Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Reusability studies with Lewis and Brønsted acid catalysts for dehydration of the primary alcohol 1-hexanol under energy-saving conditions†
Adil
Allahverdiyev
and
Harald
Gröger
 *
*
Chair of Industrial Organic Chemistry and Biotechnology, Faculty of Chemistry, Bielefeld University, Universitätsstr. 25, 33615 Bielefeld, Germany. E-mail: harald.groeger@uni-bielefeld.de
First published on 3rd February 2025
Abstract
In general, currently there is an urgent need to switch from fossil-based materials to renewable resources and this is also the case for production of alkenes with a chain length of up to C6 due to their broad application range as bulk chemicals. For instance, such C6 alkenes (hexenes) are accessible from 1-hexanol, for which recently a sustainable technical approach based on CO2 and water as renewable raw materials was reported by Siemens and Evonik Industries, making use of artificial photosynthesis in combination with microbial syngas fermentation. In continuation of our very recent comprehensive study on dehydration of 1-hexanol and other alcohols with a focus on Lewis acids and initial reusability studies over a few reaction cycles, the current study presents a simplified strategy for the reuse of the Lewis acid catalysts Cu(OTf)2 and Hf(OTf)4 and the Brønsted acid TfOH as well as a proof-of-concept for efficient recycling over 20 cycles. Over the course of the study, the catalysts demonstrated an average alkene yield of 71–77%, with no loss of activity. The production costs were calculated on the basis of industry-appropriate prices, with the lowest being 0.34 $ per kg. A successful initial lab scale-up with a 100-fold increase in reaction volume indicates a TRL4 for the developed process and enabled a product formation of 1.3 kg. Thus, these studies underline the technical feasibility of the developed dehydration process using 1-hexanol, for which catalyst recycling represents a key criterion.
Sustainability spotlightAddressing the urgent need for alternative processes for bulk chemicals such as C6-alkenes (hexenes), dehydration of 1-hexanol, which is available from the renewable resources CO2 and water as reported by Siemens and Evonik Industries, represents such an approach. Our study demonstrates an improved process from 1-hexanol to hexenes under energy-saving conditions comprising the reuse of the catalysts Cu(OTf)2, Hf(OTf)4 and TfOH, enabling an efficient recycling over 20 cycles and an average alkene yield of 71–77%. Calculated variable production costs turned out to be low at 0.34 $ per kg. A successful initial lab scale-up gave 1.3 kg product and indicates TRL4. Thus, our studies underline the technical feasibility of the developed dehydration process using 1-hexanol with efficient catalyst recycling. |
1. Introduction
Despite the implementation of sustainable measures and technological advances, the chemical industry continues to face challenges in transitioning from fossil to renewable raw materials.1 It is noteworthy that non-renewable resources2 still account for almost 80% of energy consumption, which is a cause for concern taking into account the release of CO2 from combustion of fossil raw feedstocks into the environment.3 Renewable resources based on biomass or CO2 therefore represent attractive raw materials for the future when it comes to the production of fuels and products for the chemical industry, in particular with application in the high-volume segments such as fuels and plastics.4In today's chemical industry, in terms of volume, alkenes represent the most widely used class of industrial compounds, with ethylene and propylene being the compounds with the largest production volumes and numerous applications in the field of plastics.5 Higher homologues of ethylene and propylene such as hexenes are also of utmost importance, serving as crucial intermediates for the production of a vast variety of chemicals. Hexenes are usually produced by crude oil refining or alternatively by Fischer–Tropsch synthesis,6 which, however, is associated with depletion3 or, in the case of Fischer–Tropsch synthesis, with disadvantages such as the necessity of high temperatures,7 low catalyst stability8 and low catalyst activity.7,9
An alternative approach towards hexenes offering advantages also from the perspective of sustainability has recently been reported by us. Our concept is based on combining an initial Siemens–Evonik process10 (in which carbon dioxide is converted to syngas by artificial photosynthesis using sunlight for water splitting, followed by microbial conversion to 1-hexanol) with a subsequent dehydration of 1-hexanol at low reaction temperature (and, thus, minimised energy demand) to enable the sustainable production of hexenes (Scheme 1). In the future such resulting hexenes might serve as components for aviation fuels originating from renewable resources as by 2040 at least 30% of all fuels used in transportation are to be replaced by renewable energy sources.11
 | ||
| Scheme 1 Overall process concept for producing hexenes from CO2, water and renewable energy such as sunlight and wind in a chemoenzymatic cascade with metal triflate dehydration as a key step. | ||
To date, dehydration of 1-alkanols is far from being well established, although it looks like “standard” textbook chemistry. Typically, reaction conditions are very harsh and involve exposure to very high temperatures. Most examples run at temperatures above 300 °C.12 Only a few examples are known for the dehydration of primary alcohols at relatively mild temperatures. Such examples include the work by Vorholt et al.13 and Repo et al.14 Vorholt et al. performed the dehydration at 190 °C using Brønsted acids, resulting in the formation of ethers and small quantities of alkenes.13 In addition, Repo et al. comprehensively investigated the dehydration of secondary alcohols with Lewis acids.14 They also reported two experiments of dehydration of 1-octanol at 180 °C, achieving yields of 2% and 65% when using Fe(OTf)3 and Hf(OTf)4, respectively.14 In our recent study,15 the initial attempts and proof-of-concepts of Repo et al.14 and Dombroski et al.,16 who studied the dehydration of 1-hexanol using Cu(OTf)2, served as valuable starting points for an in-depth investigation of the dehydration of primary alcohols. The use of Lewis acid-based salts is advantageous as they demonstrate the capacity to exhibit both acidic and basic sites. While (according to our proposed mechanism)15 in the hexene forming step the oxygen is coordinated by the metal ion (thus, facilitating C–O cleavage), the β-proton abstraction is accelerated by coordination to the anion.
However, the single use of catalysts in only one batch reaction, even at a low weight percentage (wt%), may impede industrial utilization, given that the transition metals in question are frequently expensive.17 In addition to economic considerations, there are also regulatory reasons that restrict the use of such catalysts, as regulations stipulate that the residual metal concentration must be below a certain ppm range.18 Hence, it is of vital importance that catalysts are separated from products and reused in order to ensure environmentally friendly19 and economically feasible20 reactions. Additional criteria include high activity, selectivity and stability when utilizing the catalysts in the corresponding process windows.21 To achieve such an objective, catalyst recycling can be facilitated by e.g., heterogenization. While the development of heterogeneous catalysts may be tedious and resource-consuming, numerous researchers have focused on this approach to reduce waste and avoid the use of hazardous reagents.19 However, heterogenization of homogeneous catalysts is typically accompanied by loss of activity and raises leaching concerns.17,18 Furthermore, the formation of soft coke during dehydration and oligomerization represents a significant impediment, as it occupies active sites and thus inhibits reactions. The removal of this undesired material has to be conducted by combustion.22
As an alternative, reusability of catalysts can also be achieved by customizing chemical reactors for synthetic processes. The in situ-removal of products by distillation procedures23 not only shifts the equilibrium to the product side and reduces required energy, but also allows for obtaining full conversion in case of reversible reactions.23 Industrial examples24 of products synthesized by the process of reactive distillation23 include methyl tert-butyl ether (MTBE) which is commonly used as an anti-knock agent25 and as a solvent for chemical synthesis. The estimated annual worldwide production of MTBE is 38 million metric tons.26
The growing interest in dehydration is also reflected in the increasing number of patents in the field of dehydration, with a particular focus on heterogeneous catalysts. For instance, alumina-based catalysts are employed in dehydration processes at temperatures ranging from 250 to 500 °C, as reported by Ziehe et al.27,28 and Koo et al.29 Accordingly, the dehydration or etherification of C4–C20 alcohols28,29 or fatty alcohols27 proceeds with high conversions and selectivities. As an example, Koo et al. reported the dehydration of 1-octanol at 250–500 °C, leading to >60% yield, >50% selectivities and an LHSV of 7 to 56 h−1.29
In this contribution, we report our study on the catalytic potential and recyclability of acid-based catalysts for energy-saving dehydration of primary alcohols at moderate temperatures using 1-hexanol as a substrate. Based on our recent successful process for performing dehydration of primary alcohols at relatively low temperatures with reasonable reaction rates15 and, thus, minimizing the energy requirements associated with this endothermic reaction, we also intensively focused on the reusability of the catalysts in order to make the reaction economically attractive from an industrial perspective. We have also set ourselves the goal of implementing a process fulfilling pilot production scale standards and, thus, technical feasibility.
2. General process concept and set up
In general, very harsh conditions are associated with dehydration of a primary alcohol as the reaction itself is thermodynamically unfavoured. Thus, in order to reduce the needed energy, the equilibrium is shifted towards the desired alkene products by distillative in situ-removal of such products being more volatile than their alcohol substrates. The dehydration can proceed through either ether-formation, which is slightly exothermic,30 and subsequent C–O cleavage or direct alkene formation which is slightly endothermic.31 The two-step route via the ether intermediate is often favoured to form the alkene as the activation energy for the C–O cleavage of the ether is lower compared to that of direct dehydration of the alcohol.32 Thus, in accordance with the literature33 we previously proposed that under mild conditions first the ether is formed, which is subsequently dehydrated.15As the boiling point of the model substrate 1-hexanol is at 157 °C, reactions were first performed at 150 °C for 1.5 hours and then further heated to 180 °C. As we previously reported,15in situ-measurements have demonstrated that the reaction occurs at 150 °C, representing, to the best of our knowledge, the lowest reported reaction temperature for the dehydration of a primary aliphatic alcohol. However, reactions on an elevated 500 mL scale have shown that the reaction proceeds at even lower temperatures (140 °C). The reaction set-up is described in detail in our previous study.15
3. Dehydration using Brønsted acids
We recently reported the dehydration of primary alcohols using Brønsted and Lewis acids, and in this study alkene formation was only observed in the case of Lewis acids.15 Among the 15 different Lewis acids, Hf(OTf)4, Ti(OTf)4 and Cu(OTf)2 turned out to be very suitable at catalyst loadings of 2 and 10 mol%, respectively, with alkene yields exceeding 70%. In contrast, the use of Brønsted acids resulted in the formation of ether, at best. Nevertheless, our findings revealed a correlation between the pKa value and ether formation. In particular, only acids with a pKa value below 2.8 resulted in the formation of the ether. Given that alkene formation had been undetected, we now extended the Brønsted acid scope with strong acids that are commercially attractive and readily accessible (Table 1). We were pleased to find that when reactions were carried out with TfOH, for the first time when using a Brønsted acid in this modest temperature range of 140–160 °C alkene formation was observed. In the presence of 10 mol% of TfOH, 73% yield for the alkenes was obtained (Scheme 2 and Table 1), thus being comparable to the reactions with metal triflates. However, when the loading was reduced to 2 mol%, yield for the alkenes dropped to 8%. In this case, mainly ether formation was found. When investigating the TfOH derivatives TfOMe and Aquivion®, lower alkene yields of 55–62% were obtained. It is noteworthy that, while the di-n-hexyl ether was observed as the predominant by-product when TfOH was used, hexyl methyl ether was also obtained when TfOMe was employed. This result was anticipated due to the fact that TfOMe is primarily utilized as a methylation reagent.34 The reaction pathway for TfOMe can thus be described as follows: 1-hexanol is first methylated and subsequently dehydrated to hexene and methanol (Scheme 3). In the case of Aquivion®, a commercial TfOH-polymer, as expected, comparable results were obtained. A potential drawback of this heterogeneous catalyst Aquivion®, however, might be that both 1-hexanol and 1-hexene as well as their oligomers can become trapped in the pores, which might promote formation of soft coke and, thus, block free acid sites. As bistriflimidic acid (HNTf2) is a comparably strong Brønsted acid, it was anticipated that it would lead to comparable alkene yields. However, less alkene formation with a low yield of 12% was observed. Thus, pKa of the Brønsted acid is not the only criterion for efficient alkene formation.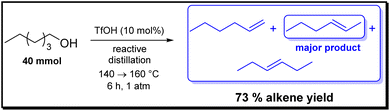 | ||
| Scheme 2 Dehydration of 1-hexanol using Lewis acids at 140–160 °C. The alkenes were formed whereas 2-hexene was the major product. In traces the ether was detected. | ||
| Entry | Catalyst | pKa | Catalyst loading (%) | Reaction time (h) | Alkene yield (%) | Ether yield (%) |
|---|---|---|---|---|---|---|
| a Previously reported.15 b Not available. | ||||||
| 1a | AcOH | 4.8 | 10 | 22 | 0 | 0 |
| 2a | H3PO4 | 2.1 | 10 | 22 | 0 | 0 |
| 3a | TFA | 0.23 | 10 | 22 | 0 | 0 |
| 4a | TsOH | −2.8 | 10 | 22 | 0 | 96 |
| 5a | H2SO4 | −3 | 10 | 22 | 0 | 87 |
| 6 | TfOH | −14 | 10 | 6 | 73 | 5 |
| 7 | TfOH | −14 | 2 | 6 | 8 | 80 |
| 8 | Aquivion® | NAb | 10 | 6 | 55 | 3 |
| 9 | TfOMe | NAb | 10 | 6 | 62 | 10 |
| 10 | HNTf2 | −14 | 10 | 6 | 12 | 81 |
4. Reusability studies of catalysts
Aiming to develop a technically feasible process for production of fuels or commodity chemicals with economically attractive catalysts costs also requires considering catalyst recycling. Furthermore, catalyst recycling strategies should also include simple catalyst separation, thus also contributing to reducing waste formation.Recently, we reported preliminary studies on the reuse of Hf(OTf)4 for 5 cycles and obtained yields of 75% on average, without any decrease in activity.15 Thus, we included this catalyst in the present study for comparison. However, in terms of an economically attractive catalyst, the Lewis acid Cu(OTf)2 and the Brønsted acid TfOH appeared to be far more suitable. Accordingly, the main focus in our extensive recycling study was on exploring these two catalysts, which also give high alkene yields (Fig. 1 and 2). In all of these recycling studies with Hf(OTf)4, Cu(OTf)2 and TfOH, 20 reaction cycles were conducted using these catalysts (Fig. 1 and 2, Scheme 4). In each cycle the reactions were carried out with 40 mmol (5 mL) of 1-hexanol resulting in the dehydration of in total 0.8 mol (100 mL) 1-hexanol, respectively. First 1-hexanol was combined with the catalyst, and then, identical to single cycle reactions, the reaction flask was connected to a distillation bridge. After the end of each reaction cycle, without adding further catalyst, additional 1-hexanol was added to the reaction flask, and then the new reaction cycle was started.
 | ||
| Fig. 1 Alkene yields of the dehydration of 1-hexanol using Cu(OTf)2, Hf(OTf)4 and TfOH over 20 cycles. | ||
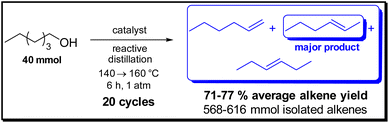 | ||
| Scheme 4 Reusability studies for the dehydration of 1-hexanol using Cu(OTf)2 (10 mol%), Hf(OTf)4 (2 mol%) and TfOH (10 mol%). | ||
Note that each catalyst remained active for over 20 cycles, resulting in the formation of 568–616 mmol (48–52 g) of alkenes (Scheme 4). Thus, it can be expected that the activity of the catalysts will remain high also for many more cycles. Taking into account some deviation of these lab scale recycling studies, which we attribute to preparative reasons, in general the alkene yields for the reactions turned out to be in a similar range over 20 cycles in the presence of the three catalysts, namely Hf(OTf)4, Cu(OTf)2 and TfOH (Fig. 1).
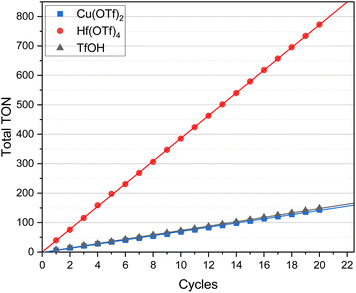
In the next step, the total TON of each catalyst was calculated (Fig. 2). As the alkene yields remained constant in a similar range for each cycle, the total TON consequently increased with each subsequent cycle.
The highest total TON of 772.5 was achieved when Hf(OTf)4 was used at 1/5 of the catalyst loading (2 mol%) compared to the one for Cu(OTf)2 and TfOH (both 10 mol%) (Fig. 2). However, taking into account the low catalyst costs for both Cu(OTf)2 and TfOH in comparison to Hf(OTf)4, the resulting total TONs of 143.2 for Cu(OTf)2 and 148.7 for TfOH are very attractive. Furthermore, it should be taken into account that for the preparation of 1 mol of Hf(OTf)4, 4 mol of TfOH would be needed. Thus, considering only catalyst loading (in mol%) alone would not necessarily give a full picture in the evaluation of the attractiveness of a catalyst.
It should be added that a different recycling method of the metal triflate was previously reported by Cullen et al.,35 exemplified for the recycling of Al(OTf)3, allowing for the completion of three reaction cycles. Catalyst recycling was performed by extraction with aqueous media, followed by removal of water under reduced pressure and at elevated temperature. However, in our recycling process such an extraction step is not applied. As mentioned above, the product is continuously removed, and reuse of the catalyst is ensured by simply adding a fresh substrate to the reactor after each cycle. Thus, a catalyst extraction step can be completely avoided.
In addition, we also studied the mass balance of our overall recycling process. As we obtain roughly 85% crude yield, consisting of 71–77% of alkenes and 8–14% ether or remaining alcohol, still 15% of the starting material is missing, which remains in original or derivatised form in the reaction flask as a crude mixture together with the catalyst. Therefore, the crude mixture was analysed by 1H-NMR, GC/FID and GC/MS. Interestingly, besides C6-compounds, also their di- and oligomers were found therein. The presence of C12 dimers and oligomers in the crude mixture can be attributed to their higher boiling point, thus making their removal via in situ-distillation difficult. Note that these C12 compounds are also of interest as fuels and might be isolated as well.
5. Calculation of costs and sustainability indices
Having demonstrated the recyclability of the catalysts over 20 cycles as a prerequisite for later utilization in a technical process, we became interested in conducting an initial calculation of the associated process expenses being related in particular to the catalyst costs. Thus, prices obtained from various available internet sources were used to calculate the production prices of 1 kg of alkene by first calculating the mmol price and then extrapolating to the amount of one kg of product. In detail, the costs were calculated according to formula (1) given in the ESI.†Although the costs decline with each successive cycle as illustrated in Fig. 6, in general and independent of the type of catalyst, the strongest impact on cost reduction came from the first five to ten cycles. Yet, only up to cycle 10 a substantial shift in costs can be observed, as the course of the costs related to subsequent cycles exhibit asymptotic behavior. The alkene yields are similar for all applied catalysts, indicating that the primary factor influencing the differences in terms of costs among the different catalysts comes from the price of the catalyst itself. The lowest cost was identified with TfOH, being available at a very low price of only 1.21 $ per kg, while the cost for Hf(OTf)4 is very high with 604.84 $ per kg. In contrast, the hexene production using Cu(OTf)2 is only slightly more expensive than the one with TfOH, which can be attributed to the low cost of also Cu(OTf)2 with a price of 3.42 $ per kg (Table 2).
| Entry | Catalysts | Costs per kg ($) | Catalyst loading per cycle | Ø alkene yield (%) | Costs of alkene per kg considering substrate ($) | Costs of alkene per kg without substrate ($) | E-Factor | PMI-value |
|---|---|---|---|---|---|---|---|---|
| 1 | Cu(OTf)2 | 3.42 | 10 mol% | 71 | 2.820 | 0.084 | 0.73 | 1.73 |
| 2 | Hf(OTf)4 | 604.84 | 2 mol% | 77 | 8.269 | 5.735 | 0.58 | 1.58 |
| 3 | TfOH | 1.21 | 10 mol% | 74 | 2.665 | 0.032 | 0.65 | 1.64 |
This impact of the type of catalyst on the cost contribution of the catalyst costs to the total costs is also visible from Fig. 3 when comparing the graphs of the costs calculated without considering the substrate costs (blue coloured graph) with those when 1-hexanol was taken into consideration (grey coloured graph).
 | ||
| Fig. 3 Costs per cycle including the price of the substrate (grey) and without it (blue). As catalyst systems, the Lewis acids Cu(OTf)2 and Hf(OTf)4 and the Brønsted acid TfOH were used. The data points for the alkene yields are shown in red colour. Costs were calculated per mmol (and then extrapolated for 1 kg of hexene, see Table 2). | ||
While for Hf(OTf)4 nearly the same graphs are obtained (indicating that catalyst costs contribute most to the total costs), in case of TfOH there is a strong difference showing that the catalyst costs here only represent a minor cost factor while the main costs are related to the substrate costs. In general, catalyst costs per kg of product are very attractive in the case of Cu(OTf)2 and TfOH, reaching 0.084 $ per kg for Cu(OTf)2 and 0.032 $ per kg for TfOH after 20 cycles. Thus, from an economic perspective, TfOH is most attractive among the studied catalysts, although 10 mol% is needed compared to 2 mol% of Hf(OTf)4. However, in the latter case, hafnium represents an expensive metal component and it also has to be considered that for one equivalent of Hf(OTf)4 four equivalents of TfOH are needed, which would then correspond to 8 mol% of TfOH as the original raw material. However, it is important to note that this initial and very preliminary cost calculation only takes into account variable costs. Accordingly, no operation costs were implied in this calculation. The operational costs can be calculated using the ESI† (3)–(6) formulae, which results in 0.27 $ per cycle. However, it should be noted that the operational costs were calculated based on a lab scale process with a magnetic stirrer, and therefore the costs on an industrial scale are likely to be significantly lower.
Subsequently, we also determined sustainability indices to evaluate not only process costs but also sustainability metrics. Thus, the E-factors were calculated showing the lowest E-factor of 0.58 for Hf(OTf)4, followed by 0.65 for TfOH and 0.73 for Cu(OTf), respectively (Table 2 and Fig. 4). It is noteworthy that all of these E-factors are below 1 and, thus, in an excellent range. The implementation of the process under neat conditions, with the complete avoidance of any solvent utilization, both at the stage of the reaction and at the stage of the work-up, is a key feature leading to such highly attractive E-factors (Fig. 4).
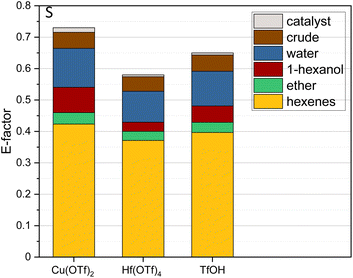 | ||
| Fig. 4 E-Factors for the dehydration of 1-hexanol over 20 cycles for Cu(OTf)2, Hf(OTf)4 and TfOH. The impact of each substance on the E-factor is shown as a percentage of the E-factor. | ||
6. Evaluation of a fed-batch strategy
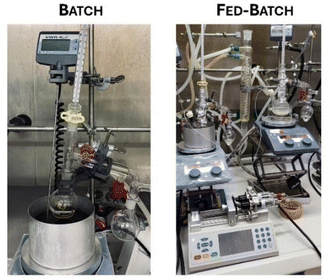
All previous reactions were carried out under batch conditions. However, continuous substrate feeding (fed-batch) can also be performed as an alternative, and our studies with this process set-up will be discussed in this chapter.Obviously, both process options have advantages and disadvantages, which will be briefly discussed first. With fed-batch, for example, the ratio of catalyst to substrate amounts can be kept high. However, the flow rate needs to be adjusted, as too low flow rates might lead to a higher degree of catalyst deactivation by, e.g., oxidation. Hence, initial experiments with several flow rates were conducted in order to explore the impact of this reaction parameter. In contrast, from a practical perspective batch reactions often represent the simplest way to perform an experiment. Nevertheless, standard batch reactions are not always the best choice, as the catalyst to substrate ratio during a reaction is not as easily adjustable as in fed-batch and space-time yields can be less. Consequently, the reactions were conducted at varying flow rates under fed-batch conditions and compared with batch conditions over five cycles. The Lewis and Brønsted acids Hf(OTf)4 and TfOH were selected as catalysts for this study. Batch conditions were identical to those in previous reactions. For fed-batch conditions, 40 mmol of 1-hexanol was initially placed with the catalyst in the reaction vessel and heated to 150 °C for 1.5 hours and then to 180 °C while additional substrate was simultaneously added (Scheme 5 and Fig. 5).
 | ||
| Scheme 5 Reusability studies for the dehydration of 1-hexanol using Cu(OTf)2 (10 mol%), Hf(OTf)4 (2 mol%) and TfOH (10 mol%). | ||
It is noteworthy that while the yield of alkene decreased considerably in the fed-batch process involving Hf(OTf)4, it remained relatively stable in the TfOH system. In contrast, an increase in the flow rate resulted in elevated alkene yields when Hf(OTf)4 was employed. This phenomenon might be attributed to degradation of the catalyst in the absence of a liquid phase. When using TfOH as a catalyst, elevated flow rates did not affect the alkene yield significantly (Fig. 6). The total TON behaved in a similar manner for both Hf(OTf)4 and TfOH, where it increased with higher flow rates for Hf(OTf)4 while it decreased slightly for TfOH (Fig. 7).
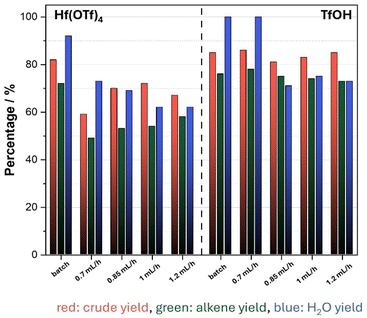 | ||
| Fig. 6 Yields of the dehydration of 1-hexanol using Hf(OTf)4 and TfOH at different flow rates and under batch conditions. | ||
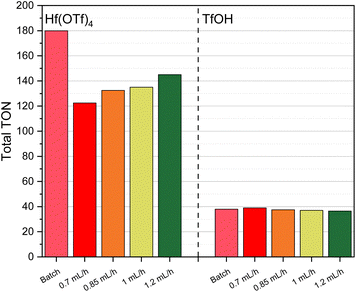 | ||
| Fig. 7 Total TON of the dehydration of 1-hexanol using Hf(OTf)4 and TfOH at different flow rates and under batch conditions. | ||
When calculating the productivity, for Hf(OTf)4 and TfOH an increase can be observed with higher flow rates, as the reaction times reduce with higher flow rates. It is noteworthy that the productivity of TfOH exhibits an increase up to 8.11 mmol h−1 when the highest flow rate is employed (Table 3). A comparison of the highest productivity of Hf(OTf)4 with the one of TfOH reveals a reduction from 8.11 mmol h−1 in case of TfOH to 6.44 mmol h−1 when using Hf(OTf)4, which can be attributed to the lower conversion of Hf(OTf)4.
| Entry | Flow rate (mL h−1) | Time (h) | Productivity (mmol h−1) | |
|---|---|---|---|---|
| Hf(OTf)4 | TfOH | |||
| 1 | Batch | 30 | 4.80 | 5.06 |
| 2 | 0.7 | 30 | 3.27 | 5.20 |
| 3 | 0.85 | 25 | 4.24 | 6.00 |
| 4 | 1 | 21.5 | 5.40 | 6.88 |
| 5 | 1.2 | 18 | 6.44 | 8.11 |
7. Catalyst re-use at an elevated lab scale & preparation of alkenes on a kg scale
Given that high alkene yields were achieved with TfOH as the most economical catalyst leading to the lowest calculated production costs, we selected TfOH for a scale-up to an elevated lab scale. In detail, the process was carried out at a 4 mol scale, which corresponds to a 100-fold increase of the reaction setup (Scheme 6, Fig. 8 and ESI†). The other reaction conditions remained similar to those of the 5 mL scale experiments, with only a change in the oil bath temperature as pre-experiments showed that a 10 °C increase was needed to obtain identical temperature of the reaction mixtures. As we previously reported,15 the temperature range inside the reaction vessel ranged from 140–160 °C when the oil bath was heated to 150 °C, and the dehydration of primary alcohols then already takes place at 150 °C. | ||
| Scheme 6 100-fold scale up of the dehydration of 1-hexanol using TfOH for 5 cycles in batch. Reaction temperatures of the oil bath were 150 → 180 °C while inside temperatures were 130–160 °C. | ||
 | ||
| Fig. 8 Alkene and crude yields of the 100-fold scale up dehydration of 1-hexanol resulting in the production of 1.3 kg alkene. | ||
We were pleased to find that this scale-up for five cycles turned out to proceed very efficiently with alkene yields being in the same range as the ones in the small scale reactions (40 mmol) (Fig. 8). Furthermore, we observed dehydration at a temperature of 140 °C, which, to the best of our knowledge, is the lowest reaction temperature reported so far for chemocatalytic dehydration of primary alcohols. In detail, within the 5 cycles of this elevated lab scale process, we performed the dehydration of in total 2.5 L of 1-hexanol as a primary alcohol component, which corresponds to an amount of 2 kg, and obtained very high dehydration yields, resulting in an overall amount of 1.3 kg of hexenes as the desired alkene product (Fig. 8 and ESI†).
8. Conclusion
In conclusion, a comprehensive feasibility study on the re-use of Brønsted and Lewis acids in the process of dehydration of primary alcohols, exemplified for 1-hexanol, has been conducted, which turned out to be an efficient method for the preparation of the resulting alkene products under mild reaction conditions. As such catalysts, Hf(OTf)4, Cu(OTf)2 and TfOH were used. A deep recycling study consisting of 20 cycles and making use of an in situ-product removal through distillation of the hexene fraction as most volatile components showed no loss of activity independent of the type of the three studied chemocatalysts. Since during both, the process and work-up, no solvent is needed, highly attractive E-factors as low as 0.58–0.73 were obtained. Also different process strategies such as batch versus fed-batch for the supply of the 1-hexanol substrate were compared.This study further revealed TfOH as the most attractive catalyst in terms of economy and sustainability. It is also noteworthy that by means of this type of Brønsted catalyst, with only 140 °C the lowest reaction temperature ever reported for such a dehydration of 1-hexanol as a primary alcohol was achieved. TfOH is also an excellent example of an economically highly attractive catalyst, leading to calculated catalyst costs of only 0.032 $ per kg of produced alkenes. In addition, sustainability metrics have been determined. The dehydration process with recycling of TfOH over 5 cycles has also been demonstrated on an elevated lab scale, resulting in the production of an overall amount of 1.3 kg of alkenes in these experiments.
Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
The authors acknowledge financial support from the European Union (EU) within the Horizon 2020 research and innovation programme for the funded project “4AirCRAFT” (grant agreement 101022633). The authors also thank Daniel Butenko, Vivian Baumert and Robin Genz for technical assistance.Notes and references
- T. Marzi, V. Knappertsbusch, A. Grevé, G. Deerberg, C. Doetsch and E. Weidner, Chem. Ing. Tech., 2018, 90, 1374–1383 CrossRef CAS.
- (a) D. X. Martínez-Vargas, L. Sandoval-Rangel, O. Campuzano-Calderon, M. Romero-Flores, F. J. Lozano, K. D. P. Nigam, A. Mendoza and A. Montesinos-Castellanos, Ind. Eng. Chem. Res., 2019, 58, 15872–15901 CrossRef; (b) S. H. Mohr, J. Wang, G. Ellem, J. Ward and D. Giurco, Fuel, 2015, 141, 120–135 CrossRef CAS.
- G. Nicoletti, N. Arcuri, G. Nicoletti and R. Bruno, Energy Convers. Manage., 2015, 89, 205–213 CrossRef.
- S. Van de Vyver, J. Geboers, P. A. Jacobs and B. F. Sels, ChemCatChem, 2011, 3, 82–94 CrossRef CAS.
- K. Weissermel and H.-J. Arpe, Industrial Organic Chemistry, Wiley-VCH, Weinheim, 2003, 4th edn Search PubMed.
- (a) B. Kamm, Angew. Chem., Int. Ed., 2007, 46, 5056–5058 CrossRef CAS PubMed; (b) E. W. Kuipers, I. H. Vinkenburg and H. Oosterbeek, J. Catal., 1995, 152, 137–146 CrossRef CAS.
- Q. Zhang, J. Kang and Y. Wang, ChemCatChem, 2010, 2, 1030–1058 CrossRef CAS.
- E. de Smit and B. M. Weckhuysen, Chem. Soc. Rev., 2008, 37, 2758–2781 RSC.
- Y. Chen, J. Wei, M. S. Duyar, V. V. Ordomsky, A. Y. Khodakov and J. Liu, Chem. Soc. Rev., 2021, 50, 2337–2366 RSC.
- T. Haas, R. Krause, R. Weber, M. Demler and G. Schmid, Nat. Catal., 2018, 1, 32–39 CrossRef CAS.
- R. W. R. Zwart, H. Boerrigter and A. van der Drift, Energy Fuels, 2006, 20, 2192–2197 CrossRef CAS.
- W. Brandenberg and A. Galat, J. Am. Chem. Soc., 1950, 72, 3275–3276 CrossRef CAS.
- J. T. Vossen, A. J. Vorholt and W. Leitner, ACS Sustainable Chem. Eng., 2022, 10, 5922–5931 CrossRef CAS.
- J. Keskiväli, A. Parviainen, K. Lagerblom and T. Repo, RSC Adv., 2018, 8, 15111–15118 RSC.
- A. Allahverdiyev, J. Yang and H. Gröger, Green Chem., 2024, 26, 7869–7878 RSC.
- K. Laali, R. J. Gerzina, C. M. Flajnik, C. M. Geric and A. M. Dombroski, Helv. Chim. Acta, 1987, 70, 607–611 CrossRef CAS.
- S. Hübner, J. G. de Vries and V. Farina, Adv. Synth. Catal., 2016, 358, 3–25 CrossRef.
- I. Vural Gürsel, T. Noël, Q. Wang and V. Hessel, Green Chem., 2015, 17, 2012–2026 RSC.
- Á. Molnár and A. Papp, Coord. Chem. Rev., 2017, 349, 1–65 CrossRef.
- C. Descorme, P. Gallezot, C. Geantet and C. George, ChemCatChem, 2012, 4, 1897–1906 CrossRef CAS.
- C. V. Satyanarayana, D. Srikant and H. R. Gurav, in Catalyst Deactivation and Regeneration, ed. C. V. Satyanarayana, D. Srikant and H. R. Gurav, Elsevier, 2016, pp. 187–219 Search PubMed.
- (a) T. Cordero-Lanzac, A. Ateka, P. Pérez-Uriarte, P. Castaño, A. T. Aguayo and J. Bilbao, Ind. Eng. Chem. Res., 2018, 57, 13689–13702 CrossRef CAS; (b) T. Cordero-Lanzac, A. T. Aguayo, A. G. Gayubo and J. Bilbao, Chem. Eng. J., 2021, 405, 126448 CrossRef CAS; (c) G. Ren, G. Li, A. Wang, Y. Cong and N. Li, Sustainable Energy Fuels, 2023, 7, 2080–2086 RSC.
- J. Stichlmair and T. Frey, Chem. Eng. Technol., 1999, 22, 95–103 CrossRef CAS.
- K. Sundmacher and A. Kienle, Reactive Distillation. Status and Future Directions, Wiley, Weinheim, 2002 Search PubMed.
- J. Flato and U. Hoffmann, Chem. Eng. Technol., 1992, 15, 193–201 CrossRef CAS.
- Annul Worldwide Production MTBE, 2023, https://www.statista.com/statistics/1067431/mtbe-production-capacity-globally/, accessed 01, 2023 Search PubMed.
- H. Ziehe, T. Gross and A. Weitze, DE102008005721C5, 2009.
- H. Ziehe, J. Schimanski, A. Brasch and E.-O. Tönsen, WO2004078336A2, 2004.
- K. Y. Koo, U. Jung, Y. Kim, H. B. Im, D. Chun, M. H. Youn, H. Jeong, G. B. Rhim, J. Park and D. Lee, US20220032272A1, 2022.
- (a) D. M. Newitt and G. Semerano, Proc. R. Soc. London, Ser. A, 1936, 348–358 CAS; (b) Dimethyl Ether, ed. A. C. Dimian, C. S. Bildea and A. A. Kiss, Elsevier, 2019 Search PubMed.
- (a) T. L. Lohr, Z. Li and T. J. Marks, Acc. Chem. Res., 2016, 49, 824–834 CrossRef CAS PubMed; (b) C. K. Narula, Z. Li, E. M. Casbeer, R. A. Geiger, M. Moses-Debusk, M. Keller, M. V. Buchanan and B. H. Davison, Sci. Rep., 2015, 5, 16039 CrossRef CAS PubMed.
- Z. Li, R. S. Assary, A. C. Atesin, L. A. Curtiss and T. J. Marks, J. Am. Chem. Soc., 2014, 136, 104–107 CrossRef CAS PubMed.
- T. K. Phung and G. Busca, Chem. Eng. J., 2015, 272, 92–101 CrossRef CAS.
- (a) Y. Chen, Chem.–Eur. J., 2019, 25, 3405–3439 CrossRef CAS PubMed; (b) D. A. Evans, A. M. Ratz, B. E. Huff and G. S. Sheppard, Tetrahedron Lett., 1994, 35, 7171–7172 CrossRef CAS; (c) D. J. Knobloch, D. Benito-Garagorri, W. H. Bernskoetter, I. Keresztes, E. Lobkovsky, H. Toomey and P. J. Chirik, J. Am. Chem. Soc., 2009, 131, 14903–14912 CrossRef CAS PubMed.
- D. B. G. Williams and A. Cullen, J. Org. Chem., 2009, 74, 9509–9512 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4su00642a |
| This journal is © The Royal Society of Chemistry 2025 |