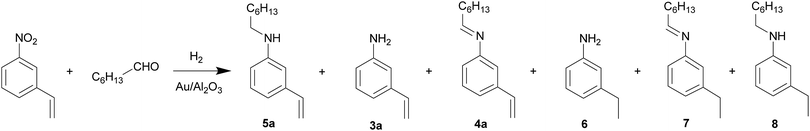
Synthesis of unsaturated secondary amines by direct reductive amination of aliphatic aldehydes with nitroarenes over Au/Al2O3 catalyst in continuous flow mode†
A. L. Nuzhdin*a,
E. A. Artiukhaab,
G. A. Bukhtiyarovaa,
S. Yu Zaytsevc,
P. E. Plyusninbc,
Yu V. Shubin bc and
V. I. Bukhtiyarovab
bc and
V. I. Bukhtiyarovab
aBoreskov Institute of Catalysis, SB RAS, Lavrentieva Ave. 5, Novosibirsk, 630090, Russia. E-mail: anuzhdin@catalysis.ru
bNovosibirsk State University, Pirogova Str. 2, Novosibirsk, 630090 Russia
cNikolaev Institute of Inorganic Chemistry, SB RAS, Lavrentieva Ave. 3, Novosibirsk, 630090 Russia
First published on 2nd September 2016
Abstract
A series of unsaturated secondary amines was successfully synthesized by direct reductive amination of aliphatic aldehydes with nitroarenes over a 2.5% Au/Al2O3 catalyst in a continuous flow reactor using molecular hydrogen as a reducing agent. In most cases, the targeted secondary amines were obtained in good to excellent yields. Interestingly, the hydrogenation of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C group is practically absent in both initial aldehydes and secondary amines under the reaction conditions. It was found that the introduction of electron-donating substituents in the para- and meta-position of nitrobenzenes increased the yield of secondary amine, while in the case of nitrobenzenes with electron-withdrawing substituents or electron-donating substituents in the ortho-position a decrease in the yield of the target product was observed.
C group is practically absent in both initial aldehydes and secondary amines under the reaction conditions. It was found that the introduction of electron-donating substituents in the para- and meta-position of nitrobenzenes increased the yield of secondary amine, while in the case of nitrobenzenes with electron-withdrawing substituents or electron-donating substituents in the ortho-position a decrease in the yield of the target product was observed.
Introduction
Secondary amines are important intermediates in pharmaceutical and agrochemical industries.1–3 Direct reductive amination of aldehydes with nitroarenes over metal catalysts (mainly based on Pd, Au and Pt) where several cascade reactions are carried out using one vessel under hydrogenation conditions is an attractive method for the synthesis of secondary aromatic amines. This approach allows the reduction of the number of synthetic steps as compared with traditional methods and minimizes waste production due to the use of molecular hydrogen as a reducing agent.2,3 The process starts with the hydrogenation of nitroarene 1 to the corresponding aniline 3, which reacts with aldehyde 2 to form imine 4 and further hydrogenation of 4 gives secondary amine 5 (Scheme 1).Various secondary aromatic amines containing alkyl, hydroxy, alkoxy, halogen, carbonyl and carboxylate groups have been obtained by the above mentioned reaction in good to excellent yields2,3 but the number of the works describing the successful synthesis of secondary amines containing C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C group is very limited3b,c because the carbon–carbon double bond is one of the most reactive functions in metal-catalyzed hydrogenation.4a To our knowledge, there are only two examples of such synthesis, and in both cases the reaction has been realized over supported gold catalysts. So, Au/TiO2 catalyst provides the one-pot synthesis of N-(3-vinylbenzyl)-aniline with a yield of about 83% by reaction between nitrobenzene and m-vinylbenzaldehyde in a batch reactor using two-stage procedure.3b Recently, we managed to realize the synthesis of N-heptyl-3-vinylaniline by direct reductive amination of n-heptaldehyde with m-nitrostyrene over Au/Al2O3 catalyst in a continuous flow reactor with a yield of 74%.3c This is a promising approach, since the reaction occurs in one step within a short time. In addition, the use of three-phase flow reactor allows an improvement of gas–liquid–solid interaction and reduction of the side products through a better control over process variables.5 Herein, we report the results of successful synthesis of a variety of unsaturated secondary amines by direct reductive amination of aliphatic aldehydes with nitroarenes over Au/Al2O3 catalyst in a flow reactor.
C group is very limited3b,c because the carbon–carbon double bond is one of the most reactive functions in metal-catalyzed hydrogenation.4a To our knowledge, there are only two examples of such synthesis, and in both cases the reaction has been realized over supported gold catalysts. So, Au/TiO2 catalyst provides the one-pot synthesis of N-(3-vinylbenzyl)-aniline with a yield of about 83% by reaction between nitrobenzene and m-vinylbenzaldehyde in a batch reactor using two-stage procedure.3b Recently, we managed to realize the synthesis of N-heptyl-3-vinylaniline by direct reductive amination of n-heptaldehyde with m-nitrostyrene over Au/Al2O3 catalyst in a continuous flow reactor with a yield of 74%.3c This is a promising approach, since the reaction occurs in one step within a short time. In addition, the use of three-phase flow reactor allows an improvement of gas–liquid–solid interaction and reduction of the side products through a better control over process variables.5 Herein, we report the results of successful synthesis of a variety of unsaturated secondary amines by direct reductive amination of aliphatic aldehydes with nitroarenes over Au/Al2O3 catalyst in a flow reactor.
Experimental
Chemicals
Hydrogen tetrachloroaurate HAuCl4 aq. (49.47 wt% Au) was purchased from Aurat (Russia) and used without further purification. Nitrobenzene (99%), o-nitrotoluene (99+%), m-nitrotoluene (99%), p-nitrotoluene (99%), p-chloronitrobenzene (99%), p-bromonitrobenzene (99%), p-nitrophenol (99%), p-nitroacetophenone (97%), 4-nitrophenyl acetate (97%), m-nitrostyrene (97%), undecylenic aldehyde (97%), n-heptaldehyde (95%), 3-methylcrotonaldehyde (97%), n-decane (99+%) from Acros Organics, as well as p-ethylnitrobenzene from Aldrich (95%), were used as supplied. Toluene (99.5%) from “ECOS” (Russia) was employed as solvent.Catalyst preparation
The γ-Al2O3 support (BET specific surface area: 162 m2 g−1, total pore volume: 0.69 mL g−1, mean pore diameter: 18.6 nm) was prepared by extrusion of a paste, obtained by mixing of 18 g of aluminum oxide (Puralox TH 100/150, Sasol), 42 g of aluminum hydroxide (Disperal 20, Sasol) and 40 mL of 0.5 wt% aqueous solution of nitric acid for peptization of alumina. The cylindrical Al2O3 granules of 1.5 mm in diameter thus obtained were dried at 110 °C overnight and calcined in a flow of air at 550 °C for 4 h. Then they were crushed and sieved to obtain support grains of 250–500 μm in diameter. The method for preparing the Au/Al2O3 catalyst was similar to those described previously:6 20 mL of 0.02 M HAuCl4 solution was adjusted to pH 7 with 0.5 M NaOH. The solution was added with stirring to 1.0 g of Al2O3 suspended in 60 mL of distilled H2O at 70 °C. After stirring for 2 h, the solid was filtered, washed by 50 mL of distilled water at room temperature, dried at 100 °C overnight, and calcined in air at 350 °C for 1 h.Catalyst characterization
The transmission electron microscopy (TEM) studies were carried out using a JEM-2010 (JEOL, Japan) electron microscope with a lattice resolution of 0.14 nm and a 200 kV accelerating voltage. The mean diameters of gold particles for each catalyst sample were determined by counting of 250–300 particles in TEM images taken with a medium magnification. XRD patterns of Au/γ-Al2O3 samples were recorded on a Shimadzu XRD-7000 diffractometer using CuKα radiation (λ = 0.15418 nm) and Ni filter on the reflected beam. The gold content in the samples was measured by Atomic absorption spectroscopy on Hitachi Z-8000 instrument. The simultaneous TG-DSC-MS measurement was performed in an apparatus consisting of a STA 449 F1 Jupiter thermal analyzer and a QMS 403D Aëolos quadrupole mass spectrometer (NETZSCH, Germany). The measurements were made in a “synthetic air” flow (80 vol% Ar and 20 vol% O2) in the temperature range of 30–800 °C using the heating rate of 10 °C min−1, the gas flow rate of 25 mL min−1 and open Al2O3 crucibles. The sample weight is 25 mg. Textural characteristics were determined from adsorption–desorption isotherms of nitrogen measured at 77 K by using an automatic volumetric device ASAP 2400 (Micromeritics).Typical procedure for reductive amination of aldehydes with nitroarenes over Au/Al2O3 catalyst
The investigation of the catalytic properties was performed using commercially available continuous flow device H-Cube Pro™ (Thalesnano, Hungary).5a A scheme of the catalytic setup is shown in Fig. 1. The liquid feed was introduced into the reactor by an HPLC pump in up-flow mode after mixing with the hydrogen flow, generated by a built-in electrolytic cell. The packed bed cartridge-like reactors CatCart®30 or CatCart®70 with an inner diameter of 4 mm and length of 30 mm or 70 mm were used.Before the experiment, the cartridge containing the catalyst (0.200 g for CatCart®30 or 0.550 g for CatCart®70) was installed in a heater unit, and the mixture of toluene with hydrogen was fed to the reactor with the rate of 0.5 mL min−1 until the required reaction parameters (temperature, pressure, flow rates of hydrogen) were reached. Afterwards, the inlet was switched to the flask with the substrate's solution and this moment was chosen as the starting point of the reaction. The solutions of nitroarene (0.025 M) and aldehyde (0.030 M or 0.0375 M) in toluene were used in the experiments with n-decane as the internal standard. In the standard experiment, the reaction was carried out at 50 bars of hydrogen pressure, 0.5 mL min−1 liquid feed rate, 60 mL min−1 hydrogen flow rate, and the temperature was changed in the range of 50–100 °C. The performance of the catalyst was evaluated by averaging the 2 samples taken at 30–33 min and 33–36 min after the start of the experiment. The novel batch of catalyst was used in each experiment.
The reaction products were analyzed by gas chromatography (Agilent 6890N instrument with a 19091S-416 HP 5-MS capillary column 60.0 m × 320 μm × 0.25 μm). The conversion of aldehydes was calculated using n-decane as the internal standard. Yields of N-containing products were calculated using GC data as the ratio of the product concentration to the sum of the concentrations of all products containing nitrogen (complete conversion of nitroarenes was observed in all experiments). The identification of the reaction products were performed by GC-MS on an Agilent 7000B Triple Quad System. In some cases the main reaction product was purified by silica gel column chromatography and characterized by 1H and 13C NMR spectroscopy (NMR spectra can be found in ESI†).
Catalyst regeneration
After the reaction was finished, a flow of pure toluene (0.5 mL min−1) was fed into the reactor instead of a reaction mixture, and the catalyst sample was washed for 30 min, and then completely dumped out from the reactor. To remove toluene the sample was placed in the vacuum heat chamber at room temperature, evacuated to 5 mbar, slowly heated up to 80 °C and dried at this temperature for 3 hours. Subsequently, the spent catalyst was heated in an air oven from room temperature to 330 °C at a heating rate of 1 °C min−1 and then maintained at this temperature for 20 hours.7Results and discussion
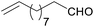
Au/Al2O3 catalyst containing 2.5 wt% Au with the mean Au particle diameter equal to 3.4 nm (Fig. 2) used herein was prepared in accordance with the so-called “deposition–precipitation” method.6 The catalyst has been studied in the reductive amination of unsaturated aliphatic aldehydes such as undecylenic aldehyde and 3-methylcrotonaldehyde with various nitroarenes (Tables 1 and 2) as well as in the reaction between m-nitrostyrene and n-heptaldehyde (Table 3). It should be noted that the complete conversion of nitroarenes was observed in all experiments. Simultaneously with the main process aldehydes 2 could produce the corresponding alcohols in a side reaction3c therefore an excess of the aldehyde to nitroarene was used. Interestingly, the hydrogenation of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C group is practically absent in both initial aldehydes and secondary amines 5 (Table 1) which has been confirmed by GC-MS and NMR data (ESI†). So, the yield of secondary amines with reduced C
C group is practically absent in both initial aldehydes and secondary amines 5 (Table 1) which has been confirmed by GC-MS and NMR data (ESI†). So, the yield of secondary amines with reduced C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C group did not exceed 2% in the reactions between 3-methylcrotonaldehyde and nitrobenzenes. Meanwhile, in the case of undecylenic aldehyde the formation of such products was not detected at all. It was found that the unsaturated secondary amines 5 could be produced with the high yields under flow conditions. The reactions of nitrobenzene and p-nitrotoluene with 3-methylcrotonaldehyde gave the targeted products with the yield of 90% (Table 1, entries 1 and 2). The adjustment of the reaction temperature and reagents ratio allowed improving the yield of secondary amine 5 up to 96% in the reaction of undecylenic aldehyde and p-nitrotoluene (Table 1, entries 6–8).
C group did not exceed 2% in the reactions between 3-methylcrotonaldehyde and nitrobenzenes. Meanwhile, in the case of undecylenic aldehyde the formation of such products was not detected at all. It was found that the unsaturated secondary amines 5 could be produced with the high yields under flow conditions. The reactions of nitrobenzene and p-nitrotoluene with 3-methylcrotonaldehyde gave the targeted products with the yield of 90% (Table 1, entries 1 and 2). The adjustment of the reaction temperature and reagents ratio allowed improving the yield of secondary amine 5 up to 96% in the reaction of undecylenic aldehyde and p-nitrotoluene (Table 1, entries 6–8).
| Entry | Nitroarene, 1 | Aldehyde, 2 | Main product | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
T, °C | Conversion of 2, % | Yieldb, % | |||
|---|---|---|---|---|---|---|---|---|---|---|
| 3 | 4 | 5 | Others | |||||||
a Catalyst (Au/Al2O3) = 200 mg, reactor CatCart®30 (30 mm × 4 mm), concentration of 1 = 0.025 mol L−1, solvent = toluene, pressure = 50 bar, hydrogen flow rate = 60 mL min−1, flow rate of liquid = 0.5 mL min−1, reaction time = 30–36 min.b GC yield. The values in parentheses are the yields of the isolated products.c n.d. = not detected.d Secondary amine with reduced C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C group.e Tertiary amine. C group.e Tertiary amine. |
||||||||||
| 1 |  |
 |
 |
1.2 | 80 | n.d.c | 6 | 2 | 90 (88) | 1d + 1e |
| 2 |  |
 |
 |
1.2 | 80 | n.d. | 4 | n.d. | 90 | 2d + 4e |
| 3 |  |
 |
 |
1.5 | 80 | 67 | 16 | 7 | 77 | n.d. |
| 4 | 1.5 | 90 | 79 | 11 | 4 | 85 | n.d. | |||
| 5 | 1.5 | 100 | 86 | 13 | 2 | 85 | n.d. | |||
| 6 |  |
 |
 |
1.5 | 80 | 83 | 7 | 2 | 91 | n.d. |
| 7 | 1.5 | 90 | 91 | 4 | 0.5 | 96 (95) | n.d. | |||
| 8 | 1.2 | 90 | 98 | 10 | 0.3 | 90 | n.d | |||
| Entry | R | Conversion of aldehyde, % | Yieldb, % | |||
|---|---|---|---|---|---|---|
| Primary amine | Imine | Secondary amine | Others | |||
| a Catalyst (Au/Al2O3) = 200 mg, concentration of nitroarene and undecylenic aldehyde: 0.025 mol L−1 and 0.0375 mol L−1, respectively, pressure = 50 bar, T = 90 °C, reaction time = 30–36 min.b GC yield.c n.d. = not detected.d Concentration of undecylenic aldehyde = 0.030 mol L−1.e Secondary amine with reduced R group.f T = 80 °C. | ||||||
| 1 | H | 79 | 11 | 4 | 85 | n.d.c |
| 2 | p-CH3 | 91 | 4 | 0.5 | 96 | n.d. |
| 3 | p-CH2CH3 | 91 | 4 | 1 | 95 | n.d. |
| 4 | p-OH | 87 | n.d. | n.d. | 99 | 1 |
| 5d | p-OH | 93 | n.d. | 2 | 98 | n.d. |
| 6 | p-OC(O)CH3 | 81 | 7 | 3 | 86 | 4e |
| 7 | p-Cl | 70 | 42 | 12 | 46 | n.d. |
| 8f | p-C(O)CH3 | 80 | 23 | 9 | 56 | 12e |
| 9 | p-Br | 77 | 8 | 8 | 82 | 2e |
| 10 | o-CH3 | 75 | 47 | 7 | 46 | n.d. |
| 11 | m-CH3 | 96 | 8 | 0.5 | 92 | n.d. |
| Entry | P, bar | Flow rate of liquid phase, mL min−1 | Yieldb, % | |||||
|---|---|---|---|---|---|---|---|---|
| 3a | 4a | 5a | 6 | 7 | 8 | |||
| a Catalyst (Au/Al2O3) = 550 mg, reactor CatCart®70 (70 mm × 4 mm), concentration of m-nitrostyrene and n-heptaldehyde: 0.025 mol L−1 and 0.0375 mol L−1, respectively, T = 50 °C, reaction time = 30–36 min.b GC yield.c Catalyst loading = 200 mg, reactor CatCart®30 (30 mm × 4 mm).d T = 60 °C. | ||||||||
| 1c | 50 | 0.50 | 24 | 7 | 65 | 1 | 0.3 | 3 |
| 2 | 50 | 0.50 | 17 | 5 | 74 | 1 | 0.2 | 3 |
| 3 | 50 | 0.35 | 3 | 1 | 82 | 1 | 0.3 | 13 |
| 4 | 40 | 0.35 | 3 | 1 | 83 | 1 | 0.2 | 12 |
| 5 | 30 | 0.35 | 6 | 2 | 83 | 1 | 0.2 | 8 |
| 6 | 20 | 0.35 | 7 | 2 | 83 | 1 | 0.2 | 7 |
| 7d | 20 | 0.35 | 4 | 1 | 76 | 2 | 0.4 | 17 |
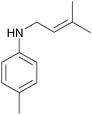
The effect of the substituent nature and its position in nitroarenes on reductive amination of aldehydes was studied on the example of reaction between undecylenic aldehyde and different nitroarenes (Table 2). It was found that introduction of electron-donating substituent (–CH3, –CH2CH3, –OH) in the para-position of the nitrobenzene increased the yield of secondary amine while in the case of nitrobenzenes with electron-withdrawing substituent (–Cl, –C(O)CH3, –Br) the decrease in the yield of the target product was observed. This can be explained by the fact that nucleophilic properties of anilines formed during the reaction grow with the introduction of electron-donating substituent8 resulting in an increase of the imine formation rate through the reaction with aldehyde. At the same time, anilines with electron-withdrawing substituent display weaker nucleophilic properties that lead to a decrease in the rate of imine formation. Additionally, the position of the substituent in nitrobenzene has a strong influence on the reaction. It was found that the yield of secondary amines in the reaction between undecylenic aldehyde and nitrotoluene isomers decreased in the following order: p-nitrotoluene > m-nitrotoluene ≫ o-nitrotoluene (Table 2, entries 2, 10 and 11). It is explained by a decrease of the nucleophilic properties of the corresponding methylanilines due to the steric hindrance created by a methyl substituent in meta- and ortho-position of the benzene ring.8
In contrast to the above-mentioned results, the significant amounts of products with reduced C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C group were formed in the reaction between m-nitrostyrene and n-heptaldehyde over Au/Al2O3 catalyst (Table 3). Decreasing the reaction temperature led to a decrease in the formation of the C–C double bond reduction products, but at the same time, significant amounts of m-vinylaniline 3a and imine 4a were formed at lower temperatures. To increase the yield of the target product 5a the contact time was increased by reducing the liquid flow rate from 0.5 to 0.35 mL min−1 and increasing the catalyst loading from 200 mg to 550 mg at 50 °C and 50 bars of H2 (Table 3, entries 1–3). As a result, the yield of 5a increased from 65% to 82%. Meanwhile, the change in hydrogen pressure from 20 to 50 bars showed no significant effect on the yield of secondary amine (Table 3, entries 3–6).
C group were formed in the reaction between m-nitrostyrene and n-heptaldehyde over Au/Al2O3 catalyst (Table 3). Decreasing the reaction temperature led to a decrease in the formation of the C–C double bond reduction products, but at the same time, significant amounts of m-vinylaniline 3a and imine 4a were formed at lower temperatures. To increase the yield of the target product 5a the contact time was increased by reducing the liquid flow rate from 0.5 to 0.35 mL min−1 and increasing the catalyst loading from 200 mg to 550 mg at 50 °C and 50 bars of H2 (Table 3, entries 1–3). As a result, the yield of 5a increased from 65% to 82%. Meanwhile, the change in hydrogen pressure from 20 to 50 bars showed no significant effect on the yield of secondary amine (Table 3, entries 3–6).
The one-pot synthesis of unsaturated secondary amines in the batch reactor by direct reductive amination of aldehydes with nitroarenes is the difficult task, because the several type of reactions should be performed at the same condition and reaction time should be properly adjusted to avoid the reduction of double bond. A similar reaction between nitrobenzene and m-vinylbenzaldehyde over Au/TiO2 catalyst was realized previously in a batch reactor but the reaction should be carried out for a long time by using two-stage procedure with different conditions to achieve a high yield of secondary amine.3b Firstly, the hydrogenation of the nitroaromatic compound was performed at 120 °C under H2 pressure until 95–98% conversion level, and then the reactor was depressurized and left at the same temperature until completion of the cascade process. In our case, the reaction occurs in one step within a short time. Therefore, continuous flow processes are more efficient than standard batch protocols for synthesis of unsaturated secondary amines by direct reductive amination of aldehydes with nitroarenes over supported gold catalysts.
The time-dependent investigation of Au/Al2O3 catalyst in reductive amination of undecylenic aldehyde with p-nitrotoluene showed a decrease in secondary amine yield from 96% to 88% after 154 minutes on-stream (Fig. 3). To elucidate the impact of the different factors on the catalyst deactivation we compared the gold content, the mean particle size of gold nanoparticles, textural properties, and the carbon deposits content in the as-prepared and spent Au/Al2O3 catalysts using atomic absorption spectroscopy, TEM, low-temperature nitrogen adsorption and TG-DSC-MS analysis, respectively. The characteristics of the as-prepared and spent catalyst are provided in the Table 4.
| As-prepared | Spent | Regenerated | |
|---|---|---|---|
| Content of Au, wt% | 2.5 ± 0.1 | 2.4 ± 0.1 | 2.5 ± 0.1 |
| Mean Au particle diameter, nm | 3.4 ± 1.4 | 3.3 ± 1.3 | 3.3 ± 1.2 |
| Content of carbon deposits, wt% | — | 4.5 | — |
| BET surface area, m2 g−1 | 142 | 139 | 143 |
| Total pore volume, cm3 g−1 | 0.63 | 0.60 | 0.66 |
| Mean pore diameter, nm | 17.8 | 17.6 | 18.1 |
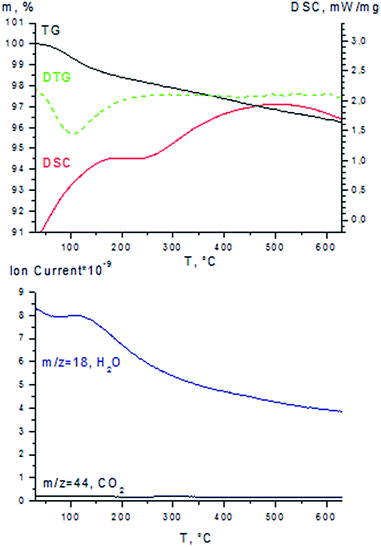
The comparison of characteristics of the as-prepared and spent catalysts allows us to conclude that Au content, Au particle size, and textural properties of Au/Al2O3 catalyst have not been changed notably after reaction (Table 4). In order to evaluate the contents of carbonaceous deposits, TG-DSC-MS analysis of the as-prepared and spent catalysts was performed in air atmosphere. The DTG curve of the as-prepared catalyst exhibit the broad peak with a maximum at ∼100 °C, which is related to removal of adsorbed water (the loss of the sample weight in the 30–200 °C range is 1.4%) (Fig. 4). Additionally, the weight loss of about 1.6% is observed in the 200–550 °C range, probably due to elimination of hydroxyl groups from the alumina surface or water removal from micropores.
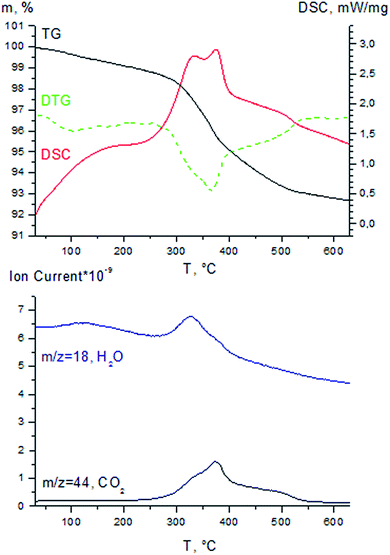
TG-DSC-MS results for spent Au/Al2O3 catalyst are presented in Fig. 5. Before TG-DSC-MS analysis the sample was washed with toluene for 30 minutes (0.5 mL min−1) and placed in the vacuum heat chamber at room temperature, evacuated to 5 mbar and then slowly heated up to 80 °C with the subsequent drying at this temperature for 3 hours. The DTG curve of spent catalyst exhibited two weight loss regions: the first (30–150 °C) was caused by the removal of adsorbed water (the weight loss in this temperature range is 1%) and the second (200–550 °C) could be explained by combustion of hydrogen-enriched carbonaceous species on the catalyst surface, that was confirmed by the exothermic peak on DSC curve and carbon dioxide and water peaks on MS curves in this region. According to TG curve the weight loss in the temperature region 200–550 °C was 6.1% and thus estimated amount of carbon deposits in spent Au/Al2O3 catalyst was approximately 4.5 wt%.
Thus, the formation of carbon-based deposits is the main reason for the Au/Al2O3 catalyst deactivation. This observation agrees with our previous results of the investigation of reductive amination of n-heptaldehyde with nitrobenzene over Au/Al2O3 catalyst in a continuous flow reactor.3c
It was shown that the activity of the spent Au/Al2O3 catalyst could be recovered completely after the oxidative treatment in air at 330 °C for 20 h (Fig. 3) which results in the removal of carbonaceous deposits accumulated on the catalyst surface. According to TG-DSC-MS data the regenerated catalyst did not contain carbon deposits and TG, DTG, DSC and MS curves of the regenerated Au/Al2O3 catalyst are similar to the corresponding curves of the as-prepared catalyst (Fig. 4 and ESI†). Importantly, no increase of gold particle size was detected after regeneration procedure which has been confirmed by TEM (Fig. 6) and XRD data (ESI†). The textural properties for the regenerated catalyst also did not deviate significantly from those characterizing the as-prepared sample (Table 4).
Conclusions
The direct reductive amination of unsaturated aliphatic aldehydes with various nitrobenzenes over Au/Al2O3 catalyst using molecular hydrogen as a reducing agent was performed in a continuous flow mode. In most cases, the unsaturated secondary amines were obtained in good to excellent yields, the hydrogenation of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C group is practically absent. It was found that introduction of electron-donating substituent in the para- and meta-position of the nitrobenzene increased the yield of secondary amine while in the case of nitrobenzenes with electron-withdrawing substituent or electron-donating substituent in the ortho-position a decrease in the yield of the target product was observed.
C group is practically absent. It was found that introduction of electron-donating substituent in the para- and meta-position of the nitrobenzene increased the yield of secondary amine while in the case of nitrobenzenes with electron-withdrawing substituent or electron-donating substituent in the ortho-position a decrease in the yield of the target product was observed.
Acknowledgements
The authors thank M. V. Shashkov for his help in carrying out this work. The work was performed with the support of Federal Agency of Scientific Organization (V.45.3.7).References
- R. N. Salvatore, C. H. Yoon and K. W. Jung, Tetrahedron, 2001, 57, 7785 CrossRef CAS.
- (a) L. Li, Z. Niu, S. Cai, Y. Zhi, H. Li, H. Rong, L. Liu, L. Liu, W. He and Y. Li, Chem. Commun., 2013, 49, 6843 RSC; (b) L. Hu, X. Cao, D. Ge, H. Hong, Z. Guo, L. Chen, X. Sun, J. Tang, J. Zheng, J. Lu and H. Gu, Chem.–Eur. J., 2011, 17, 14283 CrossRef CAS PubMed; (c) M. O. Sydnes and M. Isobe, Tetrahedron Lett., 2008, 49, 1199 CrossRef CAS; (d) M. O. Sydnes, M. Kuse and M. Isobe, Tetrahedron, 2008, 64, 6406 CrossRef CAS; (e) M. M. Dell'Anna, P. Mastrorilli, A. Rizzuti and C. Leonelli, Appl. Catal., A, 2011, 401, 134 CrossRef; (f) M. Nasrollahzadeh, New J. Chem., 2014, 38, 5544 RSC; (g) B. Sreedhar, P. S. Reddy and D. K. Devi, J. Org. Chem., 2009, 74, 8806 CrossRef CAS PubMed; (h) H. Li, Z. P. Dong, P. Wang, F. Zhang and J. Ma, React. Kinet., Mech. Catal., 2013, 108, 107 CrossRef CAS; (i) S. Wei, Z. Dong, Z. Ma, J. Sun and J. Ma, Catal. Commun., 2013, 30, 40 CrossRef CAS; (j) J. Zhou, Z. Dong, P. Wang, Z. Shi, X. Zhou and R. Li, J. Mol. Catal. A: Chem., 2014, 382, 15 CrossRef CAS; (k) F. G. Cirujano, A. Leyva-Perez, A. Corma and F. X. Llabres i Xamena, ChemCatChem, 2013, 5, 538 CrossRef CAS; (l) T. Stemmler, A.-E. Surkus, M.-M. Pohl, K. Junge and M. Beller, ChemSusChem, 2014, 7, 3012 CrossRef CAS PubMed; (m) T. Stemmler, F. A. Westerhaus, A.-E. Surkus, M.-M. Pohl, K. Junge and M. Beller, Green Chem., 2014, 16, 4535 RSC.
- (a) Y. Yamane, X. Liu, A. Hamasaki, T. Ishida, M. Haruta, T. Yokoyama and M. Tokunaga, Org. Lett., 2009, 11, 5162 CrossRef CAS PubMed; (b) L. L. Santos, P. Serna and A. Corma, Chem.–Eur. J., 2009, 15, 8196 CrossRef CAS PubMed; (c) E. A. Artiukha, A. L. Nuzhdin, G. A. Bukhtiyarova, S. Y. Zaytsev, P. E. Plyusnin, Y. V. Shubin and V. I. Bukhtiyarov, Catal. Sci. Technol., 2015, 5, 4741 RSC; (d) S.-S. Li, L. Tao, Q. Zhang, Y.-M. Liu and Y. Cao, Acta Phys.-Chim. Sin., 2016, 32, 61 CAS.
- (a) H.-U. Blaser, H. Steiner and M. Studer, ChemCatChem, 2009, 1, 210 CrossRef CAS; (b) A. Corma and P. Serna, Science, 2006, 313, 332 CrossRef CAS PubMed; (c) K. Shimizu, Y. Miyamoto, T. Kawasaki, T. Tanji, Y. Tai and A. Satsuma, J. Phys. Chem. C, 2009, 113, 17803 CrossRef CAS.
- (a) M. Irfan, T. N. Glasnov and C. O. Kappe, ChemSusChem, 2011, 4, 300 CrossRef CAS PubMed; (b) C. Wiles and P. Watts, Green Chem., 2014, 16, 55 RSC; (c) V. Hessel, Chem. Eng. Technol., 2009, 32, 1655 CrossRef CAS; (d) I. R. Baxendale, J. Chem. Technol. Biotechnol., 2013, 88, 519 CrossRef CAS.
- C. K. Costello, M. C. Kung, H.-S. Oh, Y. Wang and H. H. Kung, Appl. Catal., A, 2002, 232, 159 CrossRef CAS.
- F. Cardenas-Lizana, D. Lamey, M. Li, M. A. Keane and L. Kiwi-Minsker, Chem. Eng. J., 2014, 255, 695 CrossRef CAS.
- R. T. Morrison and R. N. Boyd, Organic chemistry, 6th edn, Prentice Hall, Englewood Cliffs N.J., 1992 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Characterization of the catalyst samples, GC chromatograms, NMR data. See DOI: 10.1039/c6ra20904a |
| This journal is © The Royal Society of Chemistry 2016 |