Dattatraya H. Dethe, Vijay Kumar B. and Rakesh Maiti
Org. Biomol. Chem., 2018,16, 4793-4796
DOI:
10.1039/C8OB01092G,
Communication
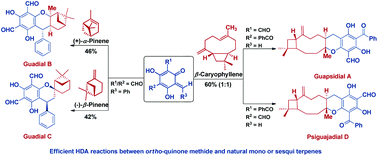
The first biomimetic total syntheses of chromane meroterpenoids, guadials B and C, guapsidial A and psiguajadial D have been completed. The key synthetic transformation involves an efficient and high yielding hetero-Diels–Alder reaction. The two structurally isomeric natural products, guadials B and C, were obtained from a common o-quinone methide in the separate reactions with α-pinene and β-pinene, respectively. The two regioisomeric natural products, guapsidial A and psiguajadial D, were achieved in a single chemical operation.