Toward enhanced pyro-catalysis performance: mechanisms, strategies and challenges
Xiaoli
Xu
ab,
Wanwan
Cheng
a,
Huan
Zhai
a,
Ying
Wang
a,
Lingbo
Xiao
 *c,
Jiahui
Hou
a,
Jianyang
Kong
a,
Laishun
Qin
*c,
Jiahui
Hou
a,
Jianyang
Kong
a,
Laishun
Qin
 a,
Yanmin
Jia
d,
Yan
Zhang
a,
Yanmin
Jia
d,
Yan
Zhang
 e,
Shun
Li
e,
Shun
Li
 f and
Da
Chen
a
f and
Da
Chen
a
aCollege of Materials and Chemistry, China Jiliang University, Hangzhou, 310018, China
bNational-Local Joint Engineering Research Center of Heavy Metals Pollutants Control and Resource Utilization, Nanchang Hangkong University, Nanchang, 330063, China
cDepartment of Applied Physics, Zhejiang University of Science and Technology, Hangzhou, 310023, China. E-mail: lbxiao@zust.edu.cn
dSchool of Physics and Information Technology, Shaanxi Normal University, Xi'an, 710121, China
eState Key Laboratory of Powder Metallurgy, Central South University, Changsha, 410083, China
fInstitute of Quantum and Sustainable Technology (IQST), School of Chemistry and Chemical Engineering, Jiangsu University, Zhenjiang, Jiangsu 212013, China
First published on 25th March 2025
Abstract
Pyro-catalysis, which can be initiated by minute temperature fluctuations in the environment, has garnered significant attention owing to its potential applications in sterilization, tumor therapy, pollutant decomposition, and hydrogen production through water splitting. The development of pyro-catalysis is beneficial for recycling and utilizing waste energy from daily life and industrial production. Although pyro-catalysis has shown promising prospects in the fields of environmental management and biomedical applications, its efficiency still needs further enhancements in terms of their thermal cycling mode, temperature fluctuation rate, and carrier separation efficiency. Reviewing the recent progress and prospects of pyro-catalysis is beneficial for further advancement in this area. This article offers a comprehensive review of the catalytic mechanisms, recent advancements, and strategies for optimizing the pyro-catalytic performance, and aims to provide an important reference for the design and application of pyro-catalytic materials while providing new insights for related research areas.
1. Introduction
With the continuous development of science and technology, the originally limited fossil energy resources continue to be consumed, creating the problems of energy depletion and environmental pollution.1–4 Low-grade heat (generally below 100 °C) energy is a common but easily overlooked source of energy in daily life, which mainly includes the diurnal temperature variation and waste heat dissipated during industrial production processes, and this energy source is difficult to be utilized effectively.5 According to statistics, nearly 68% of the primary energy is wasted in the form of low-grade heat.6 Pyroelectric materials can potentially utilize this energy as they can generate electricity by sensing minute temperature changes.Although the pyroelectric effect of tourmaline was discovered in 300 BC, its theoretical research began only in the early 20th century with the development of modern physics. In the 1960s, laser infrared technology advanced pyroelectric research, leading to the development of various pyroelectric monocrystals, ceramic and thin-film materials and detectors, infrared sensors and other new pyroelectric parts that are now widely used in military, aerospace, aviation and other fields.7 Over the past few decades, microscopic pyroelectric materials have been studied for new applications, including surface chemical catalytic reactions. Pyroelectrics are a type of non-centrosymmetric polar crystals with an inherent coupling between their electric polarization and temperature, which manifests a change in surface charge density with temperature.8 In equilibrium, the bound surface charges of polar pyroelectric materials are shielded by external or internal charges. When the temperature changes, there is an imbalance between the polarized charge and the shielding charge, resulting in a pyroelectric potential. Pyro-catalysis, which converts thermal energy into chemical energy, is a new catalytic technology. When exposed to external temperature changes, its spontaneous polarization intensity changes and charges are released, thus leading to oxidation–reduction (redox) reactions such as dye decomposition,9–11 water splitting for hydrogen generation,12–14 CO2 reduction,15–17 sterilization,18–20 and tumor treatment.21–23
Thus far, a number of outstanding reviews have been dedicated to the use of pyroelectric materials. Lang et al. introduced some lesser-known applications of pyroelectric materials in the fields of tactile devices, energy conversion, porous polymers, property measurements, pyroelectric infrared sensors, impact sensors, and space science.24 Li et al. delved into the basics of combining ferro-, piezo-, and pyroelectric effects for catalysis, highlighting the importance of internal electric fields for charge separation.25 Zhang et al. reviewed pyroelectric-electrochemical coupling in ferroelectrics for thermal energy harvesting and explored its potential in waste heat recovery.26 Huang et al. introduced key materials and effects of pyro-catalysis research, highlighting applications such as disinfection and water splitting.27 He et al. reviewed the progress in lead-free pyroelectric materials including single crystals, ceramics, inorganic films, polymers and composites.28 Wang et al. summarized the advancements in energy and environment driven by stress and temperature changes based on piezoelectric and pyroelectric effects.29 Liu et al. focused on the research progress of pyro-catalysis of perovskite oxides, introducing the structure and synthesis methods of these oxides.30 Although pyro-catalytic technology is advancing rapidly, its efficiency is still in need of enhancement, attributed to factors such as the thermal cycling mode, the rate of temperature fluctuation and carrier separation efficiency.
This article offers a comprehensive review of the pyro-catalytic mechanism, its applications, and enhancement strategies, emphasizing the importance of temperature conduction methods on the pyro-catalytic reactions. The applications of pyro-catalysis in reactive oxygen species (ROS) generation (sterilization, tumor therapy and dye decomposition) and water splitting are summarized. Strategies to improve the performance of pyro-catalysis including the heat transfer optimization, introduction of external fields such as electric, light and stress fields, as well as defect engineering, metal loading, heterojunction engineering, surface modification, and morphology control are discussed (Fig. 1). This review aims to provide important reference for the design and application of pyro-catalytic materials, while also providing fresh insights for related research endeavors.
2. Pyro-catalytic mechanism
The pyroelectric effect refers to a phenomenon that some crystals generate electric charges on their surface when the temperature (T) changes due to changes in spontaneous polarization (Ps, representing the average electric dipole moment per unit volume) within the crystal. The pyroelectric effect is a reversible physical phenomenon, that is, when the temperature increases or decreases, the surface of the crystal will produce positive and negative charges. Applications for converting temperature changes into electrical signals based on pyroelectric effects can be used in the field of sensors and detection, such as infrared sensors, temperature monitoring, pyroelectric infrared imaging and energy harvester.31–35 The pyroelectric effect can also be applied in the field of catalysis.The pyro-catalytic reaction is the product coupling of pyroelectric effect and electrochemical redox reaction. Pyro-catalysis is a process that leverages the charges produced due to pyroelectric effects to directly engage in a sequence of redox reactions.36 Under typical conditions, when the temperature of pyroelectric materials remains constant, they exhibit spontaneous polarization. However, this inherent polarization does not result in the manifestation of external electrical properties.37,38 The reason for this is that the internal electric field generated by the spontaneous polarization is neutralized by external screening charges, thereby preventing the material from displaying electrical characteristics externally (Fig. 2a).39 Although these polarized charges are shielded by compensating charges in equilibrium, transient net charges can trigger redox reactions. When the temperature of pyroelectric materials increases, the diffusion of the electric dipoles oscillating around their respective axes increases, and the spontaneous polarization, Ps, decreases.40 As a result, the equilibrium between the built-in electric field and the external screening charge is broken, resulting in the generation of free positive and negative charges at both ends of the polarization direction. The production of these free charges subsequently fosters a series of redox reactions until a new balance is established (Fig. 2b and c). When the temperature of the pyroelectric material decreases, the electric dipole oscillates in a smaller expansion angle range due to the lower thermal activity, so the spontaneous polarization, Ps, will be enhanced, and pyroelectrics must capture free charges from their surroundings to balance the polarization charge caused by the increase in polarization, resulting in the reoccurrence of redox reactions (Fig. 2d).
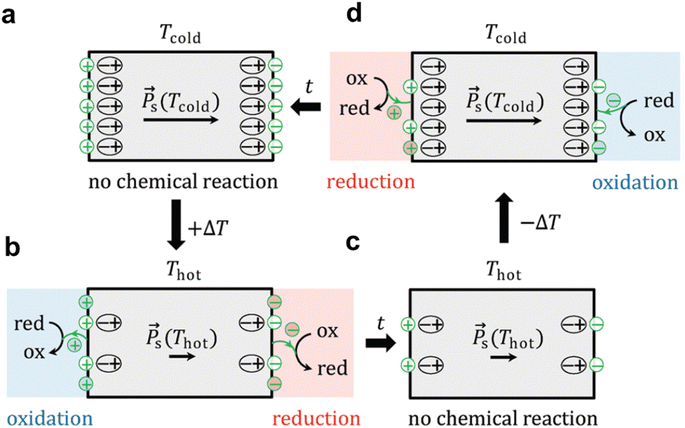 | ||
| Fig. 2 (a–d) Schematic of the pyro-catalytic reaction mechanism.39 Copyright 2020, RSC. | ||
Since the pyroelectric coefficient p is defined as the change rate of spontaneous polarization Ps with the temperature T,26,32
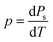 | (1) |
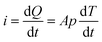 | (2) |
The factors affecting the pyro-catalytic efficiency are as follows: (1) the higher the pyroelectric coefficient, p, of the material, the more the charge generated, which is conducive to the improvement of the pyro-catalytic efficiency. (2) Pyro-catalysis depends on the charge generated by the temperature change to drive the reaction. If the temperature change rate is too slow, the amount of charge generated will be small and the catalytic efficiency will be reduced. (3) In addition, the shape and size of the material will affect its specific surface area and active site distribution, thus affecting the catalytic efficiency.
Pyroelectric effects usually occur in non-centrosymmetric crystals. Pyroelectric materials are a subclass of piezoelectric materials. There are 10 point groups of pyroelectric materials, which are 1, 2, m, mm2, 4, 4 mm, 3, 3 m, 6 and 6 mm. Typical pyroelectric materials include: triglycine sulfide (TGS), GaN, AlN, CdS, ZnO, BaTiO3, lead zirconate titanate (PZT), lead magnesium niobate–lead titanate, (PMN–PT), LiTaO3, LiNbO3, PbTiO3, PbZrO3, Bi-based layered ferroelectric materials, and polyvinylidene difluoride (PVDF).27 All ferroelectric materials have pyroelectric properties. In general, ferroelectric materials have larger pyroelectric coefficients than non-ferroelectric materials.27 For ferroelectric materials, when the temperature exceeds the Curie temperature (TC), it undergoes a phase transition in which both spontaneous polarization and pyroelectric behavior disappear. The p of the ferroelectric material reaches its peak near the Curie temperature. This occurs because when the temperature surpasses the Curie temperature, the material undergoes a phase transition from the ferroelectric to the paraelectric phase. During this transition, the rate of change of its spontaneous polarization, Ps, attains its maximum, leading to the greatest release of surface charge.31,41 A. Kakekhani and S. Ismail-Beigi used first-principles theoretical simulations to calculate the catalytic splitting of water via the pyroelectric effect of ferroelectrics.42 Taking PbTiO3 as an example, by cyclically adjusting the temperature near the Curie temperature to control the ferroelectric polarization, it is possible to catalyze the splitting of H2O into O2 and H2. The calculation indicates the control of ferroelectric polarization through the pyroelectric effect. By periodically adjusting the temperature to push the system away from equilibrium, and then using the surface's tendency to reach its thermodynamic ground state, the desired portion of the water splitting reaction is driven (Fig. 3a).
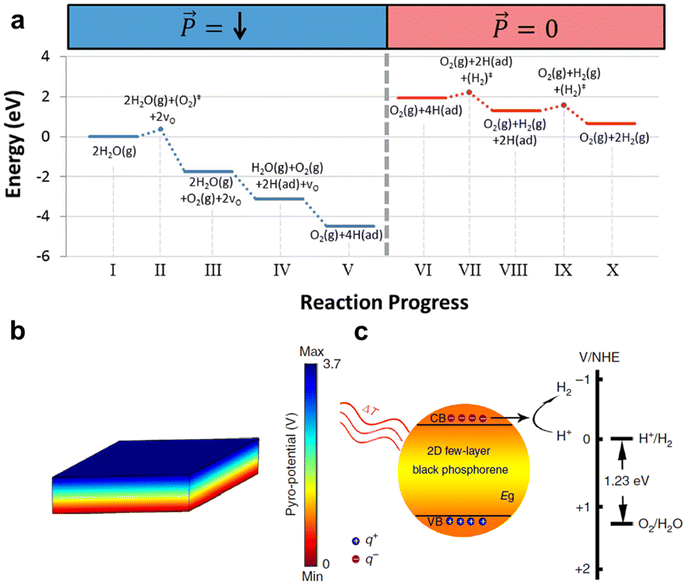 | ||
| Fig. 3 (a) Energy landscape for the cycle that includes meta-stable states (solid line segments) and transition states (circles connected by dotted lines).42 Copyright 2016, RSC. (b) COMSOL simulation of pyro-potential in black phosphorus and (c) energy diagram for hydrogen production.12 Copyright 2018, the Author(s). | ||
Pyroelectric voltage (V) can be expressed as follows:31
 | (3) |
3. Pyro-catalytic applications
3.1 ROS generation
In photocatalysis, photo-induced electron–hole pairs can form ROS (holes react with hydroxyl ions in water to form hydroxyl radicals, h+ + OH− → ·OH; electrons react with dissolved oxygen in water to form superoxide free radicals, e− + O2 → ·O2−). Unlike photocatalytic carrier generation, pyro-catalysis generates surface charges through temperature variation, and then produces ROS in a similar way to photocatalysis. In 2015, Benke et al. polarized commercial BaTiO3 nanopowders to enhance the ferroelectric domain orientation. Pd nanoparticles were added for charge transfer in pyro-catalysis.43 In aqueous solutions, the ferroelectric polarization is shielded by surface dissolved ions or dissociated water molecules. Temperature changes induce pyroelectric effects, unbalancing polarization and shielding charges, creating surface charge (σ = ΔPs). This surface charge can be used to drive electrochemical reactions between physical adsorption molecules to produce ROS (Fig. 4a). The generation of ·OH radicals was detected by the electron spin resonance (ESR) spectra of the radical trapping agent (BMPO: 5-tert-butoxycarbonyl-5-methyl-1-pyrroline N-oxide) (Fig. 4b) and the fluorescence spectra of 7-hydroxycoumarin (Fig. 4c), confirming the possibility of pyro-catalytic ROS generation. As highly reactive oxygen species, ROS can be applied in sterilization, tumor therapy and dye decomposition due to the high oxidative potential. | ||
| Fig. 4 (a) Schematic of pyro-catalytic ROS generation. (b) ESR of BaTiO3 solution. (c) Fluorescence spectra of 7-hydroxycoumarin.43 Copyright 2015, ACS. | ||
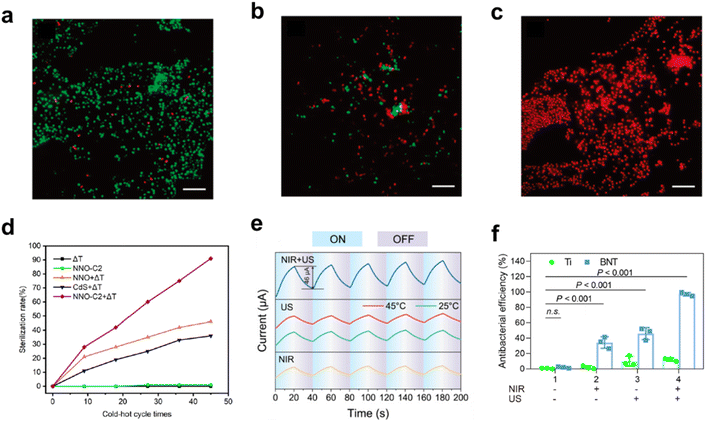 | ||
| Fig. 5 Stained fluorescence microscopic images of E. coli (a) not subjected to thermal treatment, (b) after thermal cycling for 2 h in the presence of LiTaO3 (5–10 μm) and (c) after thermal cycling for 1 h in the presence of LiTaO3 (<5 μm).20 Copyright 2012, ACS. (d) Sterilization rate of Salmonella under different conditions.19 Copyright 2022, Elsevier. (e) Response currents under different conditions. (f) Antibacterial effect of the materials on Staphylococcus aureus under different conditions.18 Copyright 2023, Elsevier. | ||
To enhance pyro-catalysis efficiency, Min et al. developed a NaNbO3/CdS heterojunction to suppress carrier recombination, boosting its pyro-catalytic antibacterial action against Salmonella.19 The NaNbO3/CdS composite achieves a 90% antibacterial rate after 45 thermal cycles between 17 and 37 °C, outperforming individual NaNbO3 and CdS (Fig. 5d). Similarly, Hu et al. utilized BiFeO3@CuBi2O4 heterostructures to markedly augment pyro-catalytic activity, with the BiFeO3@5%CuBi2O4 composite achieving 96.2% and 96.8% sterilization rates against Salmonella and Staphylococcus aureus, respectively.47 Ding et al. synthesized BaTiO3 nanotubes via anodic oxidation and hydrothermal methods, applying near-infrared (NIR) irradiation and ultrasonic therapy for bacterial eradication.18 The combo therapy significantly increased current beyond individual treatments and their theoretical sum (Fig. 5e). The synergistic pyroelectric/piezoelectric effect enhances charge carrier induction and ROS generation, achieving a 99.8% bacterial mortality rate (Fig. 5f). The use of heterojunctions and introducing external fields for pyro-catalytic enhancement will be discussed further.
 | ||
| Fig. 6 (a) ESR of SnSe-PVP solution. Confocal maps of the ablation performances of 4T1 cells in the (b) control group and (c) SnSe-PVP nanorods after laser application.48 Copyright 2018, RSC. (d) Schematic of application in pyro-catalytic antitumor therapy. (e) Pyroelectric voltage and current.49 Copyright 2022, the Author(s). (f) Schematic of the synergistic treatment of tumor via photothermal- and pyro-catalysis.22 Copyright 2023, Wiley. | ||
Combining photothermal and pyro-catalytic therapies offers synergistic tumor suppression. Zhang et al. created BaTiO3@Nb2C-PEG heterojunctions, modified with biocompatible polyethylene glycol (PEG) for biocompatibility, using 2D niobium carbide (Nb2C) for photothermal effect and barium titanate (BaTiO3) for pyro-catalytic cancer therapy (Fig. 6f).22 Under laser irradiation in the NIL-II region, Nb2C converts light into heat, and then transfers heat to BaTiO3, which triggers BaTiO3 to produce toxic ROS through pyro-catalysis, realizing the synergistic effect of photothermal and pyro-catalytic composite therapy. In vitro and in vivo studies on tumor-bearing mice show significant tumor cell ablation, with a 94.9% tumor inhibition rate. Similarly, Chang et al. designed a plasma-gold@pyroelectric barium titanate (Au@BaTiO3) core–shell nanostructure for hypoxic tumor treatment.50 Wang et al. used Bi13S18I2 as a pyro-catalyst to generate ROS, enhancing photothermal therapy by targeting heat shock proteins and tumor cell thermal tolerance.21
| ·OH/·O2− + dye → decomposition | (4) |
Wu et al. used pyroelectric BiFeO3 nanoparticles (Curie temperature TC is ∼830 °C, pyroelectric coefficient is ∼90 μC m−2 K−1) for pyro-catalytic dye decomposition via ROS.52 The TEM image shows ∼100 nm polygonal BiFeO3 nanoparticles (Fig. 7a). The ultraviolet-visible (UV-vis) spectra of Rhodamine B (RhB) solution show that an absorption peak at ∼554 nm fades by ∼85 thermal cycles (27–38 °C), signifying 99% decomposition efficiency (Fig. 7b). The fluorescence spectra at ∼425 nm confirm the ·OH generation (Fig. 7c). Similarly, Xia et al. used lead-free BaTiO3 nanofibers (pyroelectric coefficient of ∼1 × 10−7 C cm−2 K−1) for pyro-catalytic RhB dye decomposition with 99% efficiency after 72 thermal cycles (30–47 °C).54 Qian et al. decomposed RhB with ∼98.15% efficiency using ZnO nanorods (Curie temperature TC is ∼127 °C and pyroelectric coefficient is ∼−9.4 μC m−2 K−1) after 27 thermal cycles from 22 °C to 62 °C.55 Wu et al. applied sol–gel synthesized Bi0.5Na0.5TiO3 nanomaterials for dye wastewater purification at 23–63 °C fluctuations.56 Lin et al. utilized Sm3+-doped Pb(Mg1/3Nb2/3)O3–PbTiO3 relaxation ferroelectric for dye decomposition at 23–68 °C in the darkness.57 Scholars have applied pyro-catalytic dye decomposition to tooth whitening, addressing discoloration from food and beverages. Wang et al. utilized BaTiO3 to decompose dental stains via simulated oral temperature fluctuations for whitening (Fig. 7d).53 This pyro-catalytic method offers a non-destructive, safe whitening approach, with braces containing pyroelectric particles harnessing daily oral temperature changes to bleach teeth.
 | ||
| Fig. 7 (a) TEM image of BiFeO3 nanoparticles. (b) UV-vis spectra of RhB solution with BiFeO3 nanoparticles as the pyro-catalyst. The inset is a photograph of RhB. (c) Fluorescence spectra of 2-hydroxyterephthalic acid.52 Copyright 2016, RSC. (d) Pyro-catalytic tooth whitening diagram.53 Copyright 2022, the Author(s). | ||
With the development of pyro-catalysis, the research on materials has expanded to graphitic carbon nitride (g-C3N4),58 natural elbaite,59 tourmaline,9etc.Table 1 compares dye decomposition across materials, showing that pyro-catalytic performance is influenced by material morphology, aspect ratio, pyroelectric coefficient, temperature range, and rate. Subsequent chapters will delve into factors affecting pyro-catalytic performance and enhancement strategies.
| Pyrocatalyst | Morphology | Length–diameter ratio | Pyroelectric coefficient (μC m−2 K−1) | Temperature range (°C) | Cycle duration (min) | Average temperature change rate (°C min−1) | Thermal cycles | Dye | Decomposition efficiency (%) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| BiFeO3 | Nanoparticle | — | ∼90 | 27–38 | 16 | 1.375 | 85 | RhB | 99 | 52 |
| BaTiO3 | Nanofiber | ∼50 | ∼100 | 30–47 | 20 | 1.7 | 72 | RhB | 99 | 54 |
| ZnO | Nanorod | ∼13.3 | ∼−9.4 | 22–62 | 20 | 4 | 27 | RhB | 98.15 | 55 |
| Bi0.5Na0.5TiO3 | Nanoparticle | ∼100 nm | 250 | 23–63 | 10 | 8 | 85 | RhB | 96.75 | 56 |
| MB | 98.35 | |||||||||
| MO | 19.97 | |||||||||
| Pb(Mg1/3Nb2/3)O3–PbTiO3: 1 mol% Sm3+ | Micron block | ∼10 μm | >−1250 | 23–68 | — | — | 9 | Rh 6G | 94.3 ± 2.5 | 57 |
| MB | 88.52 | |||||||||
| MB | 64.32 | |||||||||
| g-C3N4 | Nanosheet | — | — | 25–60 | 20 | 3.5 | 42 | RhB | 92.6 | 58 |
| BaTiO3 | Nanowire | ∼50 | — | 25–45 | 8.75 | ∼4.57 | 80 | RhB | 95 | 60 |
| MB | 30 | |||||||||
| MO | 7 | |||||||||
| Nanoparticle | 200 nm | RhB | ∼50 | |||||||
| Nanoparticle | 100 nm | ∼30 | ||||||||
| NaNbO3 | Nanorod | >10 | 100 | 23–50 | 20 | 2.7 | 24 | RhB | ∼96 | 61 |
| MB | ∼36 | |||||||||
| Nanosheet | >1 μm | RhB | 76 | |||||||
| Nanocube | >1 μm | 33 | ||||||||
| BiOCl | Nanosphere | ∼1 μm | — | 25–65 | 20 | 4 | 54 | RhB | 85.31 | 62 |
| Nanoflower | ∼2 μm | 58.8 | ||||||||
| Nanoplate | ∼500 nm | 12.2 |
3.2 Water splitting for hydrogen generation
Pyroelectric materials can harvest waste heat energy and store it as H2, offering a solution to environmental pollution and energy shortage scarcity. Sabatier's principle dictates chemical potential equilibrium in multi-component systems, balancing adsorbate interactions for optimal catalysis. Periodic polarization changes (e.g., temperature, strain, or electric field) in ferroelectrics can manipulate binding energies, overcoming Sabatier's limitations on surface catalysis.63In 2016, Kakekhani et al. demonstrated the theoretical viability of ferroelectric PbTiO3 for water splitting to generate hydrogen via first-principles calculations.42Fig. 8a illustrates the pyro-catalytic water splitting process on PbTiO3(001) crystal surfaces. PbTiO3 undergoes phase transitions between ferroelectric and paraelectric phases with temperature cycles around its Curie temperature. In the ferroelectric phase, water molecules dissociate on the negative surface, forming atomic hydrogen. In the paraelectric phase, hydrogen atoms recombine into H2, resetting the surface. Temperature cycling controls ferroelectric polarization, splitting H2O into O2 and H2. Xie et al. utilized PZT and PVDF thin films to harness thermal energy via the pyroelectric effect for water splitting, observing continuous hydrogen bubble formation during thermal cycling.65 This setup, using pyroelectric materials as an electrolyte's external power source, spatially separates H2 and O2 products. In 2019, Zhang et al. applied PZT as an external charge source to generate a voltage of up to 2.34 V, adequate for water splitting, achieving an optimal hydrogen yield of 0.654 μmol h−1.13 Pyroelectric charges on the material's surfaces, if fully in contact with the electrolyte, can directly enhance surface chemical reactions, boosting catalytic efficiency. Belitz et al. experimentally verified the concept, detecting hydrogen at 300 ppb using a Coulomb solid electrolyte detector after exposing BaTiO3 powder to distilled water under 40–70 °C thermal cycles.14
 | ||
| Fig. 8 (a) Theoretical simulation of pyro-catalytic water splitting for hydrogen generation.42 Copyright 2016, RSC. (b) Temperature-dependence of the dielectric constant of Ba0.7Sr0.3TiO3. (c) Pyro-catalytic hydrogen evolution of Ba0.7Sr0.3TiO3.64 Copyright 2018, RSC. (d) Pyro-catalytic hydrogen evolution of 2D black phosphorene. (e) Schematic of pyro-catalytic hydrogen production.12 Copyright 2018, the Author(s). (f) Schematic of pyro-catalytic tumor therapy.23 Copyright 2021, ACS. | ||
Near the Curie temperature, pyroelectric materials exhibit enhanced effects. Xu et al. utilized a Ba0.7Sr0.3TiO3 nanomaterial near its Curie temperature (25–50 °C) for water splitting.64Fig. 8b shows the temperature dependence of the dielectric constant of Ba0.7Sr0.3TiO3, with 32 °C as its phase transition. Fig. 8c depicts hydrogen evolution with methanol addition over 36 thermal cycles, reaching 46.89 μmol g−1. Many two-dimensional materials with few layers exhibit piezo-/pyroelectricity due to structural symmetry breaking. Thickness reduction alters local atomic structure, affecting exposure ratio, coordination, and bond lengths.66 You et al. discovered pyroelectric two-dimensional few-layer black phosphorene with pyro-catalytic water splitting, reaching 540 μmol g−1 after 24 thermal cycles of 15–65 °C (Fig. 8d).12Fig. 8e illustrates the process, where heating/cooling cycles change the dipole moment, transferring q+ and q− to reactants for Redox. H+ in water reacts with q− to form H2. Methanol addition as a sacrificial agent removes positive charges, extending pyro-induced negative charge lifetime and enhancing catalytic efficiency. Hole transfer kinetics has always been the overall rate-limiting step for the efficiency of pyro-catalysis. Zhang et al. enhanced CdS's pyroelectricity with 2-mercaptobenzimidazole (2MBI) modification (CdS-2MBI), reaching 154.8 μmol g−1 after 36 thermal cycles of 25–55 °C, about 5 times that of CdS.67 2MBI's bonding and hole acceptance boost the pyroelectric response and charge separation for higher hydrogen yields. This led to the development of various pyro-catalysts for water splitting. Sun et al. tested SiC's pyro-catalytic hydrogen production at 27–60 °C, reaching 32.84 μmol g−1 in 20 thermal cycles.68 Song et al. used α-Si3N4 at 27–60 °C for pyro-catalysis, achieving 12.32 μmol g−1 in 20 thermal cycles.69
He et al. applied pyro-catalytic water splitting to tumor therapy, using CdS on Nb2C nanosheets (M/CdS) modified with tumor-targeting hyaluronic acid (HA) to create “nano-lymph” (M/CdS-HA) (Fig. 8f).23 IR-II laser-induced temperature changes trigger water splitting in tumors, reducing tumor interstitial pressure (TIP) for enhanced nanomedicine penetration. Meantime, ROS from pyro-catalysis can further damage deep tumor stem cells, leading to cell apoptosis or necrosis. In addition, overexpressed lactic acid in tumors can act as a sacrificial agent to improve ROS efficiency. This tumor penetration treatment strategy based on water splitting and ROS production as “hydrodynamic therapy” is of great significance for drug delivery.
3.3 Other applications
Pyroelectric-induced charges facilitate water splitting for hydrogen generation, ROS production for cancer therapy, sterilization, dye decomposition, CO2 reduction, synthesis and so on. CO2 is a greenhouse gas, and converting it into alternative fuels is an effective way to limit its long-term effects. Xiao et al. utilized the pyroelectric effect of bismuth tungstate (Bi2WO6) nanosheets for CO2-to-methanol conversion at 15–70 °C, achieving a max methanol yield of 55.0 μmol g−1 after 20 thermal cycles (Fig. 9a and b).15 Zou et al. prepared Ba(Ti0.8Zr0.2)O3–x(Ba0.7Ca0.3)TiO3 (BZT–xBCT, x = 0.2, 0.3, 0.4, 0.5, and 0.6) fibers via electrospinning for pyro-catalysis. Since the transition point between rhomboid phase and tetragonal phase of BZT–0.5BCT is about 30 °C, the authors chose the pyro-catalytic temperature to be 0–60 °C. After 20 thermal cycles, the yield of CO2 reduction to acetaldehyde is 382.47 μmol g−1 (Fig. 9c), and the reaction equation is as follows:16| 2CO2 + 10H+ + 10e− → CH3CHO + 3H2O | (5) |
 | ||
| Fig. 9 (a) Pyroelectric response of Bi2WO6. (b) Pyro-catalytic methanol yield via CO2 reduction.15 Copyright 2021, the Author(s). (c) Pyro-catalytic acetaldehyde yield via CO2 reduction. Inset is the GC diagram.16 Copyright 2022, Elsevier. (d) Schematic of pyro-catalytic metal salts reduction.70 Copyright 2018, ACS. (e) Schematic of pyro-catalytic bimetallic reduction.71 Copyright 2020, RSC. (f) TEM images of Au–ZnO nanorods. Inset is the HRTEM image.72 Copyright 2021, Wiley. | ||
Noble metal reduction via a single-electron reaction is simpler than CO2 reduction. Liu et al. synthesized Au nanoparticles on BaTiO3 using its pyroelectricity and temperature oscillation, offering a reducing-agent-free method for metal–pyroelectric hybrids (Fig. 9d).70 Further, they prepared gold/platinum bimetallic nanoparticles on BaTiO3via a pyro-catalytic process using chloro-auric acid and potassium tetrachloroplatinate as precursors (Fig. 9e).71 In 2021, Au reduction on ZnO surfaces was achieved, with TEM and HRTEM images showing lattice spacings of 0.26 nm for ZnO(002) planes and 0.235 nm for Au(111) planes (Fig. 9f).72 The pyro-catalytic synthesis field has promising prospects, offering an eco-friendly, efficient, and sustainable alternative to traditional methods by utilizing materials' pyroelectricity without toxic reagents, meantime utilizing the waste heat. As research advances, this method is expected to expand its application, potentially leading to the synthesis of complex organics and materials, becoming a key direction in chemical synthesis.
4. Strategies for improving pyro-catalytic performance
4.1 Heat transfer optimization
For pyro-catalysis which relies on temperature fluctuations, the discussion of device optimization and thermal conduction improvement is crucial. Generally speaking, the temperature change can be achieved by thermal conduction, thermal convection, or thermal radiation.55 Thermal conduction is the transfer of heat due to the movement of particles within a material or between touching surfaces. It is swift and stable in solids, unlike in gases and liquids where it is less efficient. Belitz's design (Fig. 10a–c) for pyro-catalytic water splitting uses Peltier heat pumps for direct, energy-efficient heating via thermal conduction.14 The unit consists of four Peltier heat pumps equipped with radiators and fans, and is fixed in an aluminum-assembled support frame. The sample is moved between hot and cold reservoirs for heating and cooling. This heating method is a typical thermal conduction method, which can directly transfer temperature to the sample, minimizing energy loss. Higher thermal conductivity, larger area, and shorter distance boost heat transfer efficiency of thermal conduction.74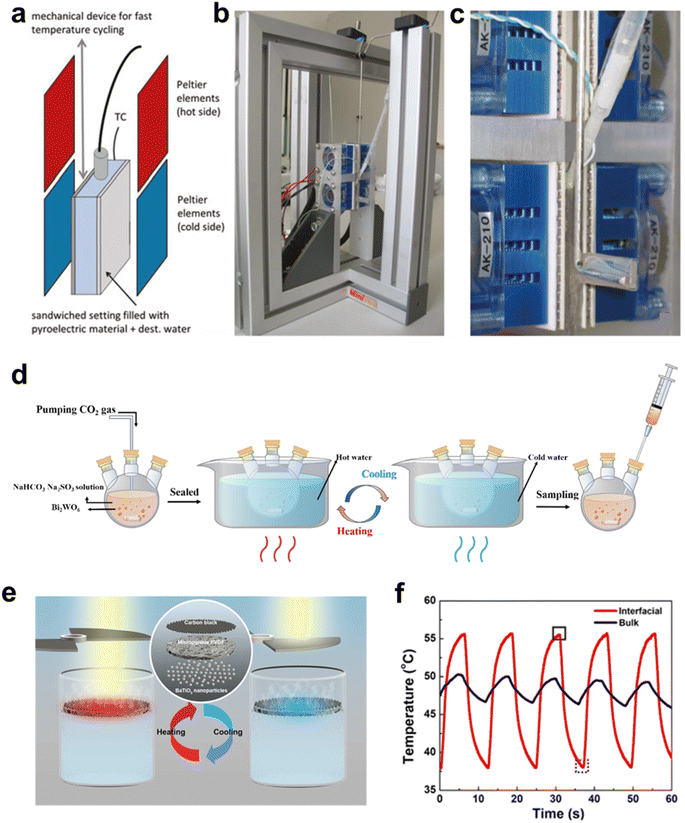 | ||
| Fig. 10 (a) Schematic of the thermal conduction device. (b) Peltier heat pumps with radiators and fans. (c) Enlarged image for detail.14 Copyright 2017, De Gruyter. (d) Schematic of thermal convection device.15 Copyright 2021, the Author(s). (e) Schematic of a device for pyro-catalytic reactions at the liquid/gas interface. (f) Time–temperature curves of pyroelectric materials of interface system and block system.73 Copyright 2018, ACS. | ||
Thermal convection is heat transfer via fluid particle movement, occurring in gases/liquids and even at rest due to density differences from temperature gradients. Most of the articles on pyro-catalysis have adopted a combination of thermal conduction and thermal convection, that is, water bath for heating and cooling (Fig. 10d).15 The temperatures of the two water baths are set to low and high, respectively. By swapping the reactor back and forth between the two water baths, the temperature can be controlled. The thermal convection efficiency depends on the medium (gas or liquid), flow rate, and flow direction, and can be enhanced by circulating flow of the pump.
Thermal radiation comprises electromagnetic waves emitted by objects based on their temperature, with the intensity rising as the temperature increases. It can transfer heat through vacuums without contact. Primarily infrared and visible light are emitted, with near-infrared thermal radiation used in medical pyro-catalysis.50,75 According to eqn (2), pyro-catalysis requires rapid temperature changes for sufficient pyroelectric current. Min et al. used carbon black/PVDF–BaTiO3 composite pyroelectric films for rapid temperature changes under light due to photothermal effects, driving efficient pyro-catalysis73 (Fig. 10e). The temperature of the BaTiO3 solution rises 4 °C in 6 s of heating, while the temperature of the film increases 17.2 °C (Fig. 10f). In order to maximize the utilization of input heat energy and optimize thermal cycle frequency, You et al. developed Au/BaTiO3 nanoparticles as a plasma heat source for laser-induced pyro-catalytic water splitting.76 Here, Au acts as a local heat source via plasma effects. COMSOL simulations show a 90 K temperature increase on Au and 40 K at the interface within 25 ns (Fig. 11). Based on the photothermal effect of plasma,77,78 pyro-catalysis can also be applied in the field of tumor therapy50 and CO2 reduction.17
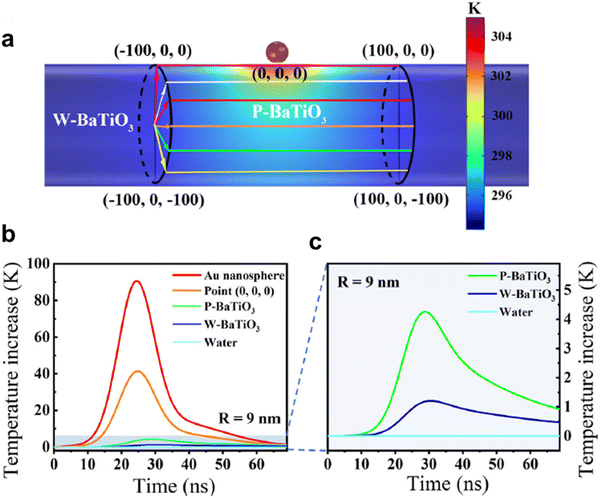 | ||
| Fig. 11 Thermal simulation. (a) Temperature distribution of Au/BaTiO3. (b) Time evolution of the temperature of the material on different positions. (c) Enlarged time evolution of the average temperatures.76 Copyright 2022, the Author(s). | ||
The photothermal effect can quickly convert light energy into heat energy, allowing materials to rapidly increase in temperature within a short period of time. By adjusting the intensity, duration, and position of the light source, the temperature and area of heating can be precisely controlled. It achieves non-contact heating, avoiding the pollution or damage that may be caused by traditional heating methods. However, it also faces challenges in terms of material selection, thermal uniformity, and system design. In general, each heat transfer method has its own unique advantages and disadvantages, fitting various scenarios, and in practice, they are often combined for desired effects.
4.2 Introducing external fields
 | ||
| Fig. 12 (a) Photocurrent response and (b) comparison of pyro-catalytic efficiency of non-polarized and polarized ceramic powders.85 Copyright 2020, AIP. (c) Effect of electric polarization intensity on pyro-catalytic activity.86 Copyright 2020, Elsevier. (d) Effect of the electric polarization intensity on the RhB decomposition rate. (e) Schematic of electric polarization effect on pyro-catalysis.51 Copyright 2020, Elsevier. (f) Weight fraction distributions of ferroelectric tetragonal and paraelectric cubic phases in polarized and unpolarized samples. (g) Pyro-catalytic activity of BaTiO3 with BET specific surface area varying with different particle sizes and polarization. (h) Relationship between the particle size and domain structure before and after polarization. (i) Relationship between the effects of polarization on different factors and particle size.36 Copyright 2020, the Author(s). | ||
Chen et al. cut and polarized commercial BaTiO3 crystals to study polarization's impact on pyro-catalytic dye decomposition.51 Unpolarized crystals have nearly 0% efficiency, while ∼5.00 kV mm−1 polarization achieves ∼56% after 140 thermal cycles (28–60 °C) (Fig. 12d). Pyro-induced charges on material surface determine the catalytic activity, related to the amount of net charges Q, according to the following formula:51,64
| Q = p·A·ΔT | (6) |
Raufeisen et al. investigated the impact of electric polarization on BaTiO3 powder's pyro-catalytic activity, examining particle size effects.36 Polarization alters phase composition, favoring ferroelectric tetragonal phases, especially in smaller particles (Fig. 12f). Polarization also impacts specific surface area, with a more pronounced effect on smaller particles, potentially boosting the pyro-catalytic performance (Fig. 12g). Below 30 nm, BaTiO3 lacks pyroelectricity, while larger particles form domains that align under polarization, enhancing residual polarization, Pr, and pyroelectric coefficient (Fig. 12h). Larger particles favor tetragonal phase but have a smaller surface area (Fig. 12i). Optimal pyro-catalytic enhancement occurs at ∼100 nm particle size of BaTiO3.
 | (7) |
 | ||
| Fig. 13 (a) J–V curves and (b) Mott–Schottky curves of the BaTiO3 photoanode. (c) H2/O2 production under both temperature fluctuation and light. The inset is a cross-section SEM image of the BaTiO3 nanorod array.87 Copyright 2020, Elsevier. (d) J–V curves of the NaNbO3 photoanode.88 Copyright 2020, Elsevier. (e) RhB decomposition ratio under different reaction conditions. (f) Combined photo/pyro-catalytic mechanism diagram.89 Copyright 2019, Elsevier. (g) Schematic of photo/pyro-catalytic dye decomposition. (h) Comparison of catalytic efficiency under different conditions.90 Copyright 2022, ACS. | ||
Chen et al. demonstrated 98.1% dye decomposition with ZnSnO3 under ultraviolet light and thermal cycling (20–65 °C), surpassing individual photocatalysis (76.8%) and pyro-catalysis (20.2%) (Fig. 13e).89 Temperature changes alter pyroelectric dipole moments, affecting polarization and carrier separation for improved catalysis (Fig. 13f).91,92 Xu et al. prepared Bi0.5Na0.5TiO3 for photo/pyro-catalytic dye decomposition (Fig. 13g).90 The setup involved mixing Bi0.5Na0.5TiO3 with RhB solution in an aluminum box with a transparent top, subjected to temperature cycling (10–70 °C) using a thermoelectric heater. With temperature variation and visible light, the RhB decomposition rate reaches 98%, outperforming individual photocatalysis and pyro-catalysis (Fig. 13h). Integrating temperature changes into photocatalysis or light into pyro-catalysis often yields mutual performance gains. Similar enhancements were seen with Bi2WO6,93 Pb(Zr0.52Ti0.48)O3,94 and Pb(Zr0.52Ti0.48)O3/Ag2O heterostructures.95
 | ||
| Fig. 14 (a) Voltage curve of the 10 μF capacitor charged using a hybrid nanogenerator.37 Copyright 2016, Wiley. (b) Comparison of the dye decomposition efficiency of NaNbO3 under different conditions.10 Copyright 2018, Elsevier. (c) Comparison of the dye decomposition efficiency of Ag2O/BaTiO3 under different conditions.97 Copyright 2020, Elsevier. (d) Current density of NaNbO3 under different conditions. Section distribution of internal (e) temperature and (f) magnetic flux density via COMSOL simulation.98 Copyright 2023, Elsevier. | ||
Pyroelectric materials, which are inherently piezoelectric, can harvest thermal energy upon temperature variation and vibrational energy and enhance catalysis via piezo-/pyro-catalysis. You et al. utilized NaNbO3 for piezo/pyro-catalysis, achieving 86.5% RhB dye decomposition, surpassing solo pyro-catalysis (63.3%) and piezo-catalysis (75.8%) efficiencies (Fig. 14b).10 The boost may stem from the synergistic effect of piezocatalysis and pyro-catalysis, rather than the simple addition of the two. Zhao et al. constructed a Ag2O/BaTiO3 heterostructure, examining four-way coupling of piezoelectric, pyroelectric, semiconductor and optical coupling, showing enhanced pyro-photocatalytic efficiency under ultrasonic stimulation, due to effective carrier separation (Fig. 14c).97 Liu et al. synthesized a Ba0.7Sr0.3TiO3 photoelectrode and assessed its performance under ultrasonic and temperature variation.103 The piezo–pyro–photocatalytic current density of Ba0.7Sr0.3TiO3 increases to 1.01 mA cm−2 with combined stimuli, surpassing individual pyro-photocatalysis, enhancing carrier separation and transport via stronger polarization-induced potentials.
 | ||
| Fig. 15 (a) Atomic diagram of the phase transformation of CoSe2 assisted by P doping.107 Copyright 2018, the Author(s). (b) SPV spectra of Ba1−xSrxTiO3 photoanodes.108 Copyright 2022, RSC. (c) SPV spectra of N-doped BiOIO3.109 Copyright 2023, Elsevier. (d) SPV spectra of Cu-doped ZnIn2S4.110 Copyright 2018, Wiley. | ||
The applications of external fields are effective methods to enhance pyro-catalysis. Among them, an external electric field can increase the built-in electric field of pyroelectric materials, thereby improving the separation efficiency of carriers. Additionally, utilizing the built-in electric field of pyroelectric materials for photothermal–pyroelectric combined catalysis can further enhance the catalytic efficiency. However, the addition of external fields undoubtedly increases the complexity and cost of the device. Therefore, reasonable design of the device is necessary for easier operation and improving the catalytic efficiency.
4.3 Defect engineering
Liu et al. demonstrated that doping enhances the built-in electric field polarization, optimizing Ba1−xSrxTiO3's pyroelectric performance and carrier separation.108 Surface photovoltage (SPV) spectra show positive curves due to an n-type semiconductor surface band curvature, thus creating a built-in electric field from the bulk phase to the surface (Fig. 15b). Wu et al. doped N into BiOIO3's IO3 polar group, altering the polar structure due to modified I–O bonds after N replaces O, and enhancing the pyroelectric polarization field of the material, thus boosting the photo/pyro-catalytic efficiency (Fig. 15c).109 In addition, optimizing doping dose is crucial: optimal doping enhances charge separation via lattice defects, but excessive doping causes structure distortion of the coordination atom, resulting in charge recombination, thereby reducing catalytic activity.117,118 SPV spectra show higher response in properly doped samples versus low response in overdoped ones, indicating more efficient charge separation (Fig. 15d).110
Doped molecules in pyro-catalytic materials create polar domains to reduce the symmetry of the host crystal, inducing polarization and pyroelectricity. Sources of polarity by doping include molecule dipole differences and asymmetric distortions.40 Luo et al. showed improved dye decomposition with Cu-doped ZnS via combined photo/pyro-catalysis.119 Sharma et al. studied Ca-doped BaTiO3 for pyro-catalysis, enhancing the pyroelectric behavior and dye decomposition efficiency.41
Liu et al. have doped BaTiO3 with Sr to form Ba0.7Sr0.3TiO3, and then induced oxygen vacancies in Ba0.7Sr0.3TiO3−xvia hydrazine hydrate reduction.128 Electron spin resonance (ESR) spectra show increased defect concentration with Sr doping and oxygen vacancies (Fig. 16a). Ba0.7Sr0.3TiO3−x exhibits 0.92 mA cm−2 current density, outperforming Ba0.7Sr0.3TiO3 and BaTiO3 by 1.35 and 2.56 times, respectively. Fig. 16b shows that Sr doping and oxygen vacancies in Ba0.7Sr0.3TiO3−x enhance the charge separation efficiency, reducing recombination and boosting catalytic activity. Qiao et al. modulated oxygen vacancy concentrations in BaTiO3 (Fig. 16c) and assessed current densities across 20–50 °C cycles.129 BaTiO3−x with oxygen vacancies achieves 0.16 mA cm−2, which is higher than 0.10 mA cm−2 of BaTiO3 (Fig. 16d). This is because the oxygen vacancy causes the imbalance of charge distribution in the crystal structure, which improves the polarization effect, aiding charge separation, but excessive vacancies can impede catalysis. Proper control of vacancy concentration can optimize catalytic activity, as seen in Yang et al.'s S-vacancy tuning in MoS2 electrocatalysis (Fig. 16e).130 The catalytic activity reaches its peak when the vacancy concentration is 5.7 × 1014 cm−2 with a turnover frequency (TOF: reaction number occurring per mole of catalyst per unit time) of 5 s−1 (Fig. 16f).
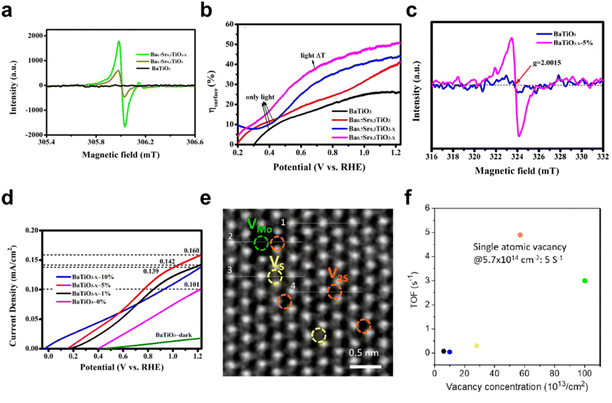 | ||
| Fig. 16 (a) ESR spectra and (b) surface charge separation efficiency of BaTiO3, Ba0.7Sr0.3TiO3 and Ba0.7Sr0.3TiO3−x.128 Copyright 2022, Wiley. (c) EPR spectra of BaTiO3 and BaTiO3−x-5%. (d) J–V curves of photoanodes with different oxygen vacancy concentrations under thermal cycling.129 Copyright 2022, Elsevier. (e) High angle annular dark-field scanning transmission electron microscopic (HAADF-STEM) image of single layer MoS2. (f) TOF of single vacancies.130 Copyright 2019, ACS. | ||
Defect engineering can alter the crystal and electronic structures of pyroelectric materials, increasing the carrier concentration within them. However, it can potentially increase chemical instability, and raise preparation costs. Therefore, it is necessary to deepen the mechanism research and to enhance the uniformity and controllability of the sample preparation process.
4.4 Metal loading
Noble metal catalysts such as Pt, Ru, and Au are esteemed in electrocatalysis and photocatalysis for their intrinsic properties and catalytic activity.131,132 They also act as charge traps to prevent pyroelectric-induced charge recombination, enhancing the redox reaction efficiency. Benke et al. utilized BaTiO3 with added Pd nanoparticles for pyro-catalytic ROS production, facilitating charge transfer.43 Xu et al. synthesized Ba0.7Sr0.3TiO3 nanoparticles and loaded Ag nanoparticles via photoreduction.133 The HRTEM image shows lattice spacings corresponding to Ba0.7Sr0.3TiO3's (101) plane and Ag's (111) and (200) planes (Fig. 17a). Ba0.7Sr0.3TiO3 decomposes 90% of RhB after 50 thermal cycles, reaching 99% with Ag coating after 50 thermal cycles (25–50 °C) (Fig. 17b). Pyroelectric polarization induced excess charges to generate ROS, decomposing dyes, with noble metals enhancing charge extraction and reducing recombination for improved pyro-catalysis efficiency (Fig. 17c).134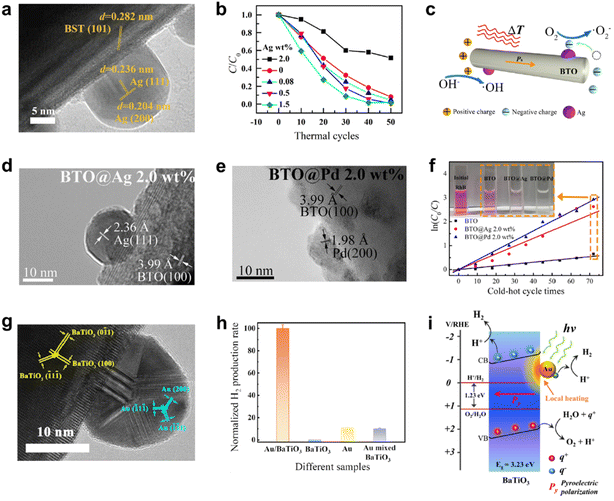 | ||
| Fig. 17 (a) HRTEM image and (b) pyro-catalytic RhB decomposition efficiency of Ba0.7Sr0.3TiO3@Ag.133 Copyright 2018, Elsevier. (c) Schematic of pyro-catalysis supported by noble metals. HRTEM image of (d) BaTiO3@Ag and (e) BaTiO3@Pd nanomaterials. (f) Relationship between ln(C0/C) and the thermal cycles. The inset is the color change of the RhB solution.134 Copyright 2018, Elsevier. (g) HRTEM image of Au/BaTiO3. (h) Normalized H2 yield of different samples (H2 yield of Au/BaTiO3 is 1). (i) Schematic of pyro-catalytic hydrogen production using Au/BaTiO3 driven by local heating of surface plasma.76 Copyright 2022, the Author(s). | ||
Ma et al. synthesized BaTiO3 nanofibers and coated them with Ag and Pd by an impregnation method to obtain BaTiO3@Ag and BaTiO3@Pd nanofibers.134 The HRTEM image confirms lattice spacings for tetragonal BaTiO3's (100) plane, cubic Ag's (111) plane, and cubic Pd's (200) plane (Fig. 17d and e). These nanofibers pyro-catalytically decompose RhB dye, with efficiencies of ∼48%, ∼92%, and ∼95% for BaTiO3, BaTiO3@Ag, and BaTiO3@Pd after 72 thermal cycles (30–52 °C). The reaction rate constants k of BaTiO3, BaTiO3@Ag and BaTiO3@Pd are calculated as 0.00769, 0.03613 and 0.04041 per cycle, respectively (Fig. 17f). Higher k values indicate better pyro-catalytic outcomes. Liu et al. utilized BaTiO3's pyroelectricity to synthesize Au nanoparticles on the surface of BaTiO3 during thermal cycling, comparing pyro-catalytic RhB decomposition efficiencies of thermal-driven synthesized Au–BaTiO3, mechanically mixed Au–BaTiO3, and pure BaTiO3.70 The Schottky barrier of thermal-driven synthesized Au–BaTiO3 at the metal–semiconductor interface separates pyroelectric-induced charges, exhibiting superior pyro-catalytic activity.
Noble metals, beyond charge separation, have some unique applications. You et al. optimized thermal energy use and cycle frequency with Au-modified BaTiO3 (Au/BaTiO3) as a plasma heat source for pyro-catalytic water splitting under pulsed laser irradiation.76 The HRTEM image confirms crystal plane alignment with BaTiO3 and Au (Fig. 17g). Au/BaTiO3 outperforms pure Au in hydrogen production under 532 nm pulsed laser, indicating Au plasma-induced photothermal effects. BaTiO3 performs pyro-catalysis, reaching peak hydrogen generation of 133.1 ± 4.4 μmol g−1 h−1 (Fig. 17h). Au acts as a local heat source in pyro-catalysis via plasma effect, heating Au/BaTiO3 surfaces to stimulate pyro-catalytic reactions. Post-pulsed laser, BaTiO3's cooling cycle engages uncompensated pyro-induced charges for catalysis (Fig. 17i). This technology offers potential for efficient clean energy production and pollutant treatment. However, some defects of noble metals, such as instability, scarcity (less than 3% of all elements), and cost, must be considered for practical application.131
Non-noble metal catalysts include transition metals (single transition metals, double transition metals, transition metal compounds and transition metal composites) and non-metals such as carbon-based materials, gaining interest in catalysis for their affordability, activity, and scalability.2,135–138 Wang et al. synthesized barium calcium titanate@carbon (Ba0.8Ca0.2TiO3@C) for pyro-catalytic RhB dye decomposition, showing improved efficiency with 3 wt% carbon addition, increasing the decomposition rate from 31.3% to 76.6% due to enhanced carrier mobility and reduced charge recombination.139
4.5 Heterojunction engineering
The selection of semiconductors with suitable band structures for heterojunctions aids carrier separation and catalytic performance. Heterojunctions, based on energy band alignment, are categorized into straddling gap (type I), staggered gap (type II), and broken gap (type III) (Fig. 18a–c).140 The same band relationship as type II, but different charge transfer modes, also includes Z-scheme-type heterojunctions (Fig. 18d)141 and S-scheme-type heterojunctions (Fig. 18e).142,143 They can be semiconductor–semiconductor, semiconductor–metal, semiconductor–carbon, or multi-component, and formed by doping (p–n junction) or altering material dimensions (3D/2D).144,145 Heterojunctions find applications in photovoltaics, transistors, photodetectors, photocatalysis, etc.146–148 | ||
| Fig. 18 Band structures of (a) straddling gap (type I), (b) staggered gap (type II) and (c) broken gap (type III) heterojunctions.140 Copyright 2017, Wiley. (d) Band structure of the Z-scheme-type heterojunction.141 Copyright 2022, Elsevier. (e) Band structure of the S-scheme-type heterojunction.142 Copyright 2020, Elsevier. (f) Schematic of BiFeO3/g-C3N4 pyro-catalysis.11 Copyright 2020, the Author(s). | ||
Chen et al. synthesized a BiFeO3/g-C3N4 heterostructure via mixed-calcination, varying the g-C3N4 contents (0–25%) to study the pyro-catalytic performance.11 The 10% g-C3N4 heterojunction achieves 94.2% dye decomposition efficiency after 18 thermal cycles of 25–65 °C, surpassing pure BiFeO3 (67.7%). Band potential differences drive electron and hole diffusion between BiFeO3 and g-C3N4, creating negative charge centers at the g-C3N4 interface and positive charge center at the BiFeO3 interface, resulting in a built-in electric field that enhances charge separation and catalytic efficiency (Fig. 18f). The work of Wang et al. confirms this conclusion.149 They prepared BaTiO3@ZnO heterojunction nanofibers by varying the ZnO coating ratios and evaluated their pyro-catalytic activity. The efficiency in RhB decomposition peaks at 97% with 2.5 wt% ZnO, which is 4 times higher than that of pure BaTiO3. The electric field inside the heterojunction redirects original carrier flow behavior, enhancing charge separation. In addition, ZnO coating roughens the BaTiO3 nanofiber surface, increasing the specific surface area and providing more active sites for catalytic reaction. Wu et al. found similar results with BaTiO3/Pr2O3 for RhB decomposition, attributing performance to effective charge separation by built-in electric field of heterojunction.150 Heterojunctions are also used to enhance pyroelectric-induced charge separation in NaNbO3/CdS sterilization,19 BiFeO3@CuBi2O4 dye decomposition and sterilization,47 and BaTiO3@Nb2C-PEG tumor therapy.22
Heterostructures extend beyond pyro-catalysis and find use in photo/pyro-electric combined or multi-field coupling catalysis, including PZT/Ag2O,95 Ag2O/BaTiO3,97 BaTiO3/CdS,151 PZT/CdS152 and Ba0.8Sr0.2TiO3/Ag/Ag2O91 for more efficient energy utilization. Heterojunctions can also be utilized to enhance the separation of charge carriers, albeit with the drawbacks of complex fabrication processes and poorer stability. Consequently, it is imperative to devote more efforts to optimizing preparation methods, enhancing stability, and exploring new material combinations.
4.6 Surface modification
The catalytic process involves adsorption, surface reaction, and desorption steps, with catalyst surfaces and interfaces being key for performance as active sites.153,154 Molecular modifications can adjust the surface electronic properties of the material. In electrocatalysis, molecular modifications can not only regulate the hydrophilicity of the catalyst surface, but also stabilize some key intermediates.155 Venugopal et al. created stable Ni–CFx bonds on NiO catalysts via polytetrafluoroethylene coating, decreasing OH* adsorption energy and increasing O* Gibbs free energy, biasing water oxidation towards H2O2 production over O2.156Hole transfer kinetics has always been the overall rate-limiting step for pyro-catalytic efficiency. Based on the good dispersion performance, the molecular co-catalyst can provide enough hole trapping sites. Zhang et al. enhanced CdS's pyroelectric properties with 2MBI, leading to a 5-fold increase in hydrogen production (Fig. 19a).67 2MBI's HOMO at 1.65 V facilitates charge transfer, oxidizing lactic acid to release the proton for hydrogen reduction and enhancing pyro-catalysis. Its bonding and hole receptor capacity amplifies the pyroelectric response of CdS, improving charge separation for enhanced hydrogen production. Yang et al. modified Sr0.3Ba0.7TiO3 with polyvinylpyrrolidone (PVP) surfactants for tribo-/pyro-catalysis.157 Thermogravimetric (TG) curves indicate ∼4% PVP on the catalyst surface (Fig. 19b). PVP-induced cathode displacement in flat band potential acts as an electron trap, enhancing electron–hole pair separation and providing more active sites for tribo-/pyro-catalysis. Moreover, a lower slope of the Mott–Schottky curve indicates a higher charge density (Fig. 19c). Finally, a maximum RhB decomposition efficiency of 98% with a reaction rate constant, k, of 0.23 h−1 under mechanical friction and low temperature fluctuations is obtained (Fig. 19d).
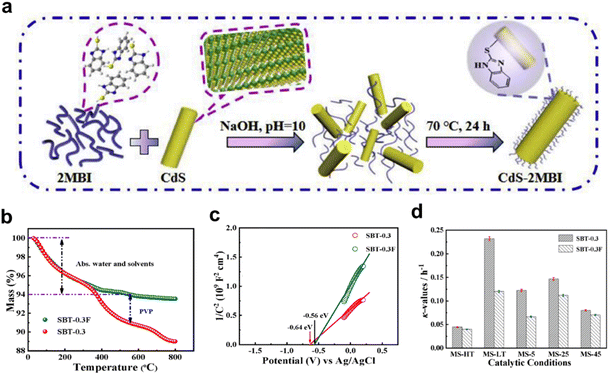 | ||
| Fig. 19 (a) Preparation process of CdS-2MBI.67 Copyright 2020, Elsevier. (b) TG/DSC curve, (c) Mott–Schottky curve, and (d) reaction rate constants of Sr0.3Ba0.7TiO3.157 Copyright 2021, Elsevier. | ||
Surface modification can enhance active sites, improve selectivity, enhance stability, and prevent catalyst agglomeration. However, it also has drawbacks, including complex preparation processes, increased costs, unstable effects, and the potential introduction of impurities. Future research directions include the development of new modifiers and in-depth theoretical studies.
4.7 Morphology control
Catalysts with the same composition but varied morphologies exhibit different exposed specific surface areas, active sites, crystal plane orientations, electronic structures or atomic arrangements, thus changing the catalytic performance.158–160 The change of particle size will affect the specific surface area and coordination environment, impacting the catalytic performance.159 Smaller particles can increase active sites but may affect carrier transport due to trap states.158 Therefore, moderate trap states can enhance the catalytic activity.161In pyro-catalysis, morphology plays a key role in determining pyroelectric potential,60,162 specific surface area,61 active crystal surface62 and the charge anisotropy distribution,3 thus affecting the pyro-catalytic performance. Wu et al. synthesized BaTiO3 nanomaterials with different morphologies and sizes for pyro-catalytic dye decomposition (Fig. 20a–c).60 After 80 thermal cycles (25–45 °C), BaTiO3 nanowires show ∼95% RhB decomposition efficiency, outperforming 200 nm (∼50%) and 100 nm (∼30%) BaTiO3 nanoparticles (Fig. 20d). The finite element simulation reveals a higher pyroelectric potential in BaTiO3 nanowires. Wu et al. regulated the growth direction and thickness of BiOIO3 photoanode for enhanced photocurrent density under thermal cycles.162 The appropriate thickness adjustment not only enhances the polarization intensity of the material, reduces the chemical reaction barrier, but also promotes the carrier separation and transfer. You et al. synthesized NaNbO3 with different morphologies for pyro-catalytic RhB decomposition, with efficiencies of ∼96% for nanorods, ∼76% for nanosheets, and ∼33% for nanoblocks after 24 thermal cycles (23–50 °C) (Fig. 20e–h).61 Larger surface area correlates higher active site density, which is conducive to the adsorption and diffusion of dye molecules and rapid charge transfer, to higher catalytic efficiency. Wu et al. also synthesized BiOCl with different shapes, showing pyro-catalytic dye decomposition efficiencies of ∼85.31% for nanospheres, ∼58.8% for nanoflowers, and ∼12.2% for nanoplates after 54 thermal cycles (25–65 °C) (Fig. 20i–l).62 Different morphologies of BiOCl expose different active crystal faces, thus impacting the catalytic performance.
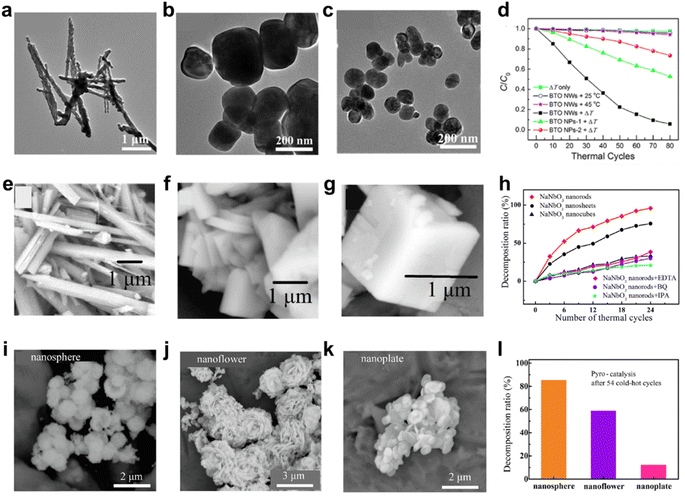 | ||
| Fig. 20 (a–c) TEM images of BaTiO3 nanowire and BaTiO3 nanoparticles of different sizes. (d) Comparison of pyro-catalytic efficiency of BaTiO3 with different morphologies.60 Copyright 2018, ACS. (e–g) SEM images of NaNbO3 nanorods, nanosheets and nanoblocks. (h) Comparison of the pyro-catalytic efficiency of NaNbO3 with different morphologies.61 Copyright 2018, Elsevier. (i–k) SEM images of BiOCl nanospheres, nanoflowers and nanoplates. (l) Comparison of the pyro-catalytic efficiency of BiOCl with different morphologies.62 Copyright 2020, Elsevier. | ||
Morphology control is a common and relatively mature method for improving the catalytic efficiency, and thus, it can be considered as a primary factor in the material preparation process. To enhance the pyro-catalytic efficiency, reliance cannot be placed on just one method; a comprehensive consideration of all factors is essential, including preparation costs, stability, and time costs. Beyond these, the operability of the setup and the effectiveness of the thermal conduction method must also be taken into account.
5. Summary and outlook
Pyroelectric effect is an intriguing phenomenon in nature, where pyroelectric materials can sense minute temperature changes and generate electrical charges as a result. It will be very meaningful to use the pyroelectric effect to collect and utilize waste low-temperature thermal energy, while addressing the energy crisis. Pyro-catalysis has become a hot research topic in recent years, showing immense potential and promising prospects in various fields, including dye decomposition, sterilization, water splitting, CO2 reduction, as well as medical applications such as teeth whitening and cancer treatment. Pyro-catalytic technology is making a swift progress, yet its performance remains to be optimized, primarily due to issues such as the thermal cycling process, the speed of temperature variations, and the efficiency of carrier separation.The temperature change rate directly influences the quantity of generated pyroelectric charge and the pyroelectric potential, which, in turn, directly impact the efficiency of pyro-catalysis. If the temperature change rate is too slow, the amount of charge generated will be small and the catalytic efficiency will be reduced. Consequently, finding more effective methods to modulate the temperature variation of pyroelectric materials is a pivotal issue that needs to be addressed. The heat transfer optimization is discussed, including thermal conduction, thermal convection, and thermal radiation. Currently, most studies on pyro-catalysis utilize a combination of thermal conduction and thermal convection, such as water bath heating and cooling. However, this method is not only inefficient in terms of energy conversion, but it also typically exhibits a prolonged cycle for temperature changes, which can hinder its practical application and overall performance. Infrared light has the advantages of strong penetration and fast heating speed. With the development of pyro-catalysis in the medical field, near-infrared radiation heating methods have been widely used and show promising prospects. In addition, it would be of significant value to investigate the utilization of low-temperature thermal energy, as well as the exploitation of daily temperature fluctuations between day and night, to drive pyro-catalysis in everyday life applications.
Other strategies to improve the performance of pyro-catalysis are thoroughly discussed, encompassing the introduction of external fields such as electric, light and stress fields, as well as defect engineering, metal loading, heterojunction engineering, surface modification, and morphology control. These strategies are fundamentally geared towards boosting the efficiency of carrier separation and migration, thereby augmenting the catalytic activity. Considering that the second pyroelectric effect is a derivative phenomenon resulting from the deformation of materials induced by temperature fluctuations, which is closely followed by the piezoelectric effect, it is imperative to take into account the piezoelectric properties of the materials when designing pyro-catalysts. Alternatively, a hybrid pyroelectric–piezoelectric catalytic approach could be adopted to further elevate the catalytic efficiency.
Currently, the majority of pyro-catalysis research papers focus on dye decomposition, with a comparatively limited number of studies addressing sterilization and cancer therapy. It is within the medical domain that the full potential of pyro-catalysis can be most effectively harnessed, particularly when integrated with infrared therapy to amplify its therapeutic impact. Consequently, there is a pressing need for more comprehensive and in-depth research into the application of pyro-catalysis within the medical field. Looking ahead, pyro-catalysis holds promise for expansion into various other areas, including nitrogen fixation, organic synthesis, and the development of self-cleaning surfaces.
In addition, the choice of materials significantly affects the efficiency of pyroelectric applications. Compared to traditional pyroelectric materials, nanomaterials exhibit several advantages such as a relatively large specific surface area, abundant active sites, and adjustable structures, which have been the focus of research. In the preparation of nanomaterials with high pyroelectric coefficients, it is crucial to consider their economic costs simultaneously. An effective approach is to grow highly active pyroelectric materials on substrates with good absorbability, which facilitates the recycling and reuse of the materials. In the biomedical field, it is essential to develop materials that are non-toxic, environmentally friendly, and possess high biocompatibility. Therefore, it is worthwhile to explore the emerging field of organic–inorganic hybrid molecular ferroelectric materials or purely organic ferroelectric materials.
The issue of thermal cycling and its potential impact on the stability and longevity of pyro-catalytic materials is also an important one. It needs a more detailed discussion on the long-term stability of these materials under operational conditions, including high temperatures and corrosive environments. Molecular modifications can adjust the surface electronic properties of the material, which can modulate catalyst hydrophilicity and stabilize some key intermediates in electrocatalysis.155 Drawing on this, future efforts to enhance the long-term stability of pyro-catalytic materials may involve mitigating the impact of prolonged operational conditions through material alterations like doping or coating.30
In summary, pyro-catalysis is an interesting and promising research direction. It can realize the utilization of low-temperature thermal energy, and has good prospects in tumor treatment and sterilization. This review aims to provide an important reference for the design and application of pyro-catalytic materials and provide new insights for related research work by summarizing the recent progress of pyro-catalysis.
Data availability
No primary research results, software or code have been included, and no new data were generated or analyzed as part of this review.Author contributions
Xiaoli Xu: conceptualization, writing – original draft and funding acquisition. Wanwan Cheng: data curation. Huan Zhai: data curation. Ying Wang: data curation. Lingbo Xiao: writing – review & editing and funding acquisition. Jiahui Hou: validation. Jianyang Kong: validation. Laishun Qin: supervision. Yanmin Jia: conceptualization, writing – review & editing. Yan Zhang: supervision. Shun Li: supervision, writing – review & editing. Da Chen: supervision, writing – review & editing.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
This work was supported by the National Natural Science Foundation of China (22309170), the Natural Science Foundation of Zhejiang Province (LQ24E020003, LQN25E020017), and open fund project of National-Local Joint Engineering Research Center of Heavy Metals Pollutants Control and Resource Utilization of Nanchang Hangkong University (ES202480182).References
- Y. Wang, X. Xu, L. Xiao, L. Li, Q. Xu, Z. Wen, L. Qin, Y. Jia, D.-L. Peng, W. Chen and D. Chen, J. Adv. Ceram., 2024, 13, 1737–1747 CrossRef CAS.
- L. Yu, Q. Zhu, S. Song, B. McElhenny, D. Wang, C. Wu, Z. Qin, J. Bao, Y. Yu, S. Chen and Z. Ren, Nat. Commun., 2019, 10, 5106 CrossRef PubMed.
- S. Chen, T. Takata and K. Domen, Nat. Rev. Mater., 2017, 2, 17050 CrossRef CAS.
- X. Xu, Y. Wang, W. Cheng, H. Zhai, L. Xiao, L. Qin and D. Chen, Surf. Interfaces, 2024, 54, 105245 CAS.
- S. Pandya, J. Wilbur, J. Kim, R. Gao, A. Dasgupta, C. Dames and L. W. Martin, Nat. Mater., 2018, 17, 432–438 CAS.
- S. Pandya, G. Velarde, L. Zhang, J. D. Wilbur, A. Smith, B. Hanrahan, C. Dames and L. W. Martin, NPG Asia Mater., 2019, 11, 26 CAS.
- I. Lubomirsky and O. Stafsudd, Rev. Sci. Instrum., 2012, 83, 051101 Search PubMed.
- Q. Wang, C. R. Bowen, R. Lewis, J. Chen, W. Lei, H. Zhang, M.-Y. Li and S. Jiang, Nano Energy, 2019, 60, 144–152 CAS.
- Z. Wu, T. Xu, X. Wang, L. Zhang, C. Zhao, W. Wu, G. Zhu and Y. Jia, Sep. Purif. Technol., 2023, 327, 124971 CAS.
- H. You, X. Ma, Z. Wu, L. Fei, X. Chen, J. Yang, Y. Liu, Y. Jia, H. Li, F. Wang and H. Huang, Nano Energy, 2018, 52, 351–359 CAS.
- M. Chen, Y. Jia, H. Li, Z. Wu, T. Huang and H. Zhang, J. Adv. Ceram., 2021, 10, 338–346 CAS.
- H. You, Y. Jia, Z. Wu, F. Wang, H. Huang and Y. Wang, Nat. Commun., 2018, 9, 2889 Search PubMed.
- Y. Zhang, S. Kumar, F. Marken, M. Krasny, E. Roake, S. Eslava, S. Dunn, E. Da Como and C. R. Bowen, Nano Energy, 2019, 58, 183–191 CAS.
- R. Belitz, P. Meisner, M. Coeler, U. Wunderwald, J. Friedrich, J. Zosel, M. Schelter, S. Jachalke and E. Mehner, Energy Harvesting Syst., 2017, 4, 107–113 Search PubMed.
- L. Xiao, X. Xu, Y. Jia, G. Hu, J. Hu, B. Yuan, Y. Yu and G. Zou, Nat. Commun., 2021, 12, 318 CAS.
- X. Zou, R. Zhu, Z. Cheng, X. Shi, L. Li, Y. Zhou, D. Wang, W. Wang, Z. Wang, Y. Shao and J. Bai, Mater. Chem. Phys., 2022, 291, 126719 CAS.
- C. Lu, X. Li, J. Li, L. Mao, M. Zhu, Q. Chen, L. Wen, B. Li, T. Guo and Z. Lou, Chem. Eng. J., 2022, 445, 136739 CAS.
- T. Ding, F. Liu, H. Xin, Y. Chen, L. Kong, J. Han, D. Ma, Y. Han and L. Zhang, Nano Today, 2024, 54, 102069 CAS.
- J. Min, H. Hao, Y. Zhao, Y. Hu, Q. Huang, F. Zhu, G. Zhang, J. Bi, S. Yan and H. Hou, Colloids Surf., A, 2022, 650, 129529 CAS.
- E. Gutmann, A. Benke, K. Gerth, H. Böttcher, E. Mehner, C. Klein, U. Krause-Buchholz, U. Bergmann, W. Pompe and D. C. Meyer, J. Phys. Chem. C, 2012, 116, 5383–5393 CAS.
- Y. Wang, X. Dai, C. Dong, W. Guo, Z. Xu, Y. Chen, H. Xiang and R. Zhang, Adv. Mater., 2022, 34, e2106773 Search PubMed.
- S. Zhang, L. Wu, W. Shi, J. Qin, W. Feng, Y. Chen and R. Zhang, Adv. Funct. Mater., 2023, 33, 2302360 CAS.
- Y. He, Z. Li, C. Cong, F. Ye, J. Yang, X. Zhang, Y. Yuan, Z. Ma, K. Zhang, Y. Lin, L. Zheng, X.-J. Liang and D. Gao, ACS Nano, 2021, 15, 10488–10501 CrossRef CAS PubMed.
- S. B. Lang and S. Muensit, Appl. Phys. A, 2006, 85, 125–134 CrossRef CAS.
- S. Li, Z. Zhao, J. Zhao, Z. Zhang, X. Li and J. Zhang, ACS Appl. Nano Mater., 2020, 3, 1063–1079 CrossRef CAS.
- Y. Zhang, P. T. T. Phuong, E. Roake, H. Khanbareh, Y. Wang, S. Dunn and C. Bowen, Joule, 2020, 4, 301–309 CrossRef CAS.
- C. Wang, N. Tian, T. Ma, Y. Zhang and H. Huang, Nano Energy, 2020, 78, 105371 CrossRef CAS.
- H. He, X. Lu, E. Hanc, C. Chen, H. Zhang and L. Lu, J. Mater. Chem. C, 2020, 8, 1494–1516 RSC.
- J. Wang, C. Hu, L. Shi, N. Tian, H. Huang, H. Ou and Y. Zhang, J. Mater. Chem. A, 2021, 9, 12400–12432 RSC.
- Q. Zhang and W. Liu, J. Mater. Chem. A, 2025, 13, 7601–7633 RSC.
- C. R. Bowen, J. Taylor, E. LeBoulbar, D. Zabek, A. Chauhan and R. Vaish, Energy Environ. Sci., 2014, 7, 3836–3856 RSC.
- C. R. Bowen, H. A. Kim, P. M. Weaver and S. Dunn, Energy Environ. Sci., 2014, 7, 25–44 RSC.
- U. Sassi, R. Parret, S. Nanot, M. Bruna, S. Borini, D. De Fazio, Z. Zhao, E. Lidorikis, F. H. L. Koppens, A. C. Ferrari and A. Colli, Nat. Commun., 2017, 8, 14311 CrossRef CAS PubMed.
- J. W. Stewart, J. H. Vella, W. Li, S. Fan and M. H. Mikkelsen, Nat. Mater., 2019, 19, 158–162 CrossRef PubMed.
- J. Yun and S.-S. Lee, Sensors, 2014, 14, 8057–8081 CrossRef PubMed.
- S. Raufeisen, P. Neumeister, J. R. Buchheim, M. Stelter and P. Braeutigam, Phys. Chem. Chem. Phys., 2020, 22, 23464–23473 RSC.
- S. Wang, Z. L. Wang and Y. Yang, Adv. Mater., 2016, 28, 2881–2887 Search PubMed.
- D. Lingam, A. R. Parikh, J. Huang, A. Jain and M. Minary-Jolandan, Int. J. Smart Nano Mater., 2013, 4, 229–245 CrossRef.
- M. U. de Vivanco, M. Zschornak, H. Stocker, S. Jachalke, E. Mehner, T. Leisegang and D. C. Meyer, Phys. Chem. Chem. Phys., 2020, 22, 17781–17790 RSC.
- E. Meirzadeh, I. Azuri, Y. Qi, D. Ehre, A. M. Rappe, M. Lahav, L. Kronik and I. Lubomirsky, Nat. Commun., 2016, 7, 13351 CrossRef CAS PubMed.
- M. Sharma, S. Patel, V. P. Singh and R. Vaish, J. Appl. Phys., 2020, 128, 095108 CrossRef CAS.
- A. Kakekhani and S. Ismail-Beigi, J. Mater. Chem. A, 2016, 4, 5235–5246 CAS.
- A. Benke, E. Mehner, M. Rosenkranz, E. Dmitrieva, T. Leisegang, H. Stöcker, W. Pompe and D. C. Meyer, J. Phys. Chem. C, 2015, 119, 18278–18286 CAS.
- S. Kumar, M. Sharma, T. Frömling and R. Vaish, J. Ind. Eng. Chem., 2021, 97, 95–110 CAS.
- Z. Y. Huo, D. M. Lee, S. Wang, Y. J. Kim and S. W. Kim, Small Methods, 2021, 5, e2100093 Search PubMed.
- E. Roake, M. Hopkins, B. Patenall and C. R. Bowen, in Joint Conf. IEEE Int. Frequency Control Symp. & IEEE Int. Symp. Applications of Ferroelectrics, IEEE, Piscataway, 2020 Search PubMed.
- Y. Hu, H. Hao, Y. Zhao, J. Min, Q. Huang, J. Zhong, G. Zhang, J. Bi, S. Yan and H. Hou, Surf. Interfaces, 2022, 33, 102191 CAS.
- Z. Tang, P. Zhao, D. Ni, Y. Liu, M. Zhang, H. Wang, H. Zhang, H. Gao, Z. Yao and W. Bu, Mater. Horiz., 2018, 5, 946–952 CAS.
- X. Wu, M. Wen, Y. Zou, X. Gao, C. Wei, R. Liu, J. Li, L. Wang, X. Li, Y. N. Liu and W. Chen, Chem. Sci., 2022, 13, 6842–6851 RSC.
- Y. Chang, Y. Cheng, R. Zheng, X. Wu, P. Song, Y. Wang, J. Yan and H. Zhang, Nano Today, 2021, 38, 101110 CAS.
- L. Chen, H. Li, Z. Wu, L. Feng, S. Yu, H. Zhang, J. Gao, Y.-W. Mai and Y. Jia, Ceram. Int., 2020, 46, 16763–16769 CrossRef CAS.
- J. Wu, W. Mao, Z. Wu, X. Xu, H. You, A. Xue and Y. Jia, Nanoscale, 2016, 8, 7343–7350 RSC.
- Y. Wang, S. Wang, Y. Meng, Z. Liu, D. Li, Y. Bai, G. Yuan, Y. Wang, X. Zhang, X. Li and X. Deng, Nat. Commun., 2022, 13, 4419 CrossRef CAS PubMed.
- Y. Xia, Y. Jia, W. Qian, X. Xu, Z. Wu, Z. Han, Y. Hong, H. You, M. Ismail, G. Bai and L. Wang, Metals, 2017, 7, 122 CrossRef.
- W. Qian, Z. Wu, Y. Jia, Y. Hong, X. Xu, H. You, Y. Zheng and Y. Xia, Electrochem. Commun., 2017, 81, 124–127 CrossRef CAS.
- Z. Wu, S. Wu, S. Hong, X. Shi, D. Guo, Y. Zhang, X. Xu, Z. Chen and Y. Jia, Nanomaterials, 2022, 12, 4091 CrossRef CAS PubMed.
- X. Lin, J. Ding, X. Li, Z. Tang, H. Chen, H. Dong, A. Wu and L. Jiang, Dalton Trans., 2023, 52, 14917–14927 RSC.
- Z. Wu, X. Shi, T. Liu, X. Xu, H. Yu, Y. Zhang, L. Qin, X. Dong and Y. Jia, Nanomaterials, 2023, 13, 1124 CrossRef CAS PubMed.
- F. Chen, J. Guo, D. Meng, Y. Wu, R. Sun and C. Zhao, Catalysts, 2021, 11, 1370 CrossRef CAS.
- J. Wu, N. Qin, B. Yuan, E. Lin and D. Bao, ACS Appl. Mater. Interfaces, 2018, 10, 37963–37973 CAS.
- H. You, Z. Wu, L. Wang, Y. Jia, S. Li and J. Zou, Chemosphere, 2018, 199, 531–537 CrossRef CAS PubMed.
- Z. Wu, W. Luo, H. Zhang and Y. Jia, Appl. Surf. Sci., 2020, 513, 145630 CrossRef CAS.
- A. Kakekhani and S. Ismail-Beigi, Phys. Chem. Chem. Phys., 2016, 18, 19676–19695 RSC.
- X. Xu, L. Xiao, Y. Jia, Z. Wu, F. Wang, Y. Wang, N. O. Haugen and H. Huang, Energy Environ. Sci., 2018, 11, 2198–2207 Search PubMed.
- M. Xie, S. Dunn, E. L. Boulbar and C. R. Bowen, Int. J. Hydrogen Energy, 2017, 42, 23437–23445 CrossRef CAS.
- C. Feng, Z. P. Wu, K. W. Huang, J. Ye and H. Zhang, Adv. Mater., 2022, 34, e2200180 CrossRef PubMed.
- M. Zhang, Q. Hu, K. Ma, Y. Ding and C. Li, Nano Energy, 2020, 73, 104810 CrossRef CAS.
- S. Sun, L. Song, S. Zhang, H. Sun and J. Wei, Ceram. Int., 2021, 47, 20486–20493 CrossRef CAS.
- L. Song, S. Sun, S. Zhang and J. Wei, Fuel, 2022, 324, 124576 CrossRef CAS.
- Y. Liu, X. Wang, Y. Qiao, M. Min, L. Wang, H. Shan, Y. Ma, W. Hao, P. Tao, W. Shang, J. Wu, C. Song and T. Deng, ACS Sustain. Chem. Eng., 2018, 7, 2602–2609 CrossRef.
- L. Wang, H. Wang, Y. Liu, X. Wang, P. Tao, W. Shang, B. Fu, C. Song and T. Deng, RSC Adv., 2020, 10, 22616–22621 RSC.
- L. Wang, H. Wang, X. Wang, P. Tao, W. Shang, B. Fu, C. Song and T. Deng, ChemistrySelect, 2021, 6, 11224–11230 Search PubMed.
- M. Min, Y. Liu, C. Song, D. Zhao, X. Wang, Y. Qiao, R. Feng, W. Hao, P. Tao, W. Shang, J. Wu and T. Deng, ACS Appl. Mater. Interfaces, 2018, 10, 21246–21253 CrossRef CAS.
- Y. Zhou, Z.-Y. Dong, W.-P. Hsieh, A. F. Goncharov and X.-J. Chen, Nat. Rev. Phys., 2022, 4, 319–335 CrossRef CAS.
- J. Huang, B. He, Z. Zhang, Y. Li, M. Kang, Y. Wang, K. Li, D. Wang and B. Z. Tang, Adv. Mater., 2020, 32, e2003382 Search PubMed.
- H. You, S. Li, Y. Fan, X. Guo, Z. Lin, R. Ding, X. Cheng, H. Zhang, T. W. B. Lo, J. Hao, Y. Zhu, H. Y. Tam, D. Lei, C. H. Lam and H. Huang, Nat. Commun., 2022, 13, 6144 CrossRef CAS PubMed.
- Y. Zhao, W. Gao, S. Li, G. R. Williams, A. H. Mahadi and D. Ma, Joule, 2019, 3, 920–937 CrossRef CAS.
- M. Sayed, J. Yu, G. Liu and M. Jaroniec, Chem. Rev., 2022, 122, 10484–10537 CrossRef CAS PubMed.
- Q. Fu, X. Wang, C. Li, Y. Sui, Y. Han, Z. Lv, B. Song and P. Xu, RSC Adv., 2016, 6, 108883–108887 RSC.
- C. Hu, F. Chen and H. Huang, Angew. Chem., Int. Ed., 2023, 62, e202312895 CrossRef CAS PubMed.
- S. Li, L. Bai, N. Ji, S. Yu, S. Lin, N. Tian and H. Huang, J. Mater. Chem. A, 2020, 8, 9268–9277 RSC.
- J. Guan, Y. Jia, J. Cao, G. Yuan, S. Huang, X. Cui, G. Li and Z. Wu, Ceram. Int., 2022, 48, 3695–3701 CrossRef CAS.
- M. Sharma and R. Vaish, J. Am. Ceram. Soc., 2020, 104, 45–56 CrossRef.
- J. Ma, L. Chen, Z. Wu, J. Chen, Y. Jia and Y. Hu, Ceram. Int., 2019, 45, 11934–11938 CrossRef CAS.
- M. Sharma, V. P. Singh, S. Kumar and R. Vaish, J. Appl. Phys., 2020, 127, 135103 Search PubMed.
- J. Ma, Y. Jia, L. Chen, Y. Zheng, Z. Wu, W. Luo, M. Jiang, X. Cui and Y. Li, J. Cleaner Prod., 2020, 276, 124218 CAS.
- S. Zhang, D. Chen, Z. Liu, M. Ruan and Z. Guo, Appl. Catal., B, 2021, 284, 119686 CAS.
- S. Zhang, B. Zhang, D. Chen, Z. Guo, M. Ruan and Z. Liu, Nano Energy, 2021, 79, 105485 CAS.
- J. Chen, W. Luo, S. Yu, X. Yang, Z. Wu, H. Zhang, J. Gao, Y.-W. Mai, Y. Li and Y. Jia, Ceram. Int., 2020, 46, 9786–9793 CAS.
- S. Xu, W. Zhu, L. Wu, X. Zhang, C. Li, Y. Wang and Y. Yang, ACS Appl. Mater. Interfaces, 2023, 15, 1276–1285 CAS.
- Z. Zhang, H. Hao, H. Yang, Y. Hu, J. Min, G. Zhang, J. Bi, S. Yan, H. Li and H. Hou, Mater. Sci. Eng., B, 2022, 279, 115678 CAS.
- M. Li, J. Sun, G. Chen, S. Wang and S. Yao, Adv. Powder Mater., 2022, 1, 100032 Search PubMed.
- Z. Qiao, Z. Liu, W. Yan, M. Ruan, Z. Guo and X. Wu, J. Alloys Compd., 2022, 892, 162203 CAS.
- Y. Zhao, Z. Liu, M. Ruan and Z. Guo, ChemistrySelect, 2022, 7, e202202373 CAS.
- Y. Zhao, C. Wang and Z. Liu, J. Alloys Compd., 2023, 944, 169221 CAS.
- Y. Yang and Z. L. Wang, Nano Energy, 2015, 14, 245–256 CAS.
- W. Zhao, Q. Zhang, H. Wang, J. Rong, L. E and Y. Dai, Nano Energy, 2020, 73, 104783 CrossRef CAS.
- T. Li, Y. Zou and Z. Liu, Appl. Catal., B, 2023, 328, 122486 CrossRef CAS.
- X. Zhong, Y. Yang, X. Wang and Z. L. Wang, Nano Energy, 2015, 13, 771–780 CrossRef CAS.
- T. Quan, X. Wang, Z. L. Wang and Y. Yang, ACS Nano, 2015, 9, 12301–12310 CrossRef CAS PubMed.
- S. Wang, X. Wang, Z. L. Wang and Y. Yang, ACS Nano, 2016, 10, 5696–5700 CrossRef CAS PubMed.
- Y. Wu, X. Zhong, X. Wang, Y. Yang and Z. L. Wang, Nano Res., 2014, 7, 1631–1639 CrossRef CAS.
- S. Liu, B. Zhang, L. E, D. Zhao and Z. Liu, Int. J. Hydrogen Energy, 2023, 48, 27269–27279 CrossRef CAS.
- Q. Yang, J. Du, X. Nie, D. Yang, L. Bian, L. Yang, F. Dong, H. He, Y. Zhou and H. Yang, ACS Catal., 2021, 11, 1242–1247 CrossRef CAS.
- X. Tan, J. Liang, H. Yu, Y. Chen, L. Shan, S. Ge, L. Zhang, L. Li and J. Yu, Chem. Eng. J., 2023, 471, 144626 CrossRef CAS.
- W. Liu, J. Xie, X. Fang, D. Wang and J. Wang, Chem. Eng. J., 2024, 483, 149262 CrossRef CAS.
- Y. R. Zheng, P. Wu, M. R. Gao, X. L. Zhang, F. Y. Gao, H. X. Ju, R. Wu, Q. Gao, R. You, W. X. Huang, S. J. Liu, S. W. Hu, J. Zhu, Z. Li and S. H. Yu, Nat. Commun., 2018, 9, 2533 CrossRef PubMed.
- S. Liu, Z. Liu and Y. Meng, New J. Chem., 2022, 46, 17292–17302 RSC.
- Y. Wu, M. Ruan, Z. Guo, C. Wang and Z. Liu, Appl. Catal., B, 2023, 339, 123169 CrossRef CAS.
- P. Wang, Z. Shen, Y. Xia, H. Wang, L. Zheng, W. Xi and S. Zhan, Adv. Funct. Mater., 2019, 29, 1807013 CrossRef.
- H. Guo, C. Y. Yang, X. Zhang, A. Motta, K. Feng, Y. Xia, Y. Shi, Z. Wu, K. Yang, J. Chen, Q. Liao, Y. Tang, H. Sun, H. Y. Woo, S. Fabiano, A. Facchetti and X. Guo, Nature, 2021, 599, 67–73 CrossRef CAS PubMed.
- A. Zhang, Y. Liang, H. Zhang, Z. Geng and J. Zeng, Chem. Soc. Rev., 2021, 50, 9817–9844 RSC.
- M. Goswami, S. Mandal and V. K. Pillai, Sci. Rep., 2023, 13, 5182 CrossRef CAS PubMed.
- X. Luo, Z. Yan, H. Luo, X. Zhou, B. Li, M. Zhang and D. Zhang, Adv. Powder Mater., 2023, 2, 100116 CrossRef.
- Y. Jia, Y. Zhang, H. Xu, J. Li, M. Gao and X. Yang, ACS Catal., 2024, 14, 4601–4637 CrossRef CAS.
- Y. Liu, Y. N. Jiang, M. Zhang, X. Zhang and Y. Ma, ACS Appl. Mater. Interfaces, 2024, 16, 12455–12466 CrossRef CAS PubMed.
- W. Zhao, M. Adeel, P. Zhang, P. Zhou, L. Huang, Y. Zhao, M. A. Ahmad, N. Shakoor, B. Lou, Y. Jiang, I. Lynch and Y. Rui, Environ. Sci.: Nano, 2022, 9, 61–80 Search PubMed.
- M. Abbas, K. Hussain, N. H. Shah, M. Ilyas, R. Batool, M. A. Ahmad, Y. Cui and Y. Wang, J. Mater. Sci. Technol., 2024, 197, 160–170 CAS.
- W. Luo, J. Ying, S. Yu, X. Yang, Y. Jia, M. Chen, H. Zhang, J. Gao, Y. Li, Y.-W. Mai and Z. Wu, Ceram. Int., 2020, 46, 12096–12101 CrossRef CAS.
- L. Sun, S. Zhao, S. Gao, R. Zhu, Y. Tan, X. Tang and H. Yi, Green Energy Environ., 2025, 10, 84–108 CAS.
- H. Li, J. Li, Z. Ai, F. Jia and L. Zhang, Angew. Chem., Int. Ed., 2018, 57, 122–138 Search PubMed.
- Z. Y. Yu, Y. Duan, J. D. Liu, Y. Chen, X. K. Liu, W. Liu, T. Ma, Y. Li, X. S. Zheng, T. Yao, M. R. Gao, J. F. Zhu, B. J. Ye and S. H. Yu, Nat. Commun., 2019, 10, 2799 Search PubMed.
- G. Ji, L. Ji, S. Wu, L. Meng, Y. Jia, Z. Liu, S. Dong, J. Tian and Y. Li, Adv. Powder Mater., 2024, 3, 100188 Search PubMed.
- J. Hu, L. Yu, J. Deng, Y. Wang, K. Cheng, C. Ma, Q. Zhang, W. Wen, S. Yu, Y. Pan, J. Yang, H. Ma, F. Qi, Y. Wang, Y. Zheng, M. Chen, R. Huang, S. Zhang, Z. Zhao, J. Mao, X. Meng, Q. Ji, G. Hou, X. Han, X. Bao, Y. Wang and D. Deng, Nat. Catal., 2021, 4, 242–250 Search PubMed.
- F. Studt, Nat. Catal., 2021, 4, 184–185 CAS.
- M. Zhu, P. Tian, X. Cao, J. Chen, T. Pu, B. Shi, J. Xu, J. Moon, Z. Wu and Y.-F. Han, Appl. Catal., B, 2021, 282, 119561 CAS.
- J. Tian, J. Li, Y. Guo, Z. Liu, B. Liu and J. Li, Adv. Powder Mater., 2024, 3, 100201 Search PubMed.
- Z. Liu, Z. Qiao, Z. Guo, M. Ruan and W. Yan, ChemCatChem, 2022, 14, e202200357 CAS.
- Z. Qiao, C. Wang, Y. Zou, X. Wu and Z. Liu, Colloids Surf., A, 2022, 647, 129073 CAS.
- J. Yang, Y. Wang, M. J. Lagos, V. Manichev, R. Fullon, X. Song, D. Voiry, S. Chakraborty, W. Zhang, P. E. Batson, L. Feldman, T. Gustafsson and M. Chhowalla, ACS Nano, 2019, 13, 9958–9964 Search PubMed.
- F. Zhang, Y. Zhu, Q. Lin, L. Zhang, X. Zhang and H. Wang, Energy Environ. Sci., 2021, 14, 2954–3009 Search PubMed.
- L. Li, X. Dai, M. Lu, C. Guo, S. M. Wabaidur, X.-L. Wu, Z. Lou, Y. Zhong and Y. Hu, Adv. Powder Mater., 2024, 3, 100170 Search PubMed.
- X. Xu, S. Chen, Z. Wu, Y. Jia, L. Xiao and Y. Liu, Nano Energy, 2018, 50, 581–588 CAS.
- J. Ma, Z. Wu, W. Luo, Y. Zheng, Y. Jia, L. Wang and H. Huang, Ceram. Int., 2018, 44, 21835–21841 CAS.
- F. Yu, H. Zhou, Y. Huang, J. Sun, F. Qin, J. Bao, W. A. Goddard 3rd, S. Chen and Z. Ren, Nat. Commun., 2018, 9, 2551 Search PubMed.
- Z. Yu, K. Mao and Y. Feng, J. Adv. Ceram., 2021, 10, 1338–1349 Search PubMed.
- L. Hu, J. Chen, Y. Wei, M. Wang, Y. Xu, C. Wang, P. Gao, Y. Liu, C. Liu, Y. Song, N. Ding, X. Liu and R. Wang, J. Hazard. Mater., 2023, 442, 130059 CAS.
- X. Li, Y. Hu, F. Dong, J. Huang, L. Han, F. Deng, Y. Luo, Y. Xie, C. He, Z. Feng, Z. Chen and Y. Zhu, Appl. Catal., B, 2023, 325, 122341 CrossRef CAS.
- H. Wang, Y. Jia, T. Xu, X. Shu, Y. He, S. Huang, G. Yuan, X. Cui, G. Li and Z. Wu, Ceram. Int., 2022, 48, 10498–10505 CrossRef CAS.
- J. Low, J. Yu, M. Jaroniec, S. Wageh and A. A. Al-Ghamdi, Adv. Mater., 2017, 29, 1601694 CrossRef PubMed.
- S. H. Park, T. Kim, A. N. Kadam, C. Bathula, A. A. Ghfar, H. Kim and S.-W. Lee, Surf. Interfaces, 2022, 30, 101910 Search PubMed.
- Q. Xu, L. Zhang, B. Cheng, J. Fan and J. Yu, Chem, 2020, 6, 1543–1559 CAS.
- A. Wang, M. Du, J. Ni, D. Liu, Y. Pan, X. Liang, D. Liu, J. Ma, J. Wang and W. Wang, Nat. Commun., 2023, 14, 6733 CrossRef CAS PubMed.
- H. Wang, L. Zhang, Z. Chen, J. Hu, S. Li, Z. Wang, J. Liu and X. Wang, Chem. Soc. Rev., 2014, 43, 5234–5244 Search PubMed.
- R. Ji, Z. Zhang, Y. J. Hofstetter, R. Buschbeck, C. Hänisch, F. Paulus and Y. Vaynzof, Nat. Energy, 2022, 7, 1170–1179 CrossRef CAS.
- X. Yan, J. H. Qian, J. Ma, A. Zhang, S. E. Liu, M. P. Bland, K. J. Liu, X. Wang, V. K. Sangwan, H. Wang and M. C. Hersam, Nat. Electron., 2023, 6, 862–869 Search PubMed.
- F. Sun, D. Xu, J. Xu, Y. Xie, H. Qi, F. Liu, H. Shao, H. Yu, W. Yu and X. Dong, J. Mater. Sci. Technol., 2025, 208, 278–291 Search PubMed.
- X. Luo, Y. Zhang, L. Liu, A. Berbille, K. Wang, G. Han, L. Zhu and Z. L. Wang, J. Mater. Sci. Technol., 2025, 206, 125–134 CAS.
- L. Wang, N. O. Haugen, Z. Wu, X. Shu, Y. Jia, J. Ma, S. Yu, H. Li and Q. Chai, Ceram. Int., 2019, 45, 90–95 CAS.
- Z. Wu, T. Xu, L. Zhang, T. Liu, Z. Wu, G. Zhu and Y. Jia, J. Adv. Ceram., 2024, 13, 44–52 CAS.
- Z. Qiao, Z. Liu, M. Ruan, Z. Guo, W. Yan and X. Wu, ChemPhotoChem, 2021, 5, 1106–1118 CAS.
- Y. Meng, Y. Zhao and Z. Liu, Ceram. Int., 2023, 49, 31144–31151 CAS.
- C. Xie, Z. Niu, D. Kim, M. Li and P. Yang, Chem. Rev., 2020, 120, 1184–1249 CAS.
- H. Fu, Z. Chen, X. Chen, F. Jing, H. Yu, D. Chen, B. Yu, Y. H. Hu and Y. Jin, Adv. Sci., 2023, e2306132 Search PubMed.
- J. Zhu, J. Li, R. Lu, R. Yu, S. Zhao, C. Li, L. Lv, L. Xia, X. Chen, W. Cai, J. Meng, W. Zhang, X. Pan, X. Hong, Y. Dai, Y. Mao, J. Li, L. Zhou, G. He, Q. Pang, Y. Zhao, C. Xia, Z. Wang, L. Dai and L. Mai, Nat. Commun., 2023, 14, 4670 CAS.
- A. Venugopal, L. H. T. Egberts, J. Meeprasert, E. A. Pidko, B. Dam, T. Burdyny, V. Sinha and W. A. Smith, ACS Energy Lett., 2022, 7, 1586–1593 CAS.
- B. Yang, H. Chen, Y. Yang, L. Wang, J. Bian, Q. Liu and X. Lou, Chem. Eng. J., 2021, 416, 128986 CAS.
- J. Yu, Z. Li, T. Liu, S. Zhao, D. Guan, D. Chen, Z. Shao and M. Ni, Chem. Eng. J., 2023, 460, 141674 CAS.
- L. Liu and A. Corma, Nat. Rev. Chem, 2021, 5, 256–276 CAS.
- P. Dong, G. Hou, X. Xi, R. Shao and F. Dong, Environ. Sci.: Nano, 2017, 4, 539–557 CAS.
- J. Kröger, A. Jiménez-Solano, G. Savasci, V. W. h. Lau, V. Duppel, I. Moudrakovski, K. Küster, T. Scholz, A. Gouder, M. L. Schreiber, F. Podjaski, C. Ochsenfeld and B. V. Lotsch, Adv. Funct. Mater., 2021, 31, 2102468 CrossRef.
- Y. Wu, M. Ruan, Z. Guo, C. Wang and Z. Liu, J. Phys. Chem. C, 2023, 127, 8493–8502 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2025 |

