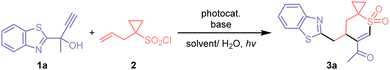Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Access to spirocyclic vinyl sulfones via radical cyclization and functional group migration†
Shan Yang,
Yasu Chen and
Chen Zhu *
*
Frontiers Science Center for Transformative Molecules, School of Chemistry and Chemical Engineering, State Key Laboratory of Synergistic Chem-Bio Synthesis, Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, Shanghai Jiao Tong University, 800 Dongchuan Road, Shanghai 200240, China. E-mail: chzhu@sjtu.edu.cn
First published on 29th April 2025
Abstract
Spirocyclic vinyl sulfones, which incorporate the three-dimensional structure inherent to spiro compounds and the Michael acceptor reactivity associated with vinyl sulfones, hold promise for novel biological activities. The lack of efficient synthetic methods, however, hinders their extensive investigations in drug discovery and development. In this work, we describe a practical and versatile approach for the synthesis of multi-functionalized spirocyclic vinyl sulfones from easily available materials. The reaction proceeds efficiently through a cascade of radical cyclization followed by (hetero)aryl migration. The protocol features mild photocatalytic conditions and provides access to a diverse range of products, enabling the construction of complex scaffolds, including medium-sized ring-fused spirocyclic vinyl sulfones.
Introduction
One widely employed strategy in drug design is to increase the complexity of molecular architectures by introducing a greater number of sp3-hybridized carbons and rings, which serves to rigidify the ligand's conformation.1 Spirocyclic compounds, distinguished by their unique and rigid three-dimensional geometry and composed of two rings connected through a single spiroatom, have been receiving increasing interest from both synthetic and medicinal chemists.2 The non-planar conformations that these compounds often adopt can lead to improved binding affinity and enhanced selectivity for biological targets, such as proteins. Spirocyclic systems incorporating a three-membered ring are notable for their capacity to improve pharmacokinetic and pharmacodynamic profiles, rendering them versatile building blocks in medicinal chemistry.3 For instance (Scheme 1A), illudin M (I)4 and illudin S(II),5 natural sesquiterpenoids with antitumor, antiviral, and genotoxic activities, are key precursors in the development of anticancer drugs; irofulven (III),6 a semi-synthetic analog of the illudin class, has advanced to clinical trials for the treatment of solid tumors such as prostate cancer, pancreatic cancer, and ovarian cancer; ptaquilosin (IV) demonstrates selective toxicity across species in NCI antitumor screening assays,7 exhibiting notable activity against human myeloid leukemia and a range of carcinoma cell lines.Vinyl sulfones represent valuable Michael acceptors in drug design, owing to their reactivity towards nucleophilic species.8 The incorporation of both a vinyl sulfone moiety and a three-membered spirocyclic subunit within a single molecular framework has the potential to unlock unique biological activities. For example (Scheme 1A), compound V demonstrates notable herbicidal efficacy at lower dosages while exhibiting favorable compatibility with crops, positioning it as a promising candidate for commercial agrochemical development.9 Nevertheless, the absence of efficient synthetic routes to access spirocyclic vinyl sulfones limits the extensive exploration of their physicochemical and physiological attributes.10
In this report, we describe a versatile and operationally simple method for the synthesis of multi-functionalized spirocyclic vinyl sulfones, employing inexpensive allylcyclopropane sulfonyl chloride and easily accessible tertiary propargyl alcohols as starting materials (Scheme 1B). The photocatalyzed reaction proceeds via a cascade process involving radical cyclization followed by (hetero)aryl migration,11,12 driven by visible-light irradiation. Notably, the use of cyclic tertiary propargyl alcohols facilitates the efficient construction of complex medium-sized ring-fused spirocyclic frameworks through ring expansion, structures that are otherwise difficult to synthesize.
Results and discussion
The investigations were performed under blue LED light irradiation at room temperature, using benzothiazole-substituted propargyl alcohol 1a and commercially available allylcyclopropane sulfonyl chloride 2 as substrates (Table 1). A range of photocatalysts were screened, with fac-Ir(ppy)3 demonstrating the best catalytic activity (Entries 1–5). Considering that HCl is the only byproduct generated during the reaction, the addition of one equivalent of base to neutralize the strong acid is essential for successful transformation. Among several organic and inorganic bases tested (Entries 5–8), Na2HPO4 was identified as the optimal one. The inclusion of a small quantity of H2O as a co-solvent enhanced the solubility of Na2HPO4 within the reaction system, thereby promoting the conversion. Subsequent evaluation of various organic solvents revealed that the reaction carried out in DCM/H2O provided the desired product 3a with a favorable yield (Entries 9–13). Furthermore, control experiments demonstrated that the reaction was completely inhibited in the absence of a photocatalyst or upon exclusion of light (Entries 14 and 15), and the yield was compromised when the reaction was performed in air or without the base (Entries 16 and 17).| Entrya | Photocatalyst | Base | Solvent | Yield b(%) |
|---|---|---|---|---|
| a Standard conditions: 1a (0.2 mmol), 2a (0.3 mmol), base (0.2 mmol), and photocatalyst (3 mol%) in solvent/H2O (v/v 2 mL/0.2 mL), irradiated with 12 W blue LEDs at room temperature under N2.b Yields of isolated products are given.c In dark.d Under air. | ||||
| 1 | Eosin Y | Na2HPO4 | CH3CN | 7 |
| 2 | Ru(bpy)3Cl2·6H2O | Na2HPO4 | CH3CN | 13 |
| 3 | Mes-Acr+ClO4− | Na2HPO4 | CH3CN | 16 |
| 4 | 4CzIPN | Na2HPO4 | CH3CN | 30 |
| 5 | fac-Ir(ppy)3 | Na2HPO4 | CH3CN | 41 |
| 6 | fac-Ir(ppy)3 | NaH2PO4 | CH3CN | 36 |
| 7 | fac-Ir(ppy)3 | Na3PO4 | CH3CN | 39 |
| 8 | fac-Ir(ppy)3 | DIPEA | CH3CN | 29 |
| 9 | fac-Ir(ppy)3 | Na2HPO4 | DCM | 74 |
| 10 | fac-Ir(ppy)3 | Na2HPO4 | MeOH | 23 |
| 11 | fac-Ir(ppy)3 | Na2HPO4 | DMSO | Trace |
| 12 | fac-Ir(ppy)3 | Na2HPO4 | PhCF3 | 38 |
| 13 | fac-Ir(ppy)3 | Na2HPO4 | EtOAc | 43 |
| 14 | — | Na2HPO4 | DCM | 0 |
| 15c | fac-Ir(ppy)3 | Na2HPO4 | DCM | 0 |
| 16d | fac-Ir(ppy)3 | Na2HPO4 | DCM | 39 |
| 17 | fac-Ir(ppy)3 | — | DCM | 58 |
With the optimized reaction conditions in hand, we investigated the compatibility of various functional groups by testing a broad range of tertiary propargyl alcohols (Scheme 2). First, we examined the impact of aliphatic substituents of different sizes surrounding the migrating group, showing that steric hindrance did not significantly impede reaction efficiency (3a–3d). A slight decrease in yield was observed with the phenyl substituent (3e). Beyond benzothiazolyl, a range of five-membered heteroaryl groups, including benzothienyl, benzofuryl, thiazolyl, thienyl, and furyl, as well as six-membered heteroaryls such as pyrimidyl, pyrazinyl, and pyridyl, demonstrated sufficient migratory ability, resulting in the formation of the corresponding products (3f–3m) in synthetically useful yields. Moreover, a diversity of functionalized phenyl groups readily participated in the migration process (3n–3y). Substrates featuring substitution on the benzene ring at the para, meta, or ortho positions were all competent to generate the desired products. Both electron-donating groups (e.g., Me, OMe, tBu) and electron-withdrawing groups (e.g., halo, CN, CF3, SO2R) proved compatible with the reaction conditions; the latter typically afforded enhanced yields, which can be attributed to the propensity of electron-deficient arenes to undergo migration promoted by nucleophilic alkyl radicals. While m-substituted substrates provided comparatively lower yields (3w and 3x), the o-chloro substrate afforded the product in high yield (3y). Furthermore, allylcyclopropane sulfonyl chloride 2 could be successfully substituted with homoallylic sulfonyl chloride, providing the desired product in a useful yield (3z). Besides tertiary propargyl alcohols, secondary propargyl alcohol (1aa) was also amenable to the formation of spirocyclic vinyl sulfone (3aa) bearing a formyl group, albeit with a low yield.
This protocol is applicable to the construction of structurally complex ring-fused spirocyclic vinyl sulfones via ring expansion (Scheme 3). When cyclic propargyl alcohols are employed, aryl or heteroaryl group migration proceeds concomitantly with ring expansion. This enables facile editing of cyclic skeletons through the addition of three carbons, transforming five- and six-membered rings into eight- and nine-membered rings.13 The reaction with the substrates (4a and 4b) derived from indanone and tetralone gave rise to the corresponding benzo-octanone and nonanone-fused products (5a and 5b). Likewise, this approach proved applicable to heteroaryl-fused analogues (4c–4g), facilitating the efficient migration of benzothienyl, benzofuryl, thienyl, thiazolyl and pyridyl moieties to afford the corresponding products (5c–5g). The substrate 4h, obtained from fluorenone, was also suitable, affording a more structurally elaborate product containing five cyclic units in a notably high yield (5h). The presence of heteroatoms, such as O or S atoms, within the substrates did not hinder the reaction outcomes. For example, substrates 4i–4m, synthesized from chromanone, thiochromanone, xanthenone, and thioxanthenone precursors, were all amenable to furnishing the corresponding products in synthetically useful yields (5i–5m).
Gram-scale preparation of 3a was successfully performed without compromising the yield, demonstrating the practicality of the method (Scheme 4a). To gain deeper insight into the reaction mechanism, a series of experiments was conducted. The addition of stoichiometric amounts of the radical scavenger 2,2,6,6-tetramethyl-1-piperidinyloxy (TEMPO) completely inhibited the formation of the target product 3a. When 2,6-di-tert-butyl-4-methylphenol (BHT) and 1,1-diphenylethylene were used as trapping reagents, the corresponding adducts (6, 7, and 8) resulting from the interception of sulfonyl radicals were isolated and characterized. This result confirmed the involvement of free-radical species in the transformation, which was initiated by a sulfonyl radical (Scheme 4b). Furthermore, light on/off experiments demonstrated that product formation occurred only during periods of continuous light irradiation, suggesting the predominance of a photocatalytic pathway (Scheme 4c). The quantum yield measurement (Φ = 0.81) indicated that the reaction proceeded primarily via a photocatalytic pathway, although the contribution of a radical chain process could not be ruled out (for details, see the ESI†).
A plausible reaction mechanism is depicted in Scheme 4d. Initially, upon irradiation with blue LED light, the excited photocatalyst IrIII* reduces sulfonyl chloride 2 via single-electron transfer (SET), generating a sulfonyl radical and an IrIV species. This sulfonyl radical then adds to the alkynyl group of propargyl alcohol 1, resulting in vinyl radical intermediate I. Subsequently, addition of the vinyl radical to the distal alkene through a six-membered cyclic transition state forms the highly reactive primary alkyl radical II. This intermediate undergoes intramolecular 1,4-(hetero)aryl migration via a five-membered cyclic transition state, leading to the formation of ketyl radical intermediate III. Oxidation of III to cation IV by the IrIV species simultaneously regenerates the IrIII species, perpetuating the photocatalytic cycle. Finally, deprotonation of cation IV affords the desired product 3.
Conclusions
In summary, we have disclosed a novel photocatalytic approach to access spirocyclic vinyl sulfones via tandem radical cyclization and functional group migration processes. The reaction features mild conditions and a broad functional group compatibility. A diverse array of complex scaffolds, including medium-sized ring-fused spirocyclic vinyl sulfones, can be readily generated in synthetically useful yields. Evaluation of the biological activities of these compounds is ongoing in our laboratories. This protocol not only facilitates the synthesis of spirocyclic vinyl sulfones that are otherwise difficult to access but also expands their potential applications in medicinal chemistry.Data availability
Data supporting the manuscript are provided in the ESI,† including the experimental methods, compound characterization, and NMR spectra for this study.Author contributions
C. Z. conceived of and directed the project, S. Y. and Y. C. conducted the experiments and analyzed the data, S. Y. and C. Z. wrote the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was supported by the National Natural Science Foundation of China (22371185 and 22171201), the Fundamental Research Funds for the Central Universities (23X010301599, 24X010301678), the Program of Shanghai Academic/Technology Research Leader (23XD1421900).Notes and references
- C. G. Wermuth, in The Practice of Medicinal Chemistry, Elsevier Academic Press, San Diego, 2003 Search PubMed.
- For selected reviews: (a) H. Lin and S. J. Danishefsky, Angew. Chem., Int. Ed., 2003, 42, 36–51 CrossRef CAS PubMed; (b) C. Galliford and K. Scheidt, Angew. Chem., Int. Ed., 2007, 46, 8748–8758 CrossRef CAS PubMed; (c) R. Rios, Chem. Soc. Rev., 2012, 41, 1060–1074 RSC; (d) K. Undheim, Synthesis, 2014, 46, 1957–2006 CrossRef CAS; (e) L. K. Smith and I. R. Baxendale, Org. Biomol. Chem., 2015, 13, 9907–9933 RSC; (f) X. Xie, W. Huang, C. Peng and B. Han, Adv. Synth. Catal., 2018, 360, 194–228 CrossRef CAS; (g) P.-W. Xu, J.-S. Yu, C. Chen, Z.-Y. Cao, F. Zhou and J. Zhou, ACS Catal., 2019, 9, 1820–1882 CrossRef CAS; (h) R. T. Ramon, Spiro Compounds: Synthesis and Applications, VCH, 2022, DOI:10.1002/9781119567646; (i) R. B. Kargbo, ACS Med. Chem. Lett., 2025, 16, 216–218 CrossRef CAS PubMed.
- For selected reviews: (a) Y. Zheng, C. M. Tice and S. B. Singh, Bioorg. Med. Chem. Lett., 2014, 24, 3673–3682 CrossRef CAS PubMed; (b) M. T. Varela, G. G. Dias, L. F. N. de Oliveira, R. G. de Oliveira, F. D. Aguiar, J. P. Nogueira, L. R. Cruz and L. C. Dias, Eur. J. Med. Chem., 2025, 287, 117368 CrossRef CAS PubMed.
- (a) R. Schobert, B. Biersack, S. Knauer and M. Ocker, Bioorg. Med. Chem., 2008, 16, 8592–8597 CrossRef CAS PubMed; (b) S. Knauer, B. Biersack, M. Zoldakova, K. Effenberger, W. Milius and R. Schobert, Anti-Cancer Drugs, 2009, 20, 676–681 CrossRef CAS PubMed; (c) R. Schobert, S. Seibt, K. Mahal, A. Ahmad, B. Biersack, K. Effenberger-Neidnicht, S. Padhye, F. H. Sarkar and T. Mueller, J. Med. Chem., 2011, 54, 6177–6182 CrossRef CAS PubMed; (d) P. Le, M. B. Nodwell, J. Eirich and S. A. Sieber, Chem.–Eur. J., 2019, 25, 12644–12651 CrossRef CAS PubMed; (e) B. Linder, M. Zoldakova, Z. Kornyei, L. H. F. Köhler, S. Seibt, D. Menger, A. Wetzel, E. Madarász, R. Schobert and D. Kögel, Int. J. Mol. Sci., 2022, 23, 9056 CrossRef CAS PubMed.
- (a) T. C. McMorris and M. Anchel, J. Am. Chem. Soc., 1965, 87, 1594–1600 CrossRef CAS PubMed; (b) M. J. Kelner, T. C. McMorris, W. T. Beck, J. M. Zamora and R. Taetle, Cancer Res., 1987, 47, 3186–3189 CAS; (c) N. G. Jaspers, A. Raams, M. J. Kelner, J. M. Ng, Y. M. Yamashita, S. Takeda, T. C. McMorris and J. H. Hoeijmakers, DNA Repair, 2002, 1, 1027–1038 CrossRef CAS PubMed; (d) V. K. Lehmann, A. Huang, S. Ibanez-Calero, G. R. Wilson and K. L. Rinehart, J. Nat. Prod., 2003, 66, 1257–1258 CrossRef CAS PubMed; (e) R. Schobert, S. Knauer, S. Seibt and B. Biersack, Curr. Med. Chem., 2011, 18, 790–807 CrossRef CAS PubMed; (f) H. Glatt, K. E. Pietsch, S. J. Sturla and W. Meinl, Arch. Toxicol., 2014, 88, 161–169 CrossRef CAS PubMed; (g) Y. Uto, K. Sasaki, M. Takahashi, K. Morimoto and K. Inoue, Anal. Sci., 2019, 35, 789–792 CrossRef CAS PubMed; (h) L. Casimir, S. Zimmer, F. Racine-Brassard, P.-É. Jacques and A. Maréchal, DNA Repair, 2023, 122, 103433 CrossRef CAS PubMed.
- (a) H. S. Friedman, S. T. Keir and P. J. Houghton, Cancer Chemother. Pharmacol., 2001, 48, 413–416 CrossRef CAS PubMed; (b) K. M. Samson, R. Taetle and M. J. Kelner, J. Org. Chem., 2001, 66, 6158–6163 CrossRef PubMed; (c) N. Senzer, J. Arsenau, D. Richards, B. Berman, J. R. MacDonald and S. Smith, Am. J. Clin. Oncol., 2005, 28, 36–42 CrossRef CAS PubMed; (d) Y.-T. Wang, T. Wiltshire, J. Senft, E. Reed and W.-X. Wang, Biochem. Pharmacol., 2007, 73, 469–480 CrossRef CAS PubMed; (e) M. D. Staake, A. Kashinatham, T. C. McMorris, L. A. Estes and M. J. Kelner, Bioorg. Med. Chem. Lett., 2016, 26, 1836–1838 CrossRef CAS PubMed; (f) J. Börcsök, Z. Sztupinszki, R. Bekele, S. P. Gao, M. Diossy, A. S. Samant, K. M. Dillon, V. Tisza, S. Spisák, O. Rusz, I. Csabai, H. Pappot, Z. J. Frazier, D. J. Konieczkowski, D. Liu, N. Vasani, J. A. Rodrigues, D. B. Solit, J. H. Hoffman-Censits, E. R. Plimack, J. E. Rosenberg, J.-B. Lazaro, M.-E. Taplin, G. Iyer, S. Brunak, R. Lozsa, E. M. V. Allen, D. Szüts, K. W. Mouw and Z. Szallasi, Clin. Cancer Res., 2021, 27, 2011–2022 CrossRef PubMed.
- (a) T. C. McMorris, M. J. Kelner, W. Wang, L. A. Esters, A. Montoya and R. Taetle, J. Org. Chem., 1992, 57, 6876–6883 CrossRef CAS; (b) A. Padwa, V. P. Sandanayaka and E. A. Curtis, J. Am. Chem. Soc., 1994, 116, 2667–2668 CrossRef CAS.
- (a) D. C. Meadows and J. Gervay-Hague, Med. Res. Rev., 2006, 26, 793–814 CrossRef CAS PubMed; (b) M. N. Noshi, A. Elawa, E. Torres and P. L. Fuchs, J. Am. Chem. Soc., 2007, 129, 11242–11247 CrossRef CAS PubMed; (c) H. Kumamoto, K. Deguchi, T. Wagata, Y. Furuya, Y. Odanaka, Y. Kitade and H. Tanaka, Tetrahedron, 2009, 65, 8007–8013 CrossRef CAS; (d) J. Morales-Sanfrutos, J. Lopez-Jaramillo and M. Ortega-Muñoz, Org. Biomol. Chem., 2010, 8, 667–675 RSC; (e) A. N. R. Alba, X. Companyó and R. Rios, Chem. Soc. Rev., 2010, 39, 2018–2033 RSC; (f) F. J. Lopez-Jaramillo, F. Hernandez-Mateo and F. Santoyo-Gonzalez, Integrative proteomics: chapter 16: vinyl sulfone: a multi-purpose function in proteomics, Leung H. C, ed. InTech, Rijeka, 2012, pp. 301–326 Search PubMed; (g) R. Ahmadi and S. Emami, Eur. J. Med. Chem., 2022, 234, 114255 CrossRef CAS PubMed; (h) Y.-C. Xiao and F.-E. Chen, Expet Opin. Drug Discov., 2023, 19, 239–251 CrossRef PubMed.
- G. Besong, (Basf SE), US Pat., 20160102103 A1, 2016 Search PubMed.
- E. K. Kozirev and V. A. Palchykov, Chemistry of Heterocyclic Compounds, New York, 2019, vol. 55, pp. 349–351 Search PubMed.
- For selected reviews: (a) Z.-M. Chen, X.-M. Zhang and Y.-Q. Tu, Chem. Soc. Rev., 2015, 44, 5220–5245 RSC; (b) W. P. Li, W. T. Xu, J. Xie, S. Y. Yu and C. J. Zhu, Chem. Soc. Rev., 2018, 47, 654–667 RSC; (c) X. Wu and C. Zhu, Acc. Chem. Res., 2020, 53, 1620–1636 CrossRef CAS PubMed; (d) X. Wu, Z. Ma, T. Feng and C. Zhu, Chem. Soc. Rev., 2021, 50, 11577–11613 RSC; (e) Y. Wei, X. Wu and C. Zhu, Synlett, 2022, 33, 1017–1028 CrossRef CAS; (f) W. Lee, I. Park and S. Hong, Sci. China Chem., 2023, 66, 1688–1700 CrossRef CAS; (g) F. Chen, Z. Cao and C. Zhu, Chem. Commun., 2024, 60, 14912–14923 RSC; (h) F. Chen, Z. Cao and C. Zhu, Angew. Chem., Int. Ed., 2025, 64, e202424667 CrossRef CAS PubMed.
- For selected recent examples: (a) Z. Wu, D. Wang, Y. Liu, L. Huan and C. Zhu, J. Am. Chem. Soc., 2017, 139, 1388–1391 CrossRef CAS PubMed; (b) X. Wu, M. Wang, L. Huan, D. Wang, J. Wang and C. Zhu, Angew. Chem., Int. Ed., 2018, 57, 1640–1644 CrossRef CAS PubMed; (c) J. Yu, Z. Wu and C. Zhu, Angew. Chem., Int. Ed., 2018, 57, 17156–17160 CrossRef CAS PubMed; (d) J. Liu, S. Wu, J. Yu, C. Lu, Z. Wu, X. Wu, X. -S. Xue and C. Zhu, Angew. Chem., Int. Ed., 2020, 59, 8195–8202 CrossRef CAS PubMed; (e) Z.-S. Wang, Y.-B. Chen, H.-W. Zhang, Z. Sun, C.-Y. Zhu and L.-W. Ye, J. Am. Chem. Soc., 2020, 142, 3636–3644 CrossRef CAS PubMed; (f) J. Yu, X. Zhang, X. Wu, T. Liu, Z.-Q. Zhang, J. Wu and C. Zhu, Chem, 2023, 9, 472–482 CrossRef CAS; (g) Z. Cao, Y. Sun, Y. Chen and C. Zhu, Angew. Chem., Int. Ed., 2024, 63, e202408177 CrossRef CAS PubMed; (h) J. Liu, J. Ma, T. Wang, X.-S. Xue and C. Zhu, JACS Au, 2024, 4, 2108–2114 CrossRef CAS PubMed; (i) J. Wang, X. Wu, Z. Cao, X. Zhang, X. Wang, J. Li and C. Zhu, Adv. Sci., 2024, 11, 2309022 CrossRef CAS PubMed; (j) T. Sephton, J. M. Large, L. S. Natrajan, S. Butterworth and M. F. Greaney, Angew. Chem., Int. Ed., 2024, 63, e202407979 CrossRef CAS PubMed; (k) J. Xu, R. H. Li, Y. J. Ma, J. Zhu, C. S. Shen and H. Jiang, Nat. Commun., 2024, 15, 6791 CrossRef CAS PubMed; (l) Y. R. Wang, H. F. Yang, Y. Zheng, M. X. Hu, J. T. Zhu, Z.-P. Bao, Y. Y. Zhao and X.-F. Wu, Nat. Catal., 2024, 7, 1065–1075 CrossRef CAS; (m) E. Derat, G. Masson and A. Claraz, Angew. Chem., Int. Ed., 2024, 63, e202406017 CrossRef CAS PubMed; (n) S.-Y. Wen, J.-J. Chen, Y. Zheng, J.-X. Han and H.-M. Huang, Angew. Chem., Int. Ed., 2025, 64, e202415495 CrossRef CAS PubMed.
- (a) L. Li, Z.-L. Li, F.-L. Wang, Z. Guo, Y.-F. Cheng, N. Wang, X.-W. Dong, C. Fang, J. Liu, C. Hou, B. Tan and X.-Y. Liu, Nat. Commun., 2016, 7, 13852 CrossRef PubMed; (b) N. Wang, Q.-S. Gu, Z.-L. Li, Z. Li, Y.-L. Guo, Z. Guo and X.-Y. Liu, Angew. Chem., Int. Ed., 2018, 57, 14225–14229 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5sc02555a |
| This journal is © The Royal Society of Chemistry 2025 |