.60) compared to the non-immobilized ZnPc25 (ΦΔ = 0.36 and ΦT = 0.54). In the antibacterial tests under UV light exposure, the ZnPc25@NCD hybrid achieved a 99.9% bacterial kill rate, outperforming free ZnPc25 (99.86%). This aligns with its higher singlet oxygen production. In 2024, Özçesmeci et al. reported the synthesis of new nanoconjugates through the non-covalent conjugation of copper(II) phthalocyanine, substituted with 2,3-dihydroxypropoxy groups in the non-peripheral positions, with carbon dots (CDs) and carbon-boron quantum dots (CBDs). The antimicrobial photodynamic therapy (aPDT) efficacy of each hybrid was evaluated against Gram-positive bacteria (E. hirae and E. faecalis), Gram-negative bacteria (E. coli, P. aeruginosa, and B. subtilis), and microfungi (C. parapsilosis and C. albicans). The selected phthalocyanine was obtained by deprotecting the (2,2-dimethyl-1,3-dioxolan-4-yl)methoxy groups in 1,8(11),15(18),22(25)-tetrakis[(2,2-dimethyl-1,3-dioxolan-4-yl)methoxy]phthalocyaninato copper(II) using acetic acid. The CBDs were synthesized via microwave-assisted synthesis, involving citric and boric acids. The aPDT evaluation revealed the lowest minimum inhibitory concentration (MIC) of 4 mg L−1 against Gram-positive bacteria, which decreased further post-irradiation, significantly enhancing antimicrobial effects. Additionally, the study demonstrated promising biological applications for the new conjugates including radical scavenging activity, antidiabetic effects, DNA cleavage activity, and inhibition of biofilm formation by S. aureus and P. aeruginosa.
A recent study explored the combination of CDs functionalized with NiPc and Cu–In–Zn–S quantum dots (CIZS QDs) for surface proton generation and reduction. The synthesis of the ternary CIZS QD/NiPc@CD composite is presented in Fig. 9A.136 The size of the CIZS QDs and CDs was determined by transmission electron microscopy (TEM), corresponding to 3.5 ± 0.5 and 2.5 ± 0.5 nm, respectively (Fig. 9B and C). The nanocomposite CIZS QDs/NiPc@CDs revealed the formation of smaller NiPc@CDs and larger CIZS QDs. The HRTEM analysis of the CIZSQDs/NiPc@CDs confirms the presence of both nanostructures with lattice spacings of 0.310 nm, corresponding to the CIZS QDs, and 0.210 nm, corresponding to the CDs (Fig. 9D and E). Elemental mapping images of the CIZS QDs/NiPc@CDs further confirmed that In, Cu, Zn, S, Ni, and C were well merged, indicating that CIZS QDs and NiPc@CDs were well integrated to form the nanocomposite material (Fig. 9F-J). The performance of the developed ternary nanocomposites CIZS QDs/NiPc@CDs was evaluated in photocatalytic hydrogen evolution coupled with organic oxidation electron/hole extraction. A preliminary study, based on the photocatalytic degradation of rhodamine B (RhB), showed (Fig. 9K) that the CIZS QD/NiPc@CD nanocomposite presented higher photodegradation activity. The authors demonstrated that the dominant mechanism was based on photogenerated holes because with the addition of ethylenediaminetetraacetic acid (hole quencher), the photodegradation reaction was completely inhibited (Fig. 9I). This clearly illustrates that the photogenerated holes mainly contribute to the degradation of RhB in the CIZS QD/NiPc@CD composite photocatalyst system, which also proves the effectiveness of the hole-extraction strategy by NiPc@CD modification. The performance of the CIZS/NiPc@CD nanocomposite was further assessed for the photocatalytic hydrogen evolution coupled with the oxidation of different alcohols. The photocatalytic hydrogen production rate by CIZS QDs/NiPc@CDsvia MeOH oxidation was found to be the highest among all the tested nanomaterials, reaching 1.121 mmol g−1 h−1 (Fig. 9M and N). Moreover, the oxidation of biomass-derived furfuryl alcohol (FFA) resulted in hydrogen production that reached 2.516 mmol g−1 h−1 (Fig. 9O and P).
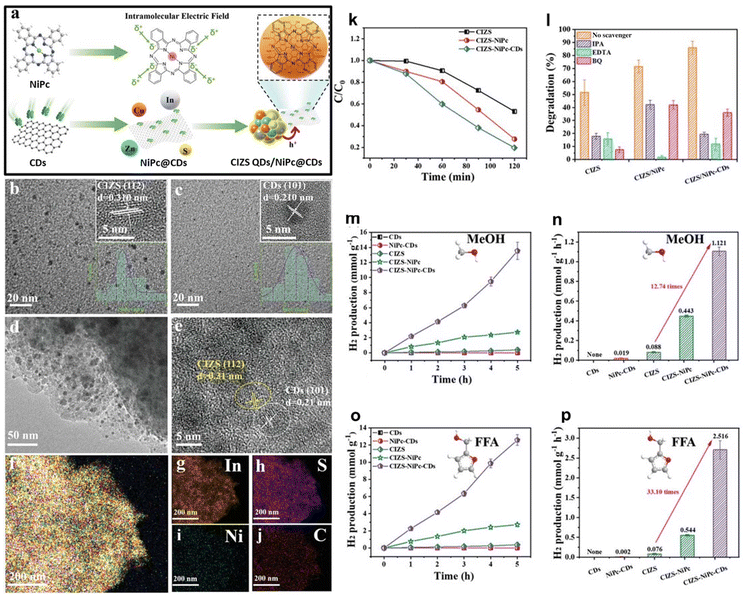 | ||
| Fig. 9 (a) Schematic diagram for the construction of the ternary CIZS QD/NiPc@CD composite. (b) TEM images of CIZS QDs and (c) CDs and the corresponding HRTEM images (top insets) and size distribution histograms (bottom insets) obtained by counting 100 particles. (d) TEM and (e) HRTEM images of CIZS QDs/NiPc@CDs. (f–j) The corresponding elemental mapping images of CIZS QDs/NiPc@CDs. (k) Photocatalytic degradation ratio of RhB over CIZS QDs, CIZS QDs/NiPc, and CIZS QDs/NiPc@CDs under visible-light irradiation. (l) Active species trapping experiment during the photocatalytic degradation of RhB over CIZS QDs/NiPc@CDs with the addition of quenchers of h+ (EDTA), ˙O2− (BQ), and ˙OH (isopropanol, IPA). Photocatalytic hydrogen evolution performance over CDs, NiPc@CDs, CIZS QDs, CIZS QDs/NiPc, and CIZS QDs/NiPc@CDs coupled with (m), (n) methanol oxidation and (o and p) FFA oxidation reactions. Reproduced with permission.136 Copyright 2023, John Wiley & Sons, Inc. | ||
 | ||
| Fig. 10 Preparation of ZnPc26A-C substituted with eight carbazole units used in the synthesis of ZnPc26A-C@CD nanoassemblies further immobilized in polystyrene films.137 | ||
The immobilization of the ZnPc in CDs obtained from the pyrolysis of citric acid in the presence of NaOH afforded the required nanoassemblies ZnPc26A-C@CDs that after being embedded in PS films showed an average thickness of 0.45–0.62 mm. These thin films of ZnPc26A-C@CDs-PS showed promising optical limiting parameters in different solvents, with improved optical limiting performance, characterized by increased third-order susceptibilities and hyperpolarizabilities, as well as lower limiting threshold values.
In 2021, AlMarzouq et al. selected the analogue manganese phthalocyanine with eight carbazole units (MnPc26C) to conjugate with CDs, which were obtained through the pyrolysis of calcium acetate at 200 °C, to evaluate the influence of the carbazole substituents and CDs on the redox mechanism of MnPc.138 The electrochemical performance of MnPc26C and the resulting conjugate was investigated using cyclic voltammetry (CV), square wave voltammetry (SWV), and spectroelectrochemistry (SEC), showing extended metal-based redox processes at low potentials. Furthermore, the carbazole substituents provided additional redox activity, facilitating the electropolymerization of the complex onto the electrode surface, simplifying the electrode modification.
2.2. Covalent functionalization
 | ||
| Fig. 11 (A) NCDs covalently linked to ZnPc27: (1) differential absorption 3D map obtained by fsTAS of ZnPc27@NCDs in CH3OH at room temperature upon excitation at λ = 387 nm; (2) time absorption profiles and the corresponding line fittings at λ = 951 (magenta), 803 (dark green), 755 (orange), and 550 nm (purple); (3) species-associated differential spectra of SB-CT (black), SB-CS (red), S1 (green), and T1 (blue) excited states; and (4) concentration evolution over time.139 (B) Synthesis of CoPc28 and its functionalization with rCDs to obtain CoPc28@rCD and CoP28(π–π)@rCD hybrids.140 (C) Strategy used in the preparation of a [ZnPc29@NCDs@PSCO2H]m membrane.141 | ||
| CDs type | Chemical conjugation | Phthalocyanine | Φ CD | Φ Pc | Φ Hybrid | Application | Ref. |
|---|---|---|---|---|---|---|---|
| a Φ f in DMSO. b Φ T in DMSO. c Φ Δ in DMSO. | |||||||
| NCDs | Covalent | 9(10),16(17),23(24)-Tri-tert-butyl-2-carboxy-5,28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14,19-diimino-7,12 14,19-diimino-7,12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 21,26-dinitrilotetrabenzo[c,h,m,r][1,6,11,16]tetraazacycloeicosinato-(2−)-N29,N30,N31,N32 zinc(II) (ZnPc27) 21,26-dinitrilotetrabenzo[c,h,m,r][1,6,11,16]tetraazacycloeicosinato-(2−)-N29,N30,N31,N32 zinc(II) (ZnPc27) |
NCDs (0.17)a | ZnPc27 (0.15)a | * | Charge transfer | 139 |
| CDs, rCDs and NCDs | Covalent and/or non-covalent (π–π stacking) | 2-Mono[(4-carboxyphenoxy)]-9(10),16(17),23(24)-Tris[tert-butylphenoxy]phthalocyaninato cobalt(II) (CoPc28) | * | * | * | Electrooxidation of hydrazine | 141 |
| NCDs | Covalent and/or non-covalent (π–π stacking) | 2-Mono[(4-carboxyphenoxy)]-9(10),16(17),23(24)-Tris[phenyl]phthalocyaninato zinc(II) (ZnPc29) | NCDs (0.16)a | ZnPc29 (0.16)a | πZnPc29@NCDs (0.18)a | Determination of ΦΔ values | 140 |
| πZnPc29@NCDs (0.70)b | |||||||
| ZnPc29(0.70)b | πZnPc29@NCDs (0.33)c | ||||||
| ZnPc29@NCDs (0.19)a | |||||||
| ZnPc29(0.56)c | ZnPc29@NCDs (0.49)b | ||||||
| ZnPc29@NCDs (0.44)c | |||||||
| NCDs | Covalent | 2,9(10),16(17),23(24)-Tetrakis[(3-carboxyphenoxy)]phthalocyaninato cobalt(II) (CoPc30) | * | * | * | Electrochemical sensors for dopamine, norepinephrine, and epinephrine detection | 142 |
Knowing that the elimination of oxygen-containing groups from the surface of CDs is responsible for reducing the energy band gap of reduced carbon dots (rCDs) compared to amino-functionalized CDs (NCDs), resulting in materials with improved electron transfer processes and conductivity, Centane et al.141 developed conjugates based on CoPc28 (Fig. 11B) covalently linked to NCDs and rCDs and evaluated their performance during the electrocatalysis of hydrazine on a glassy carbon electrode (GCE). For comparison, the authors also considered the development of analogous nanoassemblies obtained via π–π interactions. Unsymmetrical CoPc28 was obtained by the condensation of 4-(p-tert-butylphenoxy)phthalonitrile (Pht7) and 4-(4-carboxyphenoxy)phthalonitrile in pentanol at 160 °C and in the presence of CoCl2·6H2O and DBU (CoPc28, Fig. 11C).140 NCDs were synthesized by oxidative cutting of GO sheets with KMnO4 and H2SO4, followed by the reaction of the obtained CDs with an ammonia solution in the presence of EDC/N-hydroxysuccinimide (NHS). On the other hand, rCDs were obtained by treating the CDs in water with hydrazine hydrate to eliminate the high number of oxygen-containing functionalities. The CoPc28@rCDs and CoPc28@NCDs hybrids were obtained by reacting CoPc28 and the required CDs in DMF and using EDC/NHS as activating carboxyl reagents. The analogous π–π hybrids (CoP28(π–π)@rCDs and CoPc28(π–π)@NCDs) were obtained by sonication of the required constituents. The efficiencies of the precursors and conjugates covalently and non-covalently linked in the electrocatalysis of hydrazine were evaluated using a GCE. The procedure was conducted by placing each hybrid on the GCE, followed by drying at 70 °C to obtain the following electrodes: CoPc28@GCE/rCDs, CoPc28@GCE/NCDs, CoPc28(π–π)@GCE/rCDs, and CoPc28(π–π)GCE/NCDs. The rCDs@GCE, NCDs@GCE, and CoPc@GCE electrodes were prepared by the same procedure. Low LOD values for hydrazine oxidation were obtained in the presence of electrodes containing the hybrids prepared by covalent conjugation, CoPc28@GCE/rCDs and CoPc28@GCE/NCDs. It was also observed that the hybrids composed of rCDs gave better LOD values than the corresponding NCD hybrids. The results obtained confirmed that the elimination of excessive oxygen-containing groups from the surface of the CDs does indeed tune the energy band gap, improving the required electron transfer and conductivity.
To compare how the efficiency of a Pc at generating 1O2 was affected by the type of bond linked to the CDs, Nyokong et al. (2019) developed polystyrene membranes [ZnPc29@NCDs@PSCO2H]m bearing NCDs covalently linked to the unsymmetrical ZnPc29 bearing a carboxyphenoxy unit and to carboxyl-functionalized polystyrene (PSCO2H) through amide bonds; membranes resulting from the π–π interactions between ZnPc29 and NCDs@PSCO2H were also prepared, [πZnPc29@NCDs@PSCO2H]m (Fig. 11B).141 The NCDs were obtained through the hydrothermal treatment of GO with ammonia, and during the covalent conjugation process, their coupling with ZnPc29 and then to PSCO2H was performed in DMF in the presence of DCC. The observation of shifts in the region of the amide peak in the FTIR spectra, from 1667 cm−1 in ZnPc29@NCDs to 1657 cm−1 in ZnPc29@NCDs-(COOH)-PS, suggested successful conjugation. Initially, the NCDs were monodispersed with a size of approximately 14 nm, but after conjugate formation, the size increased to 21 nm. The XRD diffractograms showed that the synthesized nanoconjugates were amorphous, like their constituents. Photophysical/photochemical characterization revealed that the hybrid materials exhibited better performance prior to incorporation into the membranes (e.g., ΦΔ of 0.33 (π) and 0.44 (c) compared to 0.16 (π) and 0.29 (c) after incorporation). Moreover, hybrids with covalent linkages consistently outperformed those with π–π interactions under both conditions.
3. Conclusions
CDs have emerged as versatile nanoplatforms capable of addressing key limitations associated with molecular compounds such as Pcs. By combining CDs with Pcs, the resulting hybrid macrocycles exhibit improved light sensitivity, water solubility, stability, and environmental compatibility. This review allows us to conclude that this integration can significantly expand the applications of Pcs, with the resulting hybrids showing great promise in diverse fields, including biosensing, phototherapeutic applications, photocatalysis, electrocatalysis, and oxygen reduction reactions (ORRs) among others.Most of the research focused on developing Pcs and CDs with compatible functionalities. This involved tailoring the structural features of Pcs, such as peripheral substituents (α and/or β), varying the types and number of functional groups, and modulating charge distributions (neutral, positive, or negative). These modifications enabled the resulting hybrids to achieve in general appropriate performance in terms of ΦΔ, ΦF, photostability, hydrophilicity, and targeted functionality.
The conjugation of CDs with Pc dyes through covalent or non-covalent interactions yielded Pc@CD hybrids with distinct photophysical properties. Covalent strategies were primarily based on amidation and esterification. The wide structural variety of Pcs facilitated the functionalization of oxygen and nitrogen-containing groups on the CDs. While covalently bonded hybrids exhibit greater chemical stability and higher reaction yields, their practical applications are hindered by synthetic complexity, generally requiring the preparation of non-symmetrical Pcs. Nevertheless, covalent functionalization influences the interaction between the two structures, modulating photoluminescence, preserving the capacity for 1O2 generation and introducing interesting features favorable for mediating electron transfer, conductivity and ORR processes. Although the number of reported examples is limited, we believe that continued efforts to develop these types of hybrids can significantly impact renewable energy technologies, advance environmental sustainability, improve catalytic efficiency, and drive innovations in therapeutic applications. On the other hand, non-covalent conjugation, achieved through electrostatic interactions, complexation, or π–π stacking, facilitates the easy formation of supramolecular structures. Considering that some Pc@CD hybrids exhibit pH-dependent behavior, some groups were prompted to explore electrostatic interactions to optimize Pc–CD binding. The non-covalent conjugation approach offers the advantage of faster and simpler reaction steps while preserving the structural and electronic characteristics of the starting materials. However, non-covalent strategies generally compromise the photoluminescence of Pc@CD hybrids due to quenching effects. These quenching effects were extensively explored in sensing applications, where luminescence was recovered upon interaction with the target analyte.
Despite the observed decrease in the fluorescence quantum yield (ΦF) of the conjugates and the corresponding increase in triplet quantum yield (ΦT), these changes did not always translate into improved efficiency in generating 1O2 due to competing screening processes that reduce light absorption or oxygen access to the active sites of the photosensitizer. Nevertheless, depending on the structural features of the Pcs, these limitations were overcome in some cases, leading to promising outcomes in PDT and aPDT. The ability to generate ROS was also explored in oxidative processes in water, although the number of studies remains very limited. Similarly, studies related to hydrogen production are scarce.
Despite significant progress, the development of highly efficient Pc@CD hybrids remains an open research frontier. The primary challenge lies in mitigating the partial or total quenching of Pcs by CDs, an effect observed in both covalent and non-covalent conjugation approaches. This quenching typically results in reduced ΦF values and ROS production, primarily due to Förster resonance energy transfer (FRET), when compared to free-base Pcs. A critical question to address this issue is how to prevent planar coupling between CDs and Pcs. One promising approach involves the exploration of metalated phthalocyanines with bulky aromatic or aliphatic substituents linked by N, O, or S atoms at the peripheral positions (α- and/or β-). These substituents can introduce steric hindrance to aromatic CDs, thereby minimizing quenching effects. Also, the metalation of Pcs with transition metals that require axial ligands, such as In3+, Ga3+, or Si4+, can further minimize the direct contact between CDs and Pcs, enhancing the overall performance of the hybrid systems.
Another important aspect is the wide range of synthetic approaches and precursors available to produce CDs in the development of Pc@CD hybrids, which significantly influence their structural and photophysical/photochemical properties. Therefore, ensuring reproducibility and scalability remains a critical challenge. To address this, it is essential to have well-documented and standardized synthetic protocols that provide clear details on reaction conditions, precursor ratios, and processing steps. This will promote reproducibility and comparability across studies. Advanced characterization techniques and quality control measures will also be vital for confirming structural integrity and performance. In this context, emerging technologies like machine learning (ML) and deep learning (DL) can offer powerful tools for the optimization and automated identification of new synthetic conditions. By analyzing large datasets, ML and DL can identify patterns, predict material properties, optimize the process and ultimately enable more efficient synthetic protocols. These approaches have the potential to significantly enhance the performance of these hybrids, unlocking new possibilities for their applications across diverse fields.
Abbreviations
| 0D | Zero dimensional |
| 1O2 | Singlet oxygen |
| 2D | Two dimensional |
| 6FBPA | Bisphenol A hexafluoride |
| AA | Ascorbic acid |
| AFM | Atomic force microscopy |
| AmGQDs | Aminated graphene quantum dots |
| aPDT | Antimicrobial photodynamic therapy |
| Au-IPA | Isophthalic electrografted gold electrode |
| CDs | Carbon dots |
| CNPs | Carbonized nanoparticles |
| CNTs | Carbon nanotubes |
| CPDs | Carbonized polymer dots |
| CQDs | Carbon quantum dots |
| CV | Cyclic voltammetry |
| DA | Dopamine |
| DBN | 1,8-Diazabicyclo[5.4.0]undec-7-ene (DBU) or 1,5-diazabicyclo[4.3.0]non-5-ene |
| DBU | 1,8-Diazabicyclo[5.4.0]undec-7-ene |
| DCC | N,N’-Dicyclohexylcarbodiimide |
| DMAE | 2-Dimethylaminoethanol |
| DMAP | 4-Dimethylaminopyridine |
| DMF | N,N′-dimethylformamide |
| DMMP | Methylphosphonate |
| DMSO | Dimethyl sulfoxide |
| DPV | Differential pulse voltammetry |
| ds-DNA | Double-stranded DNA |
| EDC | 1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide |
| EIS | Electrochemical impedance spectroscopy |
| EP | Epinephrine |
| EPR | Enhanced permeation retention |
| FRET | Förster resonance energy transfer |
| FTIR | Fourier transform infrared spectroscopy |
| GCE | Glassy carbon electrode |
| GO | Graphene oxide |
| GQDs | Graphene quantum dots |
| H2Pc | Free-base phthalocyanine |
| HFIP | hexafluoroisopropanol |
| ICT | Inter-charge transfer |
| IPCA | 5-Oxo-1,2,3,5-tetrahydroimidazo-[1,2-α]-pyridine-7-carboxylic acid |
| LOD | Limit of detection |
| MCF-7 cell lines | Human breast cancer cell lines |
| MPc | Metalophthalocyanine |
| MW | Microwave treatment |
| NCNDs | N-doped carbon nanodots |
| NCDs | Nitrogen doped-carbon dots |
| NHS | N-Hydroxysuccinimide |
| NMR | Nuclear magnetic resonance |
| NOR | Norepinephrine |
| NSCDs | Nitrogen–sulfur doped-carbon dots |
| ORR | Oxygen reduction reaction |
| PACT | Photodynamic antimicrobial chemotherapy |
| PBS | Phosphate-buffered saline |
| Pc(s) | Phthalocyanine(s) |
| PDT | Photodynamic therapy |
| PET | Photo-induced electron transfer |
| PL | Photoluminescence |
| Por | Porphyrin |
| QY | Quantum yield |
| rGQDs | Reduced graphene quantum dots |
| SDT | Sonodynamic therapy |
| SEC | Spectroelectrochemistry |
| SEM | Scanning electron microscopy |
| SWV | Square wave voltammetry |
| t-CDs | Thermally-reduced carbon dots |
| TEM | Transmission electron microscopy |
| TEMPOL | (4-Hydroxy-2,2,6,6-tetramethylpiperidine-1-oxyl 2,2,6,6-tetramethylpiperidin-1-yl)oxyl |
| TEMPO-Pht | Phthalonitrile bearing the radical scavenger TEMPOL |
| XPS | X-ray photoelectron spectroscopy |
| XRD | X-ray diffraction |
| J | Spectral overlap |
| τ f | Fluorescence lifetime |
| Φ F | Fluorescence quantum yield |
| Φ PL | Photoluminescence quantum yield |
| Φ T | Triplet quantum yield |
| Φ Δ | Singlet oxygen quantum yield |
Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Authors acknowledge to University of Aveiro, Instituto Superior Técnico (IST, Portugal) and FCT/MCTES (Fundação para a Ciência e Tecnologia/Ministério da Ciência e Tecnologia, Portugal) for the support to LAQV-REQUIMTE and CQE-IST to the project: 2022.05950.PTDC (https://doi.org/10.54499/2022.05950.PTDC) through national funds, where applicable, co-financed by European Union, FEDER, QREN, and COMPETE within the PT2020 Partnership Agreement, and to the Portuguese NMR Network. C. I. M. Santos is also grateful to her research contract (REF.IST-ID/95/2018, 2023.08400.CEECIND). Funding: This research work received financial support from Instituto Superior Técnico (IST, Portugal), the University of Aveiro and FCT/MCTES (Fundação para a Ciência e Tecnologia (FCT) and Ministério da Ciência Tecnologia e Ensino Superior) to support the LAQV-REQUIMTE (LA/P/0008/2020 https://doi.org/10.54499/LA/P/0008/2020, UIDP/50006/2020 https://doi.org/10.54499/UIDP/50006/2020 and UIDB/50006/2020 https://doi.org/10.54499/UIDB/50006/2020), by IMS (LA/P/0056/2020, https://doi.org/10.54499/LA/P/0056/2020), CQE (UIDB/00100/2020, https://doi.org/10.54499/UIDP/00100/2020 and UIDP/00100/2020, https://doi.org/10.54499/UIDP/00100/2020), and TEMA (UIDB/00481/2020 https://doi.org/10.54499/UIDB/00481/2020 and UIDP/00481/2020 https://doi.org/10.54499/UIDP/00481/2020), research units through national funds and was co-financed by the FEDER, within the PT2020 Partnership Agreement, and to the Portuguese NMR Network. The authors also thanks to the FCT for the financial support of the project CarboNCT - 2022.03596.PTDC (https://doi.org/10.54499/2022.03596.PTDC).References
- M. W. Jones, A. I. Coppola, S. H. Doerr, T. A. Quine, C. Santín, T. Dittmar and R. Jaffé, Nat. Commun., 2020, 11, 1 CrossRef PubMed.
- Z. Zhao, W. Zhang, J. Zhang, Y. Li, H. Bai, F. Zhao, Z. Jin, J. Tang, Y. Xiao, W. Xu and Y. Lü, Adv. Photonics Res., 2024, 5, 2400010 CrossRef CAS.
- Q. Xu, W. Li, L. Ding, W. Yang, H. Xiao and W. Ong, Nanoscale, 2019, 11, 1475 RSC.
- K. Jiang, S. Sun, L. Zhang, Y. Lu, A. Wu, C. Cai and H. Lin, Angew. Chem., 2015, 127, 5450 CrossRef.
- L. Đorđević, F. Arcudi, M. Cacioppo and M. Prato, Nat. Nanotechnol., 2022, 17, 112 CrossRef PubMed.
- A. Das, E. V. Kundelev, A. A. Vedernikova, S. A. Cherevkov, D. V. Danilov, A. V. Koroleva, E. V. Zhizhin, A. N. Tsypkin, A. P. Litvin, A. V. Baranov, A. V. Fedorov, E. V. Ushakova and A. L. Rogach, Light:Sci. Appl., 2022, 11, 1 CrossRef PubMed.
- Q. Zhang, R. Wang, B. Feng, X. Zhong and K. (Ken) Ostrikov, Nat. Commun., 2021, 12, 1 CrossRef CAS PubMed.
- B. Bartolomei, A. Bogo, F. Amato, G. Ragazzon and M. Prato, Angew. Chem., Int. Ed., 2022, 61, e202200038 CrossRef CAS PubMed.
- S. Hu, A. Trinchi, P. Atkin and I. Cole, Angew. Chem., Int. Ed., 2015, 54, 2970 CrossRef CAS PubMed.
- G. E. Lecroy, F. Messina, A. Sciortino, C. E. Bunker, P. Wang, K. A. S. Fernando and Y. P. Sun, J. Phys. Chem. C, 2017, 121, 28180 CrossRef CAS.
- F. Yan, Z. Sun, H. Zhang, X. Sun, Y. Jiang and Z. Bai, Microchim. Acta, 2019, 186, 1 CrossRef PubMed.
- H. Ali, S. Ghosh and N. R. Jana, Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol., 2020, 12, e1617 CAS.
- J. Zhu and S. Mu, Adv. Funct. Mater., 2020, 30, 2001097 CrossRef CAS.
- Y. P. Sun, B. Zhou, Y. Lin, W. Wang, K. A. S. Fernando, P. Pathak, M. J. Meziani, B. A. Harruff, X. Wang, H. Wang, P. G. Luo, H. Yang, M. E. Kose, B. Chen, L. M. Veca and S. Y. Xie, J. Am. Chem. Soc., 2006, 128, 7756 CrossRef CAS PubMed.
- X. T. Tian and X. B. Yin, Small, 2019, 15, 1901803 CrossRef CAS PubMed.
- Z. Wang, F. Yuan, X. Li, Y. Li, H. Zhong, L. Fan and S. Yang, Adv. Mater., 2017, 29, 1702910 CrossRef PubMed.
- D. Li, P. Jing, L. Sun, Y. An, X. Shan, X. Lu, D. Zhou, D. Han, D. Shen, Y. Zhai, S. Qu, R. Zbořil and A. L. Rogach, Adv. Mater., 2018, 30, 1705913 CrossRef PubMed.
- M. T. Hasan, R. Gonzalez-Rodriguez, C. Ryan, N. Faerber, J. L. Coffer and A. V. Naumov, Adv. Funct. Mater., 2018, 28, 1804337 CrossRef.
- B. Ju, H. Nie, Z. Liu, H. Xu, M. Li, C. Wu, H. Wang and S. X. A. Zhang, Nanoscale, 2017, 9, 13326 RSC.
- H. Nie, M. Li, Q. Li, S. Liang, Y. Tan, L. Sheng, W. Shi and S. X. A. Zhang, Chem. Mater., 2014, 26, 3104 CrossRef CAS.
- H. Ding, J. S. Wei, P. Zhang, Z. Y. Zhou, Q. Y. Gao and H. M. Xiong, Small, 2018, 14, 1800612 CrossRef PubMed.
- S. Miao, K. Liang, J. Zhu, B. Yang, D. Zhao and B. Kong, Nano Today, 2020, 33, 100879 CrossRef CAS.
- X. Li, Y. Fu, S. Zhao, J. F. Xiao, M. Lan, B. Wang, K. Zhang, X. Song and L. Zeng, Chem. Eng. J., 2022, 430, 133101 CrossRef CAS.
- S. Yoo, Y. Song and S. Hahn, Light:Sci. Appl., 2022, 11, 1 CrossRef PubMed.
- J. Tang, J. Zhang, Y. Zhang, Y. Xiao, Y. Shi, Y. Chen, L. Ding and W. Xu, Nanoscale Res. Lett., 2019, 14, 1 CrossRef CAS PubMed.
- M. Bottrill and M. Green, Chem. Commun., 2011, 47, 7039 RSC.
- K. M. Tsoi, Q. Dai, B. A. Alman and W. C. W. Chan, Acc. Chem. Res., 2013, 46, 662 CrossRef CAS PubMed.
- L. Ye, K. T. Yong, L. Liu, I. Roy, R. Hu, J. Zhu, H. Cai, W. C. Law, J. Liu, K. Wang, J. Liu, Y. Liu, Y. Hu, X. Zhang, M. T. Swihart and P. N. Prasad, Nat. Nanotechnol., 2012, 7, 453 CrossRef CAS PubMed.
- A. M. Derfus, W. C. W. Chan and S. N. Bhatia, Nano Lett., 2004, 4, 11 CrossRef CAS PubMed.
- Z. L. Wu, Z. X. Liu and Y. H. Yuan, J. Mater. Chem. B, 2017, 5, 3794 RSC.
- F. Arcudi, L. Ďorďević and M. Prato, Acc. Chem. Res., 2019, 52, 2070 CrossRef CAS PubMed.
- F. Arcudi, L. Đorđević, F. Arcudi and L. Đorđević, Small, 2023, 19, 2300906 CrossRef CAS PubMed.
- L. Đorđević, F. Arcudi and M. Prato, Nat. Protoc., 2019, 14, 2931 CrossRef PubMed.
- Y. Dong, J. Shao, C. Chen, H. Li, R. Wang, Y. Chi, X. Lin and G. Chen, Carbon, 2012, 50, 4738 CrossRef CAS.
- I. L. Medintz, H. T. Uyeda, E. R. Goldman and H. Mattoussi, Nat. Mater., 2005, 4, 435 CrossRef CAS PubMed.
- H. Zhu, X. Wang, Y. Li, Z. Wang, F. Yang and X. Yang, Chem. Commun., 2009, 5118 RSC.
- H. Peng and J. Travas-Sejdic, Chem. Mater., 2009, 21, 5563 CrossRef CAS.
- D. V. Talapin, A. L. Rogach, E. V. Shevchenko, A. Kornowski, M. Haase and H. Weller, J. Am. Chem. Soc., 2002, 124, 5782 CrossRef CAS PubMed.
- Y. Yan, D. Zhai, Y. Liu, J. Gong, J. Chen, P. Zan, Z. Zeng, S. Li and W. Huang, ACS Nano, 2018, 12, 3523 CrossRef CAS PubMed.
- J. Schneider, C. J. Reckmeier, Y. Xiong, M. Von Seckendorff, A. S. Susha, P. Kasak and A. L. Rogach, J. Phys. Chem. C, 2017, 121, 2014 CrossRef CAS.
- F. Yuan, Y. K. Wang, G. Sharma, Y. Dong, X. Zheng, P. Li, A. Johnston, G. Bappi, J. Z. Fan, H. Kung, B. Chen, M. I. Saidaminov, K. Singh, O. Voznyy, O. M. Bakr, Z. H. Lu and E. H. Sargent, Nat. Photonics, 2019, 14, 171 CrossRef.
- L. Wang, Y. Wang, T. Xu, H. Liao, C. Yao, Y. Liu, Z. Li, Z. Chen, D. Pan, L. Sun and M. Wu, Nat. Commun., 2014, 5, 1 Search PubMed.
- E. Haque, J. Kim, V. Malgras, K. R. Reddy, A. C. Ward, J. You, Y. Bando, M. S. A. Hossain and Y. Yamauchi, Small Methods, 2018, 2, 1800050 CrossRef.
- C. I. M. Santos, I. F. A. Mariz, S. N. Pinto, G. Gonçalves, I. Bdikin, P. A. A. P. Marques, M. G. P. M. S. Neves, J. M. G. Martinho and E. M. S. Maçôas, Nanoscale, 2018, 10, 12505 RSC.
- C. I. M. Santos, L. Rodríguez-Pérez, G. Gonçalves, S. N. Pinto, M. Melle-Franco, P. A. A. P. Marques, M. A. F. Faustino, M.Á Herranz, N. Martin, M. G. P. M. S. Neves, J. M. G. Martinho and E. M. S. Maçôas, Carbon, 2020, 166, 164 CrossRef CAS.
- C. I. M. Santos, L. Rodríguez-Pérez, G. Gonçalves, C. J. Dias, F. Monteiro, M. D. A. F. Faustino, S. I. Vieira, L. A. Helguero, M.Á Herranz, N. Martín, M. G. P. M. S. Neves, J. M. G. Martinho and E. M. S. Maçôas, ACS Appl. Nano Mater., 2021, 4, 13079 CrossRef CAS.
- A. Braun and J. Tcherniac, J. Ann. Ber., 1907, 40, 2709 CrossRef CAS.
- H. Von der Weid and E. Diesbach, Helv. Chim. Acta, 1927, 10, 886 CrossRef.
- N. B. McKeown and N. B. McKeown, Phthalocyanine Materials: Synthesis, Structure and Function, Cambridge University Press, Cambridge, UK, 1998, vol. 1 Search PubMed.
- A. L. Thomas, Phthalocyanine Research and Applications, CRC Press, 1990 Search PubMed.
- A. G. Dandridge, H. A. E. Drescher and J. Thomas, In Dyes, 1929, 169 Search PubMed.
- J. M. Robertson, J. Chem. Soc., 1935, 615 RSC.
- J. M. Robertson, J. Chem. Soc., 1936, 1195 RSC.
- R. P. Linstead and J. M. Robertson, J. Chem. Soc., 1936, 1736 RSC.
- J. M. Robertson and I. D. A. Woodward, J. Chem. Soc., 1937, 219 RSC.
- J. M. Robertson and I. Woodward, J. Chem. Soc., 1940, 36 RSC.
- R. P. Linstead, J. Chem. Soc., 1934, 1016 RSC.
- G. T. Byrne, R. P. Linstead and A. R. Lowe, J. Chem. Soc., 1934, 1017 RSC.
- C. E. Dent and R. P. Linstead, J. Chem. Soc., 1934, 1027 RSC.
- C. E. Dent, R. P. Linstead and A. R. Lowe, J. Chem. Soc., 1934, 1033 RSC.
- D. Gounden, N. Nombona and W. E. van Zyl, Coord. Chem. Rev., 2020, 420, 213359 CrossRef CAS.
- S. Yang, Y. Yu, X. Gao, Z. Zhang and F. Wang, Chem. Soc. Rev., 2021, 50, 12985 RSC.
- D. Li, S. Cai, P. Wang, H. Cheng, B. Cheng, Y. Zhang and G. Liu, Adv. Healthc. Mater., 2023, 12, 2300263 CrossRef CAS PubMed.
- T. Furuyama, K. Satoh, T. Kushiya and N. Kobayashi, J. Am. Chem. Soc., 2014, 136, 765 CrossRef CAS PubMed.
- H. Tomoda, E. Hibiya, T. Nakamura, H. Ito and S. Saito, Chem. Lett., 1976, 5, 1003 CrossRef.
- T. Haruhiko, S. Shojiro, O. Shojiro and S. Shinsaku, Chem. Lett., 2006, 9, 1277 Search PubMed.
- S. W. Oliver and T. D. Smith, J. Chem. Soc., Perkin Trans. 2, 1987, 1579 RSC.
- P. J. Brach, S. J. Grammatica, O. A. Ossanna and L. Weinberger, J. Heterocycl. Chem., 1970, 7, 1403 CrossRef CAS.
- H. J. Callot, F. Metz and C. Piechocki, Tetrahedron, 1982, 38, 2365 CrossRef CAS.
- G. de la Torre, G. Bottari, U. Hahn and T. Torres, in Functional Phthalocyanine Molecular Materials, 2010, pp. 1–44 Search PubMed.
- C. C. Leznoff, M. Hu and K. J. M. Nolan, Chem. Commun., 1996, 1245 RSC.
- J. Follana-Berná, R. Farran, W. Leibl, A. Quaranta, Á. Sastre-Santos and A. Aukauloo, Angew. Chem., Int. Ed., 2021, 60, 12284 CrossRef PubMed.
- O. L. Kaliya, E. A. Lukyanets and G. N. Vorozhtsov, J. Porphyrins Phthalocyanines, 1999, 3, 592 CrossRef CAS.
- B. A. Bench, W. W. Brennessel, H.-J. Lee, S. M. Gorun, H. Ali and J. E. van Lier, Angew. Chem. Int. Ed., 2002, 41, 747 CrossRef CAS PubMed.
- O. Tsaryova, A. Semioshkin, D. Wöhrle and V. I. Bregadze, J. Porphyrins Phthalocyanines, 2012, 9, 268 CrossRef.
- X. Duan, Y. Zhang, H. Wang, F. Dai, G. Yang and Y. Chen, New J. Chem., 2020, 44, 13240 RSC.
- C. P. S. Ribeiro and L. L. M. O. Lourenço, J. Photochem. Photobiol., 2021, 48, 1 Search PubMed.
- S. R. D. Gamelas, A. T. P. C. Gomes, M. A. F. Faustino, A. C. Tomé, J. P. C. Tomé, A. Almeida and L. M. O. Lourenço, ACS Appl. Bio Mater., 2020, 3, 4044 CrossRef CAS PubMed.
- L. M. O. Lourenço, D. M. G. C. Rocha, C. I. V. Ramos, M. C. Gomes, A. Almeida, M. A. F. Faustino, F. A. Almeida Paz, M. Neves, A. Cunha, J. P. C. Tome, L. M. O. Lourenço, Deisy, M. G. C. Rocha, C. I. V. Ramos, M. C. Gomes, M. A. F. Faustino, F. A. Almeida Paz, M. G. P. M. S. Neves, Â. Cunha and J. P. C. Tomé, ChemPhotoChem, 2019, 3, 251 CrossRef.
- L. M. O. Lourenço, A. Sousa, M. C. Gomes, M. A. F. Faustino, A. Almeida, A. M. S. Silva, M. G. P. M. S. Neves, J. A. S. Cavaleiro, Â. Cunha and J. P. C. Tomé, Photochem. Photobiol. Sci., 2015, 14, 1853 CrossRef PubMed.
- K. A. D. F. Castro, J. A. Prandini and L. M. O. Lourenço, Front. Chem., 2022, 10, 1 Search PubMed.
- L. M. O. Lourenço, P. M. R. Pereira, E. Maciel, M. Válega, F. M. J. Domingues, M. R. M. Domingues, M. G. P. M. S. Neves, J. A. S. Cavaleiro, R. Fernandes and J. P. C. Tomé, Chem. Commun., 2014, 50, 8363 RSC.
- L. M. O. Lourenço, M. G. P. M. S. Neves, J. A. S. Cavaleiro and J. P. C. Tomé, Tetrahedron, 2014, 70, 2681 CrossRef.
- J. Lopes-Nunes, J. Carvalho, J. Figueiredo, C. I. V. Ramos, L. M. O. Lourenço, J. P. C. Tomé, M. G. P. M. S. Neves, J. L. Mergny, J. A. Queiroz, G. F. Salgado and C. Cruz, Bioorg. Chem., 2020, 100, 103920 CrossRef CAS PubMed.
- C. I. V. Ramos, S. P. Almeida, L. M. O. Lourenço, P. M. R. Pereira, R. Fernandes, M. A. F. Faustino, J. P. C. Tomé, J. Carvalho, C. Cruz and M. G. P. M. S. Neves, Molecules, 2019, 24, 733 CrossRef CAS PubMed.
- L. M. O. Lourenço, B. A. Iglesias, P. M. R. Pereira, H. Girão, R. Fernandes, M. G. P. M. S. Neves, J. A. S. Cavaleiro and J. P. C. Tomé, Dalton Trans., 2015, 44, 530 RSC.
- S. Dutta, B. G. Ongarora, H. Li, M. G. H. da Vicente, B. K. Kolli and K. P. Chang, PLoS One, 2011, 6, e20786 CrossRef CAS PubMed.
- J. M. D. Calmeiro, J. P. C. Tomé and L. M. O. Lourenço, J. Mater. Chem. C, 2020, 8, 8344 RSC.
- L. Wibmer, L. M. O. Lourenço, A. Roth, G. Katsukis, M. G. P. M. S. Neves, J. A. S. Cavaleiro, J. P. C. Tomé, T. Torres and D. M. Guldi, Nanoscale, 2015, 7, 5674 RSC.
- L. M. O. Lourenço, A. Hausmann, C. Schubert, M. G. P. M. S. Neves, J. A. S. Cavaleiro, T. Torres, D. M. Guldi and J. P. C. Tomé, ChemPlusChem, 2015, 80, 832 CrossRef PubMed.
- L. C. Nene and T. Nyokong, Diamond Relat. Mater., 2023, 131, 109549 CrossRef CAS.
- B. Uprety and H. Abrahamse, Front. Chem., 2022, 10, 946574 CrossRef CAS PubMed.
- L. Lei, B. Dai, T. Han, C. Zhou, Z. Gong and P. Zhang, Front. Mater., 2022, 9, 744 Search PubMed.
- X. Xu, R. Ray, Y. Gu, H. J. Ploehn, L. Gearheart, K. Raker and W. A. Scrivens, J. Am. Chem. Soc., 2004, 126, 12736 CrossRef CAS PubMed.
- C. Xia, S. Zhu, T. Feng, M. Yang, B. Yang, C. Xia, T. Feng, M. Yang, B. Yang and S. Zhu, Adv. Sci., 2019, 6, 1901316 CrossRef CAS PubMed.
- D. Yakovlev, E. Kolesova, S. Sizova, K. Annas, M. Tretyak, V. Loschenov, A. Orlova and V. Oleinikov, Nanomaterials, 2022, 12, 3874 CrossRef CAS PubMed.
- A. C. S. Samia, X. Chen and C. Burda, J. Am. Chem. Soc., 2003, 125, 15736 CrossRef CAS PubMed.
- Ş. Eryiğit, A. Gelir, E. Budak, C. Ünlü, A. Gömleksiz, İ. Özçeşmeci and A. Gül, Luminescence, 2022, 37, 268 CrossRef PubMed.
- L. Fan, Y. Hu, X. Wang, L. Zhang, F. Li, D. Han, Z. Li, Q. Zhang, Z. Wang and L. Niu, Talanta, 2012, 101, 192 CrossRef CAS PubMed.
- O. J. Achadu, I. Uddin and T. Nyokong, J Photochem Photobio A: Chem, 2016, 317, 12 CrossRef CAS.
- I. Scalise and E. N. Durantini, Bioorg. Med. Chem., 2005, 13, 3037 CrossRef CAS PubMed.
- M. K. Nazeeruddin, R. Humphry-Baker, M. Grätzel, M. K. Nazeeruddin, R. Humphry-Baker, M. Grätzel and B. A. Murrer, Chem. Commun., 1998, 6, 719 RSC.
- O. J. Achadu and T. Nyokong, New J. Chem., 2016, 40, 8727 RSC.
- S. E. Korkut, D. Akyüz, K. Özdoʇan, Y. Yerli, A. Koca and M. K. Şener, Dalton Trans., 2016, 45, 3086 RSC.
- O. J. Achadu and T. Nyokong, J. Fluoresc., 2016, 26, 283 CrossRef CAS PubMed.
- O. J. Achadu and T. Nyokong, Talanta, 2017, 166, 15 CrossRef CAS PubMed.
- O. J. Achadu and T. Nyokong, Dyes Pigm., 2017, 145, 189 CrossRef CAS.
- A. M. Santiago, C. I. M. Santos, L. M. O. Lourenço, I. F. A. Mariz, J. P. C. Tomé and E. Maçôas, Nanomaterials, 2022, 12, 1892 CrossRef CAS PubMed.
- O. J. Achadu and T. Nyokong, Dyes Pigm., 2019, 160, 328 CrossRef CAS.
- A. Ogunsipe, J. Y. Chen and T. Nyokong, New J. Chem., 2004, 28, 822 RSC.
- O. J. Achadu and T. Nyokong, Dyes Pigm., 2019, 160, 328 CrossRef CAS.
- W. Jiang, M. Jiang, T. Wang, X. Chen, M. Zeng, J. Yang, Z. Zhou, N. Hu, Y. Su and Z. Yang, RSC Adv., 2021, 11, 14805 RSC.
- W. Jiang, X. Chen, T. Wang, B. Li, M. Zeng, J. Yang, N. Hu, Y. Su, Z. Zhou and Z. Yang, RSC Adv., 2021, 11, 5618 RSC.
- S. M. Mousavi, M. Y. Kalashgrani, N. Javanmardi, M. Riazi, M. H. Akmal, V. Rahmanian, A. Gholami and W. H. Chiang, J. Mater. Chem. B, 2024, 12, 7041 RSC.
- A. Karagianni, N. G. Tsierkezos, M. Prato, M. Terrones and K. V. Kordatos, Carbon, 2023, 203, 273 CrossRef CAS.
- R. Matshitse, K. E. Sekhosana, O. J. Achadu and T. Nyokong, J. Coord. Chem., 2017, 70, 3308 CrossRef CAS.
- T. Fan, W. Zeng, W. Tang, C. Yuan, S. Tong, K. Cai, Y. Liu, W. Huang, Y. Min and A. J. Epstein, Nanoscale Res. Lett., 2015, 10, 1 CrossRef PubMed.
- N. Nwahara, R. Nkhahle, B. P. Ngoy, J. Mack and T. Nyokong, New J. Chem., 2018, 42, 6051 RSC.
- L. C. Nene, M. E. Managa, D. O. Oluwole, D. M. Mafukidze, A. Sindelo and T. Nyokong, Inorg. Chim. Acta, 2019, 488, 304 CrossRef CAS.
- L. C. Nene, M. Managa and T. Nyokong, Dyes Pigm., 2019, 165, 488 CrossRef CAS.
- L. C. Nene and T. Nyokong, Photodiagn. Photodyn. Ther., 2021, 36, 102573 CrossRef CAS PubMed.
- K. Celep, G. Gökçil, P. Şen, F. Şahin, A. Erdoğmuş and G. Y. Atmaca, Appl. Organomet. Chem., 2025, 39, e70007 CrossRef CAS.
- G. Gökçil, G. Y. Atmaca, P. Şen, F. Şahin and A. Erdoğmuş, J. Photochem. Photobiol., A, 2025, 459, 116108 CrossRef.
- G. Gökçil, K. Celep, P. Şen, F. Şahin, A. Erdoğmuş and G. Y. Atmaca, Polyhedron, 2025, 272, 117468 CrossRef.
- R. Matshitse and T. Nyokong, J. Fluoresc., 2018, 28, 827 CrossRef CAS PubMed.
- R. Matshitse, N. Nwaji, M. Mananga, E. Prinsloo and T. Nyokong, J. Photochem. Photobiol., A, 2018, 367, 253 CrossRef CAS.
- M. De, S. Ghosh, T. Sen, M. Shadab, I. Banerjee, S. Basu and N. Ali, Mol. Ther. – Nucleic Acids, 2018, 10, 9 CrossRef CAS PubMed.
- G. van der Horst, A. F. van de Merbel, E. Ruigrok, M. H. van der Mark, E. Ploeg, L. Appelman, S. Tvingsholm, M. Jäätelä, J. van Uhm, M. Kruithof-de Julio, G. N. Thalmann, R. C. M. Pelger, C. H. Bangma, J. L. Boormans, G. van der Pluijm and E. C. Zwarthoff, Mol. Oncol., 2020, 14, 3121 CrossRef CAS PubMed.
- Y. I. Openda, P. Sen, M. Managa and T. Nyokong, Photodiagn. Photodyn. Ther., 2020, 29, 101607 CrossRef CAS PubMed.
- L. Hui, J. Huang, G. Chen, Y. Zhu and L. Yang, ACS Appl. Mater. Interfaces, 2016, 8, 20 CrossRef CAS PubMed.
- P. M. Sivakumar, G. Sheshayan and M. Doble, Chem. Biol. Drug Des., 2008, 72, 303 CrossRef CAS PubMed.
- P. Sen, N. Nwahara and T. Nyokong, Main Group Chem., 2021, 20, 175 CrossRef CAS.
- M. Özçeşmeci, S. Gümrükçü, C. Ünlü, İ.Y Coşkun, S. Özdemir, M. S. Yalçın, G. Tollu and İ. Özçeşmeci, Appl. Organomet. Chem., 2024, 38, e7423 CrossRef.
- D. M. Mafukidze and T. Nyokong, J. Coord. Chem., 2017, 70, 3598 CrossRef CAS.
- O. J. Achadu, M. Managa and T. Nyokong, J. Photochem. Photobiol., A, 2017, C, 174 CrossRef.
- Q. Chen, Y. Liu, B. Mao, Z. Wu, W. Yan, D. Zhang, Q. Li, H. Huang, Z. Kang and W. Shi, Adv. Funct. Mater., 2023, 33, 2305318 CrossRef CAS.
- S. A. Majeed, N. Nwaji, J. Mack, T. Nyokong and S. Makhseed, J. Lumin., 2019, 213, 88 CrossRef CAS.
- D. AlMarzouq, S. A. Majeed, Ö. Budak and A. Koca, Inorg. Chim. Acta, 2021, 527, 120558 CrossRef CAS.
- M. Cacioppo, T. Scharl, L. Đorđević, A. Cadranel, F. Arcudi, D. M. Guldi and M. Prato, Angew. Chem., Int. Ed., 2020, 59, 12779 CrossRef CAS PubMed.
- D. M. Mafukidze and T. Nyokong, J. Mol. Struct., 2019, 1180, 307 CrossRef CAS.
- S. Centane, E. K. Sekhosana, R. Matshitse and T. Nyokong, J. Electroanal. Chem., 2018, 820, 146 CrossRef CAS.
- C. Luhana, I. Moyo, K. Tshenkeng and P. Mashazi, Microchem. J., 2022, 180, 107605 CrossRef CAS.
- H. M. Sharma, D. K. Das and A. Kumar, Int. J. Hydrogen Energy, 2024, 110, 159 CrossRef.
- K. H. Koh, S. H. Noh, T. H. Kim, W. J. Lee, S. C. Yi and T. H. Han, RSC Adv., 2017, 7, 26113 RSC.
- Y. Lu, Y. Jiang, X. Gao, X. Wang and W. Chen, J. Am. Chem. Soc., 2014, 136, 11687 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2025 |

