Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Strategic integration of nitroimino and dinitromethyl explophores onto tetrazole: K2DNMNAT as a material with enhanced thermal stability and optimized oxygen balance†
Parul Sainia,
Jatinder Singh a,
Richard J. Staples
a,
Richard J. Staples b and
Jean'ne M. Shreeve
b and
Jean'ne M. Shreeve *a
*a
aDepartment of Chemistry, University of Idaho, Moscow, ID 83844-2343, USA. E-mail: jshreeve@uidaho.edu
bDepartment of Chemistry, Michigan State University, East Lansing, Michigan 48824, USA
First published on 6th May 2025
Abstract
Enhancing the energy output of tetrazole-based materials through nitro functionalization often compromises thermal stability, posing a significant challenge in developing advanced energetic materials. In this study, we address this issue by strategically integrating both nitroimino and dinitromethyl high-energy functional groups onto the tetrazole ring, along with the incorporation of two potassium ions, yielding dipotassium 4-(dinitromethaneidyl)-5-(nitroimino)-4,5-dihydrotetrazol-1-ide (K2DNMNAT). Structural characterization confirmed the successful one-step nitration, while thermal and energetic assessments demonstrated an optimal balance between stability and performance. Comparative analysis with previously reported tetrazole-based nitroimino salts reveals that K2DNMNAT exhibits superior oxygen balance without compromising the decomposition temperature. Furthermore, when evaluated against tetrazole-based dinitromethyl-containing compounds, remarkable thermal resilience and enhanced energetic properties are found. Sensitivity tests indicate that this new nitroimino tetrazole exhibits mechanical stability within established safety thresholds in comparison with potassium-based primary explosives. Combining a straightforward synthesis, potassium-assisted stabilization, improved oxygen balance, and robust thermal stability positions, K2DNMNAT is seen to be a promising contender for next-generation energetic materials with enhanced performance and safety.
Introduction
Tetrazole and its derivatives have garnered significant attention in the field of energetic materials due to their high nitrogen content (80%) and favorable positive heat of formation (ΔHf = 4.77 kJ g−1). These characteristics make tetrazole a valuable backbone for high-performance energetic compounds, particularly for applications requiring high energy density, rapid decomposition rates, and environment friendliness.1–6 However, functionalizing the tetrazole ring remains a formidable challenge due to the limited number of reactive sites available for substitution. Traditionally, modifications have been restricted to the C5 and N1 positions, with some reported functionalization at both positions. Despite these efforts, expanding the tunability of tetrazole-based compounds for enhanced energetic performance while maintaining structural stability remains a crucial objective.7–28Due to the limited functionality of tetrazole, its modification has primarily relied on linear substitutions, incorporating energetic groups such as additional tetrazole rings, azo (–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–) linkages, and methylene (–CH2–) bridges. While these modifications have improved energetic performance in some cases, they often have led to reduced molecular density. Additionally, integrating high-energy groups, such as nitro (–NO2) or dinitromethyl [–C(NO2)2], frequently results in decreased thermal stability, thereby limiting their practical applications. This trade-off between energy and stability poses a major challenge in the design of next-generation environmentally friendly energetic materials. To overcome this limitation, an innovative and facile approach that strategically integrates high-energy functional groups without compromising thermal resilience is essential.
N–) linkages, and methylene (–CH2–) bridges. While these modifications have improved energetic performance in some cases, they often have led to reduced molecular density. Additionally, integrating high-energy groups, such as nitro (–NO2) or dinitromethyl [–C(NO2)2], frequently results in decreased thermal stability, thereby limiting their practical applications. This trade-off between energy and stability poses a major challenge in the design of next-generation environmentally friendly energetic materials. To overcome this limitation, an innovative and facile approach that strategically integrates high-energy functional groups without compromising thermal resilience is essential.
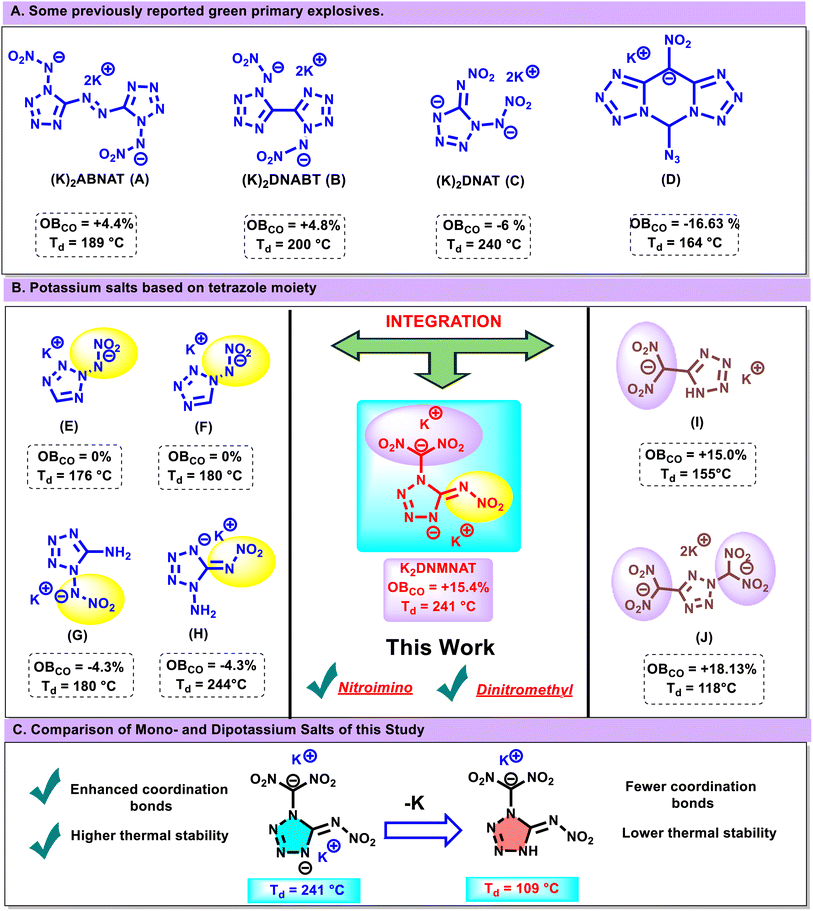
Coordination chemistry principles enable the precise organization of molecules or metal ions into stable, well-defined structures, allowing researchers to fine-tune these arrangements to optimize properties such as sensitivity, thermal stability, and energy output in energetic materials.29–33 Recent advances have focused on potassium salts with tetrazole backbones, since the tetrazole ring behaves as an environmentally friendly and versatile ligand, forming coordination bonds with various metal ions (Fig. 1A). Compounds such as 5,5′-azobis(1-nitroimino tetrazolate) (K2ABNAT), potassium dinitroimino-5,5′-bistetrazolate ((K)2DNABT), potassium 4,5-bis(dinitromethyl)furoxannate (K2BDNMF), and compound D (potassium 5-azido-10-nitro-bis(tetrazolo)[1,5-c:5′,1′-f]pyrimidinium) have shown promise as potential replacements for environmentally harmful lead azide-based primary explosives.34–38
(K2ABNAT), potassium dinitroimino-5,5′-bistetrazolate ((K)2DNABT), potassium 4,5-bis(dinitromethyl)furoxannate (K2BDNMF), and compound D (potassium 5-azido-10-nitro-bis(tetrazolo)[1,5-c:5′,1′-f]pyrimidinium) have shown promise as potential replacements for environmentally harmful lead azide-based primary explosives.34–38
Further investigations into potassium-substituted tetrazole compounds have revealed important insights into their thermal stability and oxygen balance. Potassium salts featuring a single nitroimino functionalization, such as compounds E (potassium 2-(N-nitramino)-5H-tetrazolate) and F (potassium 1-(N-nitramino)-5H-tetrazolate), exhibit decomposition temperatures of 176 °C and 180 °C, respectively, with an oxygen balance (OB) of 0% (Fig. 1B). However, attempts to enhance thermal resilience by introducing an amino (–NH2) group vicinal to nitroimino functionalities, as observed in compounds G (potassium 1-nitrimino-5-aminotetrazolate) and H (potassium 1-amino-5-nitriminotetrazolate), resulted in a decreased OB of −4.3%, further emphasizing the challenge of optimizing both stability and energetic output. Efforts to improve oxygen balance through the incorporation of dinitromethyl (–C(NO2)2) groups, as seen in compounds I (potassium 5-(dinitromethyl)-1H-tetrazolate) and J (dipotassium (2H-tetrazole-2,5-diyl)bis(dinitromethanide), failed to maintain adequate thermal stability, illustrating the inherent difficulty in achieving a synergy between oxygen balance and decomposition temperature. While increasing the number of nitro groups generally enhances oxygen balance, density, and detonation parameters—factors crucial for applications in rocket propulsion and high-performance explosives—the corresponding reduction in thermal stability remains a significant drawback. These findings underscore the pressing need for a rational functionalization strategy that maximizes both energy and structural integrity.39–43
In this study, tetrazole was functionalized strategically by the successful incorporation of both nitroimino and dinitromethyl functional groups onto the tetrazole ring, while simultaneously introducing potassium metal to enhance structural stability. The incorporation of potassium ions facilitated the formation of new coordination bonds, thereby contributing to the overall stability of the compound. This innovative strategy resulted in the synthesis of dipotassium 4-(dinitromethaneidyl)-5-(nitroimino)-4,5-dihydrotetrazol-1-ide (K2DNMNAT), which exhibits a remarkable balance of thermal stability and oxygen balance, addressing the limitations encountered in previous derivatives effectively.
Results and discussion
To synthesize K2DNMNAT, two strategies are considered. The first strategy involves the isolation of the sensitive nitramine compound 4 (Scheme 1). This route consists of four steps, including two nitration steps. In contrast, the second strategy begins with the facile synthesis of stable derivative 2. Upon the reaction of compound 2 with mixed acid (HNO3/H2SO4), double nitration occurs, resulting in the formation of compound 3. The crude mixture is treated directly with NH2OH·HCl/KOH to yield K2DNMNAT in quantitative yield.44,45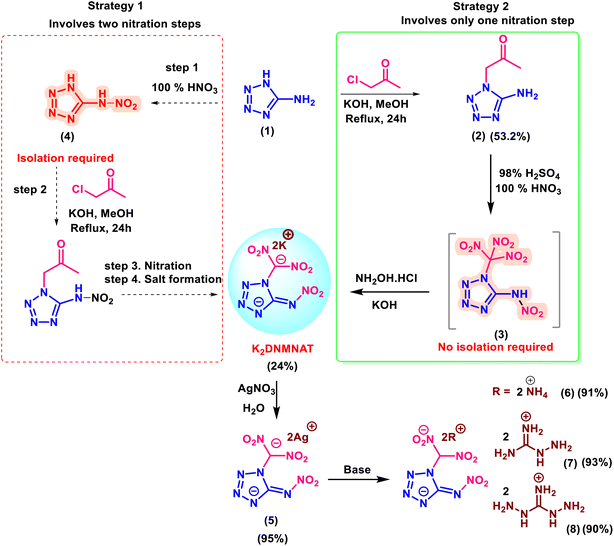 | ||
| Scheme 1 Synthesis of dipotassium-4-(dinitromethaneidyl)-5-(nitroimino)-4,5-dihydrotetrazol-1-ide (K2DNMNAT) and its salts (5–8). | ||
K2DNMNAT is reacted with AgNO3 to make the corresponding silver salt (5). The subsequent treatment of 5 with NH4Cl gives the ammonium salt (6). The energetic salts 7 and 8 were synthesized by reacting compound K2DNMNAT with aminoguanidine·HCl and diamminoguanidine·HCl, respectively. It is observed that neutralization of K2DNMNAT with dilute acid to make a neutral derivative resulted in the selective isolation of the mono potassium salt 9. Interestingly, compound 9 precipitates due to strong intramolecular hydrogen bonding (Scheme 2A).
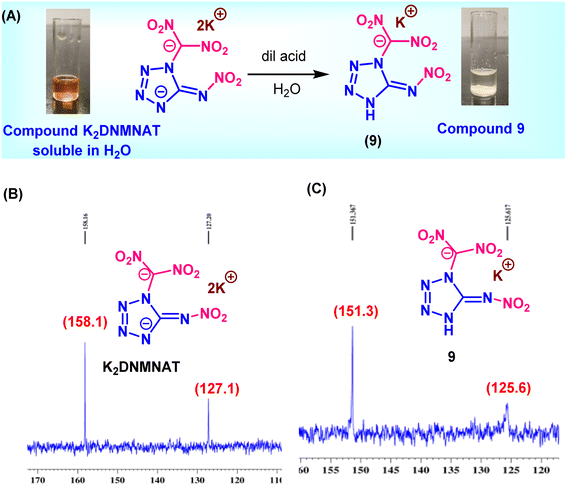 | ||
| Scheme 2 (A) Synthesis of potassium dinitro(5-(nitroimino)-4,5-dihydro-1H-tetrazol-1-yl) methanide (9). (B) 13C NMR of compound K2DNMNAT, (C) 13C NMR of compound 9. | ||
The characterization of compounds K2DNMNAT and 9 was performed using NMR spectroscopy. In the 13C NMR spectrum of compound K2DNMNAT, two distinct peaks were seen at 158.1 ppm (tetrazole carbon) and 127.1 ppm (dinitromethyl group carbon). In contrast, for compound 9, the tetrazole carbon peak is shifted to 151 ppm, while the dinitromethyl group carbon peak exhibited only a slight change to 125.6 (Schemes 2B and C). These results suggest that neutralization first occurs at the nitramine functional group rather than the dinitromethylene carbon.
Single-crystal X-ray crystallography
Attempts to obtain high-quality single crystals of compound K2DNMNAT suitable for X-ray diffraction were conducted using various organic solvents. However, the crystalline nature of the compound was lost upon solvent evaporation. Crystallization from DMSO yielded suitable single crystals with two molecules of DMSO. Compound K2DNMNAT·2DMSO crystallizes in the monoclinic space group P21/c with a calculated density of 1.785 g cm−3 at 101 K. As illustrated in Fig. 2A, the tetrazole ring and the nitroimino group are nearly coplanar, whereas the dinitromethyl group is oriented in a different plane. | ||
| Fig. 2 (A) Drawing at 50% ellipsoids of compound K2DNMNAT·2DMSO. (B) Drawing at 50% ellipsoids of compound 9·H2O. (DMSO and H2O are omitted for clarity). | ||
Compound 9·H2O crystallizes in the monoclinic space group C2/c with a crystal density of 2.025 g cm−3 at 100 K (Fig. 2B). As depicted in Fig. 2A, similar to the crystal structure of compound K2DNMNAT·2DMSO, the tetrazole and nitroimino groups in compound 9·H2O are nearly coplanar, while the nitromethyl group is positioned in a different plane. Hydrogen bonding interactions are observed with a maximum donor–acceptor (D⋯D) distance of 3.11 Å and a minimum bond angle of 110°. The specific hydrogen bonding interactions include O1W–O2_1: 3.006 Å, O1W–O4_2: 2.866 Å, O1W–O6_3: 2.728 Å, and N1–O1W_4: 2.626 Å.
Physicochemical and detonation properties
The thermal behavior of compounds K2DNMNAT, 6, 7·H2O, 8·2H2O, and 9·H2O was analyzed using differential scanning calorimetry (DSC) at a heating rate of 5 °C min−1 under an N2 atmosphere (Table 1). Among these, the dipotassium salt (K2DNMNAT) exhibited the highest decomposition temperature at 241 °C. The decomposition temperatures of compounds 6, 7·H2O, 8·2H2O, and 9·H2O are 145, 137, 119, and 107 °C, respectively.| Comp. | Tda (°C) | OBCO/CO2 (%) | ρb (g cm−3) | ΔHfc (kJmol−1) | Pd (GPa) | Dve (m s−1) | ISf (J) | FSg (N) |
|---|---|---|---|---|---|---|---|---|
| a Temperature (onset) of decomposition.b Density at 25 °C using gas pycnometer.c Molar enthalpy of formation, calculated using isodesmic reactions with the Gaussian 03 suite of programs (revision D.01).d Detonation pressure.e Detonation velocity (calculated using EXPLO5 version 7.01.01).f Sensitivity to impact (IS).g Sensitivity to friction (FS).h X-ray density calculated at room temperature (RT).i ref. 49.j ref. 50.k ref. 51.l ref. 32.m ref. 28. | ||||||||
| K2DNMNAT | 241 | +15.4/+5.1 | 2.08 | −106.7 | 24.6 | 7661 | 1 | 10 |
| 6 | 145 | 0/−11.9 | 1.75 | 157.9 | 33.2 | 8985 | 1 | 20 |
| 7·H2O | 137 | −19.9/−35.9 | 1.65 | 199.6 | 26.5 | 8471 | 1 | 40 |
| 8·2H2O | 119 | −21.4/−35.6 | 1.68 | 186.9 | 29.0 | 8835 | 2 | 40 |
| 9·H2O | 107 | +16.5/+5.5 | 1.96h | −6.4 | 30.2 | 8447 | 3 | 20 |
| LAi | 315 | −11/−11 | 4.08 | 450.1 | 33.8 | 5920 | 2.5–4 | 1 |
| LSj | 282 | −5.7/−8.0 | 3.06 | −835 | — | 5200 | 2.5–5 | 1.5 |
| KDNPk | 260 | 0/−34.2 | 1.95 | −461.7 | 20.01 | 6952 | 0.05 | 9.8 |
| DTAT-Kl | 163.6 | −19.4/−38.4 | 1.88 | 326.4 | 31.7 | 7917 | 1 | 20 |
| (K)2DNABTm | 200 | 4.8/−4.8 | 2.11 | 970.97 | 25.2 | 8330 | 5 | <1 |
Based upon peak decomposition temperatures measured by differential scanning calorimetry (DSC) for compound K2DNMNAT at different heating rates of 5, 10, 15, and 20 °C min−1 (Fig. 3A), the activation energies (Ek and E0), and linear correlation coefficients (Rk and R0) were calculated using Kissinger's46 and Ozawa's47 methods and are given in Tables S5 and S6 (ESI).† The results show that the activation energy calculated by either method is essentially the same (Ek = 190.4 kJ mol−1; E0 = 189.3 kJ mol−1). Depending upon the calculated numerical values of Ek, the rate constant of decomposition can be expressed as ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) k = ln
k = ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ak − (190.4 × 103)/RT. The low activation energy of K2DNMNAT, which falls in the range of reported primary explosives, indicates that it can be easily stimulated for intense chemical reactions.48
Ak − (190.4 × 103)/RT. The low activation energy of K2DNMNAT, which falls in the range of reported primary explosives, indicates that it can be easily stimulated for intense chemical reactions.48
 | ||
| Fig. 3 (A) DSC plots of K2DNMNAT at different heating rates. (B) Comparison graph of oxygen balance and thermal stability for A–J and K2DNMNAT. | ||
The comparative study of oxygen balance and thermal stability of K2DNMNAT with previously reported potassium salts is presented in Fig. 3B. This study demonstrates that K2DNMNAT exhibits an optimal balance between energy and safety compared to the previously reported compounds (A–J). The Hirshfeld surface analyses for K2DNMNAT and 9 were performed and are shown in Fig. 4A and B.
 | ||
| Fig. 4 (A and B) Hirshfeld surfaces for K2DNMNAT and 9 (C and D) 2D fingerprint plots for compound K2DNMNAT and 9. | ||
On the edges of the Hirshfeld surface of K2DNMNAT and 9, many red regions (high close-contact population) were observed, which are mainly due to coordination bonds, i.e., K⋯O contacts, and contribute 26.7% and 20.3% for K2DNMNAT and 9, respectively to the total interactions (Fig. 3C and D). The larger value of K⋯O contacts stabilizing interactions enhances molecular stability. The impact and friction sensitivities of compounds K2DNMNAT, 6, 7·H2O, 8·2H2O, and 9·H2O were assessed using BAM standard methods, and the results are summarized in Table 1. All compounds demonstrated sensitivity to mechanical stimuli. The experimental densities of the compounds were measured using a gas pycnometer at a controlled temperature of 25 °C. Notably, compound K2DNMNAT exhibited better friction sensitivity (FS) than LA, LS, KDNP, and (K)2DNABT. K2DNMNAT also showed an oxygen balance (OB) of +15.5%, which is significantly higher than that of LA, LS, KDNP, DTAT-K, and (K)2DNABT, making it more environmentally favorable compared to these previously reported compounds. The experimental densities and calculated enthalpies of formation were further utilized in the EXPLO5 (v7.01.01) software to predict the detonation properties of these compounds. The results indicated that compound 6 exhibited the highest calculated detonation velocity of 8985 m s−1. K2DNMNAT's detonation velocity surpassed that of LA, LS, and KDNP, suggesting enhanced explosive characteristics. Its detonation velocity is also comparable to that of DTAT-K.
LA, LS, and KDNP demonstrate good thermal stability. They have lower oxygen balances and detonation velocities compared to K2DNMNAT. On the other hand, DTAT-K and (K)2DNABT exhibit better detonation velocities but have lower thermal stability and oxygen balance.
Experiments such as the “red-hot needle” and “heated plate” tests were conducted on a 10 mg sample of K2DNMNAT to evaluate its energetic behavior (Fig. 5A and B). The red-hot needle test revealed a sharp deflagration accompanied by a visible flame, as shown in Fig. 5A. Similarly, the heated plate test also demonstrated deflagration, confirming the energetic nature of the compound.
In conclusion, the synthesis of dipotassium 4-(dinitromethaneidyl)-5-(nitroimino)-4,5-dihydrotetrazol-1-ide (K2DNMNAT) illustrates a significant advance in the development of potassium metal-based energetic salts. The careful tuning of nitro groups has proven crucial in achieving a desirable equilibrium between energy output and stability, a challenge that continues to define the landscape of energetic materials. We have synthesized the 6, 7, and 9 energetic salts, which also show good energy properties. Furthermore, their compatibility with other energetic materials enhances their potential as promising candidates for new high-energy-density materials with practical applications.
Data availability
All data relevant to the work described here are available in the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
The diffractometer (Rigaku Synergy S) for SC-XRD was purchased with support from the National Science Foundation (MRI program) under grant no. 1919565. We are grateful to the Fluorine-19 fund for support.References
- J. Zhou, J. Zhang, B. Wang, L. Qiu, R. Xu and A. B. Sheremetev, FirePhysChem, 2024, 2, 83–139 CrossRef.
- J. Cai, T. Fei, R. Li, J. Xiong, J. Zhang, P. Yin and S. Pang, ACS Appl. Mater. Interfaces, 2022, 14, 52951–52959 CrossRef CAS PubMed.
- T. M. Klapötke, Chemistry of High-Energy Materials, Walter de Gruyter & Co, KG, Berlin-New York, 2012, 2nd edn Search PubMed.
- T. M. Klapötke, D. G. Piercey, N. Mehta, K. D. Oyler, M. Jorgensen, S. Lenahan, J. S. Salan, J. W. Fronabarger and M. D. Williams, Anorg. Allg. Chem., 2013, 639, 681–688 CrossRef.
- C. L. He and J. M. Shreeve, Angew. Chem., Int. Ed., 2016, 55, 772–775 CrossRef CAS.
- M. H. V. Huynh, M. A. Hiskey, T. J. Meyer and M. Wetzler, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 5409–5412 CrossRef CAS PubMed.
- M. Krawiec, S. R. Anderson, P. Dubé, D. D. Ford, J. S. Salan, S. Lenahan, N. Mehta and C. R. Hamilton, Propellants, Explos., Pyrotech., 2015, 40, 457–459 CrossRef CAS.
- L. Zhai, X. Fan, B. Wang, F. Bi, Y. Li and Y. Zhu, RSC Adv., 2015, 5, 57833–57841 RSC.
- J. A. Garrison and R. M. Herbst, J. Org. Chem., 1957, 22, 278–283 CrossRef CAS.
- X. Yu, J. Tang, C. Lei, C. Xue, G. Cheng, C. Xiao and H. Yang, J. Mater. Chem. A, 2024, 12, 19513–19520 RSC.
- X. Yu, J. Tang, C. Lei, C. Xue, H. Yang, C. Xiao and G. Cheng, J. Mater. Chem. A, 2024, 12, 29638–29644 RSC.
- J. Liu, Y. Dong, M. Li, Y. Liu, W. Huang, C. Xiao, G. Cheng and Y. Tang, J. Org. Chem., 2025, 90(11), 4054–4061 CrossRef CAS.
- B. Wang, X. Qi, W. Zhang, K. Wang, W. Lia and Q. Zhang, J. Mater. Chem. A, 2017, 5, 20867–20873 RSC.
- J. E. Zuckerman, M. C. St Myer, M. Zeller and D. G. Piercey, ChemPlusChem, 2025, 90, e202400164 CrossRef PubMed.
- J. Singh, R. J. Staples and J. M. Shreeve, J. Mater. Chem. A, 2023, 11, 12896–12901 RSC.
- Y. Yang, W. Zhang, S. Pang, H. Huang and C. Sun, J. Org. Chem., 2024, 89, 12790–12794 CrossRef CAS PubMed.
- F. Meng, R. Zhou, Z. Xu, P. Wang, Y. Xu and M. Lu, J. Org. Chem., 2025, 90, 3964–3973 CrossRef CAS PubMed.
- A. F. Tarchoun, D. Trache, T. M. Klapötke and K. Khimeche, Chem. Eng. J., 2020, 400, 125960 CrossRef CAS.
- N. Fischer, D. Fischer, T. M. Klapötke, D. G. Piercey and J. Stierstorfer, J. Mater. Chem., 2012, 22, 20418–20422 RSC.
- N. Fischer, D. Izsák, T. M. Klapötke, S. Rappengluck and J. Stierstorfer, Chem. - Eur. J., 2012, 18, 4051–4062 CrossRef CAS PubMed.
- N. Fischer, L. Gao, T. M. Klapötke and J. Stierstorfer, Polyhedron, 2013, 51, 201–210 CrossRef CAS.
- N. Fischer, T. M. Klapötke, M. Reymann and J. Stierstorfer, Eur. J. Inorg. Chem., 2013, 2013, 2167–2180 CrossRef CAS.
- K. Hafner, T. M. Klapötke, P. C. Schmid and J. Stierstorfer, Eur. J. Inorg. Chem., 2015, 2015, 2794–2803 CrossRef CAS.
- X. Wang, S. Jin, C. Zhang, L. Li, S. Chen and Q. Shu, Chin. J. Chem., 2015, 33, 1229–1234 CrossRef CAS.
- M. Benz, T. M. Klapötke and J. Stierstorfer, Org. Lett., 2022, 24, 1747–1751 CrossRef CAS.
- T. M. Klapötke, C. M. Sabaté and J. Stierstorfer, New J. Chem., 2009, 33, 136–147 RSC.
- J. A. Garrison and R. M. Herbst, J. Org. Chem., 1957, 22, 278–283 CrossRef CAS.
- P. Kumar, V. D Ghule and S. Dharavath, Dalton Trans., 2023, 52, 747–753 RSC.
- M. Jujam, R. Rajak and S. Dharavath, Adv. Funct. Mater., 2025, 35, 2412638 CrossRef CAS.
- J. Singh, A. K. Chinnam, R. J. Staples and J. M. Shreeve, Inorg. Chem., 2022, 61, 16493–16500 CrossRef CAS PubMed.
- S. Li, Y. Wang, C. Qi, X. Zhao, J. Zhang, S. Pang and S. Zhang, Angew. Chem., Int. Ed., 2013, 52, 14031–14035 CrossRef CAS.
- J. Zhang, Y. Du, K. Dong, H. Su, S. Zhang, S. Li and S. Pang, Chem. Mater., 2016, 28, 1472–1480 CrossRef CAS.
- J. Singh, R. J. Staples and J. M. Shreeve, J. Mater. Chem. A, 2025, 13, 11475–11485 RSC.
- T. M. Klapötke and J. Stierstorfer, Helv. Chim. Acta, 2007, 90, 2132–2150 CrossRef.
- D. Fischer, T. M. Klapötke and J. Stierstorfer, Angew. Chem., Int. Ed., 2014, 53, 8172–8175 CrossRef CAS PubMed.
- D. Fischer, T. M. Klapötke and J. Stierstorfer, Angew. Chem., Int. Ed., 2015, 54, 10299–10302 CrossRef CAS PubMed.
- Y.-N. Li, B.-Z. Wanga, Y.-J. Shu, L.-J. Zhai, S.-Y. Zhang, F.-Q. Bi and Y.-C. Li, Chin. Chem. Lett., 2017, 28, 117–120 CrossRef CAS.
- N. Szimhardt, M. H. H. Wurzenberger, P. Spieß, T. M. Klapötke and J. Stierstorfer, Propellants, Explos., Pyrotech., 2018, 43, 1203–1209 CrossRef CAS.
- M. Benz, T. M. Klapötke, J. Stierstorfer and M. Voggenreiter, ACS Appl. Eng. Mater., 2023, 1, 3–6 CrossRef CAS.
- Q. Yu, P. Yin, J. Zhang, C. He, G. H. Imler, D. A. Parrish and J. M. Shreeve, J. Am. Chem. Soc., 2017, 139, 8816–8819 CrossRef CAS.
- J. Singh, R. J. Staples and J. M. Shreeve, Sci. Adv., 2023, 9, eadk3754 CrossRef CAS.
- S. G. Zlotin, I. L. Dalinger, N. N. Makhova and V. A. Tartakovsky, Russ. Chem. Rev., 2020, 89, 1–54 CrossRef CAS.
- O. T. O'Sullivan and M. J. Zdilla, Chem. Rev., 2020, 120, 5682–5744 CrossRef.
- Q. Yu, G. H. Imler, D. A. Parrish and J. M. Shreeve, Org. Lett., 2019, 21, 4684–4688 CrossRef CAS PubMed.
- V. Thottempudi, H. Gao and J. M. Shreeve, J. Am. Chem. Soc., 2011, 133, 6464–6471 CrossRef CAS.
- H. E. Kissinger, Anal. Chem., 1957, 29, 1702–1706 CrossRef CAS.
- T. A. Ozawa, Bull. Chem. Soc. Jpn., 2006, 38, 1881–1886 CrossRef.
- D. J. Whelan, R. J. Spear and R. W. Read, Thermochim. Acta, 1984, 80, 149–163 CrossRef CAS.
- Q.-u.-N. Tariq, S. Manzoor, M.-u.-N. Tariq, W.-L. Cao and J.-G. Zhang, Def. Technol., 2022, 18, 1945–1959 CrossRef.
- M. A. Ilyushin, I. V. Tselinsky and I. V. Shugalei, Cent. Eur. J. Energ. Mater., 2012, 9, 293–328 CAS.
- K. Pandey, A. Tiwari, J. Singh, P. Bhatia, P. Das, D. Kumar and J. M. Shreeve, Org. Lett., 2024, 26, 1952–1958 CrossRef CAS PubMed.
Footnote |
| † CCDC 2430362 and 2430363. For crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d5ta02595h |
| This journal is © The Royal Society of Chemistry 2025 |