Open Access Article
Open Access ArticleA doxorubicin loaded chitosan–poloxamer in situ implant for the treatment of breast cancer†
Guru Prasanna Sahooa,
Vineet Kumar Raia,
Deepak Pradhana,
Jitu Haldera,
Tushar Kanti Rajwara,
Ritu Mahantya,
Ivy Sahaa,
Ajit Mishraa,
Priyanka Dasha,
Chandan Dasha,
Jameel Al-Tamimic,
Salim Manoharadas b,
Biswakanth Kara,
Goutam Ghosha and
Goutam Rath
b,
Biswakanth Kara,
Goutam Ghosha and
Goutam Rath *a
*a
aDepartment of Pharmaceutics, School of Pharmaceutical Sciences, Siksha ‘O’ Anusandhan (Deemed to be University), Bhubaneswar, Odisha, India. E-mail: goutamrath123@gmail.com; Tel: +91-9888206383
bDepartment of Botany and Microbiology, College of Science, King Saud University, P. O. Box. 2454, Riyadh, 11451, Saudi Arabia
cZoology Department, College of Science, King Saud University, P. O. Box 2455, Riyadh, Saudi Arabia
First published on 25th October 2024
Abstract
Breast cancer is a serious concern for many women worldwide. Drug-loaded implants have shown several benefits over systemic administrations. To provide anti-cancer drugs with controlled release and reduced systemic toxicity, biodegradable in situ implants have attracted a lot of attention. In the present study, we aimed to design and optimize a doxorubicin-loaded chitosan–poloxamer in situ implant for breast cancer treatment. Utilizing Box–Behnken Design and a Quality-by-Design (QbD) methodology, the in situ implant was prepared with chitosan (X1), poloxamer 407 concentration (X2), and stirring time (X3) as the independent variables. It was characterized for its in vitro gelation time, pH, rheology, and morphology, and evaluated based on drug release profile, in vitro cytotoxicity activities, in vitro anti-inflammatory potential, in vitro cellular uptake, and in vivo anti-inflammatory and pharmacokinetics to ensure their therapeutic outcomes. The results revealed that the prepared formulation showed a gelation time of 26 ± 0.2 s with a viscosity of 8312.6 ± 114.2 cPs at 37 °C. The developed formulation showed better cytotoxic activity in MCF-7 cell lines compared to the free drug solution. It demonstrated reduced levels of pro-inflammatory cytokines in RAW 264.7 macrophages. Further, the prepared in situ implant increases the intracellular accumulation of DOX in the MCF-7 cells. The in vivo pharmacokinetic investigations depicted an increase in t1/2 and a decrease in AUC of the developed formulation resulting in prolonged drug release and there could be a lower drug concentration in the bloodstream than for the free drug. Therefore, the developed in situ implant may offer a viable option for breast cancer treatment.
1. Introduction
One of the most common and dangerous health issues that women face globally is breast cancer. Every year, over a million new cases are diagnosed. According to the World Health Organization, 670![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 individuals worldwide lost their lives to breast cancer in 2022, while 2.3 million women received a diagnosis.1 As a result, one of the greatest hazards to humanity is cancer, a global health issue with no known bounds in terms of death and incidence rates. The primary cause of death for patients with breast cancer is metastasis.2 The prognosis for metastatic patients remains unsatisfactory and dismal despite advancements in breast cancer treatment.
000 individuals worldwide lost their lives to breast cancer in 2022, while 2.3 million women received a diagnosis.1 As a result, one of the greatest hazards to humanity is cancer, a global health issue with no known bounds in terms of death and incidence rates. The primary cause of death for patients with breast cancer is metastasis.2 The prognosis for metastatic patients remains unsatisfactory and dismal despite advancements in breast cancer treatment.
Systemic treatments like chemotherapy involve intravenous delivery of anti-cancer drugs at maximum tolerable doses that cause serious toxicities in healthy tissues, such as cardiomyopathy and neutropenia3,4 and the drug reaches the tumor site in low concentrations, which decreases the tumor treatment's effectiveness.5,6 Further, surgery is a non-aggressive procedure that is regarded as the primary strategy in the early stages of breast cancer.7 Even with the advancements in surgical methods, there is still a chance that residual tumor cells will stay in the surgical margins or the circulation following the surgical interventions, increasing the risk of metastasis and cancer recurrence.8,9 As a result, alternative approaches are required to reduce the chance of distant metastasis, stop the tumor from growing back, and increase the effectiveness of tumor-targeted drugs.10 The use of targeted drug delivery has the potential to achieve therapeutic concentrations within the tumor site, which would reduce systemic levels and, in turn, reduce the likelihood of unwanted side effects.11
Implantable drug delivery devices are the better approaches for local drug administration to the tumor site.12 These implants use biopolymer components that are both biodegradable and non-biodegradable. The non-biodegradable implants require surgical procedures twice, as they need to be inserted and removed. Using biodegradable polymers in implantable devices could be a better approach as the body can naturally remove it after use, eliminating the need for surgical extraction and increasing the patient's acceptance.13 In recent decades, many biomaterials have been widely used to develop implantable drug-delivery devices against cancer.14 Particularly biopolymers have attracted more attention because of their beneficial qualities, which include biocompatibility, biodegradability, and ease of processing.15 In the past decade, drug delivery systems that are implanted have advanced significantly and have shown to be an extremely effective way to deliver drugs to specified areas.16 They also reduce the total amount of drug in blood circulation by providing accurate spatial control, recurrence probability can be reduced, enhancing the rate of overall survival, and shielding healthy cells from harm.17 By maintaining therapeutically relevant drug levels within the tumor for extended durations, they can overcome the limitations of short half-lives, dose-dependent systemic toxicity, and rapid clearance associated with conventional chemotherapy agents.18 Implantable drug delivery systems include hydrogel, nanofibers, in situ gel, and osmotic pumps used for local chemotherapeutics delivery.19 As an injectable drug delivery technique, in situ implants (ISI) have garnered a lot of interest as these systems are injectable into the body with a syringe, and after injection, they solidify to form a semisolid depot.20 When a stimulus (such as ions, temperature, or pH) is applied, the liquid combination precipitates, cross-links, or polymerizes to solidify into an “implant”.21 Among these, temperature-responsive in situ gel has been widely used over the past few years.
Temperature changes cause temperature-sensitive in situ gel to shift from a solution to a gel.
Poloxamer 407, a commercially accessible and standardized excipient approved for parenteral use by the FDA, is a well-known thermosensitive component focused by many researchers.22 Poloxamer (PO), being a biocompatible polymer composed of polyoxy ethylene (PEO), polyoxy propylene (PPO), and polyoxy ethylene chains (PEOn–PPOn–PEOn) to form nonionic triblock polymers.23 It provides flexibility and control in drug delivery system design, allowing for forming formulations with customized release profiles and improved therapeutic efficacy.24 Additionally, the literature suggests that chitosan (CH) has good biodegradability, low toxicity, and suitable biocompatibility.25 Furthermore, chitosan itself has significant effects on several tumor types that are anti-metastatic anti-proliferative, anti-angiogenetic, and pro-apoptotic.26,27 A thermosensitive chitosan-based injectable hydrogel was developed by Ahsan et al. showing an inhibiting effect on tumor development and used as an effective anticancer drug carrier.28 Hence, we aimed to develop in situ gel as an implant comprising chitosan and poloxamer 407 for treating breast cancer.
Here, we have used doxorubicin (DOX) as a model drug. It is a powerful cytotoxic anthracycline antibiotic that damages DNA and induces cancer cells to undergo apoptosis. This study highlights the potential role of the in situ implant incorporating chitosan and poloxamer 407 using the QbD approach in the treatment of breast cancer by assuring improved cytotoxicity activity, anti-inflammatory activity, and improvised therapeutic potential which would reduce the systemic toxicity of chemotherapy and cancer recurrence after surgical procedure.
2. Materials and methods
2.1. Materials
Chitosan (molecular weight 190–310 kDa, deacetylation degree 75–85%) was obtained from Loba Chemie Pvt Ltd, poloxamer 407 was purchased from Hi Media Pvt Ltd. Doxorubicin was procured as a gift sample from Sisco Research Laboratory Pvt. Ltd (Mumbai). Glacial acetic acid, 3-(4,5-dimethylthiazol2-yl)-2,5-diphenyl tetrazolium bromide (MTT, Sigma), and Dulbecco's modified Eagle's medium (DMEM), dimethyl sulfoxide (DMSO) were obtained from Hi Media Pvt Ltd MCF-7 cells and RAW264.7 cells were bought from National Centre for Cell Science, Pune. All the remaining substances were of analytical grade.2.2. Optimization
Using Design Expert® Software (version 13, Stat Ease Inc., Minneapolis, USA), the Box–Behnken design was chosen to optimize the in situ gel formulation using 33 factorial design with 3 factors, 3 levels, and 17 runs. In this study, chitosan concentration (X1), poloxamer 407 concentration (X2), and stirring time (X3) were selected as independent variables. The three factorial levels for these variables were designated as follows: −1, 0, and +1 for low, medium, and high levels, respectively as shown in Table 1. The viscosity at 25 °C (Y1), viscosity at 37 °C (Y2), and gelation time (Y3) were chosen as the dependent variables. Using response surface analysis, it was possible to understand the relationship and interaction terms between the parameters as well as the predicted points of the independent variables that would comprise the optimum formulation. A one-way ANOVA was used for the statistical study to ascertain how formulation parameters affected the response variables. Following the software's recommendation, we created one optimal formulation in triplicate. After identifying the range that the ideal conditions might fall into using overlay plots and the desirability function, we measured the dependent variables. To verify the accuracy of the model, we conducted a comparison between the predicted viscosity at 25 °C, viscosity at 37 °C, and gelation time with the observed viscosity at 25 °C, viscosity at 37 °C, and the gelation time.| Independent variables | Symbol | Coded levels | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| Chitosan concentration (%) | X1 | 0.3 | 0.5 | 0.7 |
| Poloxamer 407 concentration (%) | X2 | 10 | 20 | 30 |
| Stirring time (second) | X3 | 60 | 105 | 150 |
2.3. Validation of data analysis and response surface technique
Design Expert software from Stat-Ease was modified to utilize the gathered results. By employing Analysis of Variance (ANOVA), the proposed models were verified. The program generated primary and interaction effects of the variables in the form of “3D response surface plots, 2D perturbation curves, and contour plots.” We selected the combination that predicted the highest degree of desirability, and we compared the projected values with the experiment results.3. Preparation and physiochemical characterization of DOX–CH–PO in situ implant
Poloxamer 407 solutions were prepared by the cold method process. The optimized amount of polymer was dispersed in distilled, deionized water and stirred continuously for one hour at room temperature. The poloxamer solutions that had partially dissolved were kept in the refrigerator at 4 °C until the polymer had entirely dissolved, which took around 24 hours. The chitosan solution was made by adding the optimized amount in a 2% w/v acetic acid solution and stirring continuously until the chitosan was completely dissolved. Aqueous solution of doxorubicin was added to the chitosan solution with continuous stirring. Finally, this drug solution was added to the poloxamer solution at room temperature and evaluated for different parameters.293.1. Fourier transform infrared spectroscopy (FTIR)
The drug and polymers in the prepared formulation and solid mixture were examined for possible interactions using infrared spectroscopy (FT/IR-4600, Jasco, Japan). The metal structure of the device was loaded with roughly 10 mg of powdered samples, and FT-IR readings were taken. The spectra of poloxamer 407, pure doxorubicin, chitosan, and the prepared formulation were compared at 400 to 4000 cm−1. A reference spectrum was compared to identify distinctive peaks representing any drug–polymer interaction.3.2. Differential scanning colorimetry (DSC)
Using reference material as a sample, this approach estimates the temperature-dependent difference in heat required to raise the sample's temperature. Using a differential scanning calorimeter (DSC-60, Shimadzu, Kyoto, Japan), thermograms of poloxamer 407, pure doxorubicin, chitosan, and formulation were recorded. The sample weight ranged from 2 to 3 mg, and the DSC measurements were carried out in a nitrogen-purging atmosphere with a scanning rate of 10 °C min−1. The samples underwent a heating cycle of 10 °C min−1, ranging from 0 to 350 °C.3.3. X-ray diffraction (XRD)
XRD is an essential analytical tool for determining the crystalline or amorphous solid state in which drugs may occur. X-ray diffraction investigation of the pure drug, polymers, and formulation was carried out using a Rigaku X-ray diffractometer. The glass slide containing the powdered materials under evaluation was put on the X-ray diffractometer. Within a 2θ range of 10 to 90°, the scanning rate was 10 minutes.3.4. Pharmaceutical characterization of DOX–CH–PO in situ implant
A 5 mL stoppered test tube with an inner diameter of 10 mm was filled with 1 mL of each prepared solution. Following that, the test tubes were submerged in a water bath with a thermostat set to 37 °C. By flipping the tube once every second until no flow was seen, the sol–gel transition was tracked.30
where Wi denotes the sample's starting weight and Wf its final weight following degradation.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1 (6 cm long). Before being put in a dissolution tester, each dialysis bag was fastened with two clamps on either end. Before sealing and placing the dialysis bags in a dissolution tester, DOX–CH–PO solution and free DOX solution were added. Using 500 mL of PBS as a release medium, the test condition involved 50 rpm stirring speed at 37 ± 0.5 °C. Fresh medium was added to the release medium at predetermined intervals. Similarly, it was performed at 0.1 M acetate buffer pH 5.5. The amount of drug released was measured using HPLC at a maximum wavelength of 254 nm.
000 g mol−1 (6 cm long). Before being put in a dissolution tester, each dialysis bag was fastened with two clamps on either end. Before sealing and placing the dialysis bags in a dissolution tester, DOX–CH–PO solution and free DOX solution were added. Using 500 mL of PBS as a release medium, the test condition involved 50 rpm stirring speed at 37 ± 0.5 °C. Fresh medium was added to the release medium at predetermined intervals. Similarly, it was performed at 0.1 M acetate buffer pH 5.5. The amount of drug released was measured using HPLC at a maximum wavelength of 254 nm.3.5. Biological characterization of DOX–CH–PO in situ implant
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm and 4 °C in an Eppendorf 5425 R G ultracentrifuge. The ELISA kits' instructions for performing antigen–antibody reactions on supernatants were followed to measure the levels of pro-inflammatory cytokines (TNF-α, IL-6, and IL-1β).36 Two groups were assessed for total protein (pg mL−1): group II, which received treatment, and group I, which did not get treatment (disease control).
000 rpm and 4 °C in an Eppendorf 5425 R G ultracentrifuge. The ELISA kits' instructions for performing antigen–antibody reactions on supernatants were followed to measure the levels of pro-inflammatory cytokines (TNF-α, IL-6, and IL-1β).36 Two groups were assessed for total protein (pg mL−1): group II, which received treatment, and group I, which did not get treatment (disease control).3.6. Statistical analysis
A statistical analysis was performed on the data using version 9 of GraphPad Prism. One-way analysis of variance (ANOVA) was used to compare group variances, with a significance level of P = 0.05. The sample sizes range from n = 3 to n = 6, and the data is shown as mean ± standard deviation (SD).4. Results and discussion
4.1. Statistical optimization of DOX–CH–PO in situ implant
Based on the Box–Behnken factorial design, the design expert projected a total of 17 runs for three components, which were modified at three distinct levels: chitosan concentration (X1), poloxamer 407 concentration (X2), and stirring time (X3). These runs were varied at three distinct levels (coded as −1, 0, and +1). The viscosity at 25 °C (Y1), viscosity at 37 °C (Y2), and gelation time (Y3) were studied as responses shown in Table 2.| Formulation code | X1 | X2 | X3 | Y1 | Y2 | Y3 |
|---|---|---|---|---|---|---|
| Chitosan (%) | Poloxamer 407 (%) | Stirring time (s) | Viscosity at 25 °C (cPs) | Viscosity at 37 °C (cPs) | Gelation time (s) | |
| F1 | 0.3 | 20 | 150 | 141.5 | 7563.2 | 23 |
| F2 | 0.5 | 20 | 105 | 151.8 | 8245.3 | 30 |
| F3 | 0.7 | 20 | 150 | 145.3 | 7845.6 | 25 |
| F4 | 0.7 | 10 | 105 | 138.6 | 6945.6 | 35 |
| F5 | 0.3 | 20 | 60 | 149.3 | 8015.4 | 23 |
| F6 | 0.5 | 20 | 105 | 153.6 | 8612.3 | 27 |
| F7 | 0.5 | 30 | 150 | 176.5 | 9784.6 | 10 |
| F8 | 0.3 | 30 | 105 | 172.1 | 9542.1 | 15 |
| F9 | 0.7 | 30 | 105 | 183.6 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 453.3 453.3 |
12 |
| F10 | 0.3 | 10 | 105 | 140.2 | 7398.8 | 37 |
| F11 | 0.7 | 20 | 60 | 153.5 | 8512.4 | 27 |
| F12 | 0.5 | 10 | 150 | 135.6 | 6712.2 | 40 |
| F13 | 0.5 | 20 | 105 | 149.2 | 8098.2 | 28 |
| F14 | 0.5 | 20 | 105 | 152.2 | 8412.3 | 26 |
| F15 | 0.5 | 20 | 105 | 150.7 | 8145.9 | 28 |
| F16 | 0.5 | 30 | 60 | 183.2 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 356.9 356.9 |
11 |
| F17 | 0.5 | 10 | 60 | 144.6 | 7789.8 | 35 |
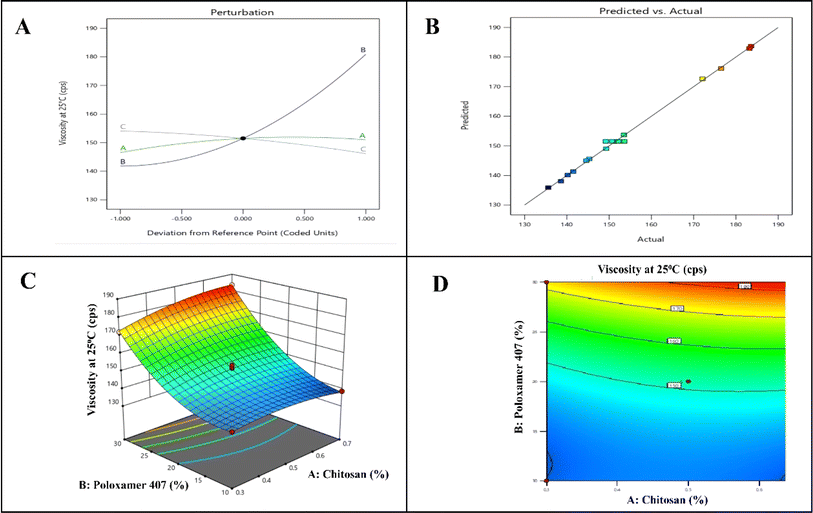
| Y1 = 151.5 + 2.2375X1 + 19.55 X2 − 3.9625 X3 + 3.275 X1X2 − 0.1 X1X3 + 0.575 X2X3 − 2.725 X12 + 9.85 X22 − 1.375 X32 | (1) |
The equation showed that chitosan (X1) and poloxamer 407 (X2) have a favorable impact on the in situ implant while stirring time (X3) has a negative impact on it. The fact that X3's magnitude was most readily apparent led to the conclusion that its detrimental effects were most severe. The combined interaction effect of X1X2 and X2X3 was also found to be synergistic, with X1X3 having the largest detrimental effect on the gel. As a result, it can be inferred that increasing the stirring time (X3), there is a decrease of viscosity at 25 °C and as the concentration of poloxamer 407 (X2) increases, there is an increase in the viscosity at 25 °C of the in situ implant.
With a p-value of less than 0.05 (<0.0001), the ANOVA for the quadratic response surface model of viscosity at 25 °C was determined to be significant. With a p-value of 0.0020, factor A (chitosan concentration) was found to have a significant impact on viscosity at 25 °C. On the other hand, factor C and B's p-values of less than 0.0001 indicate that they significantly affect viscosity at 25 °C. There was a significant correlation between the actual and expected results, as evidenced by the variations of less than 0.2 between the adjusted R2 (0.9925) and predicted R2 (0.9898) for the viscosity at 25 °C quadratic model. It is recommended to utilize this model to navigate the design space. The contour plot of the reaction and response surface for Y1 are shown in Fig. 1.
| Y2 = 8302.8 + 154.675 X1 + 1411.31 X2 − 346.112 X3 + 341.1 X1X2 − 53.65 X1X3 + 126.325 X2X3 − 197.288 X12 + 479.437 X22 − 121.362 X32 | (2) |
The equation showed that the chitosan (X1) and poloxamer 407 (X2) have a positive impact on the viscosity at 37 °C, whereas the stirring time (X3) has a negative impact. Similarly, it was found that the interaction between X1X2 and X2X3 was synergistic, while X1X3 had a detrimental effect on viscosity at 37 °C. It indicates that as the concentration of poloxamer 407 (X2) and chitosan (X1) increases, there is an increase in the viscosity at 37 °C. Micelle production and micellar aggregation occur in response to an increase in poloxamer 407 concentration. The concentration must be higher than the micellar concentration for the gel phase to occur. Hydrophobic parts of the poloxamer 407 are kept apart in cold water by hydrogen bonding between POP chains and water. With an increase in temperature, the hydrogen bonding is broken and hydrophobic interactions are forming the gel.
The p-value for the model, which is less than 0.05, is less than 0.0001, indicating that the ANOVA for the quadratic response surface model for viscosity at 37 °C is significant. With a p-value of 0.0450, X1 was found to have a significant impact on viscosity at 37 °C. X2 and X3 had p-values of less than 0.0001 and 0.0010, respectively, indicating a substantial impact on viscosity at 37 °C. The adjusted R2 (0.9728) and projected R2 (0.9448), which displayed discrepancies of less than 0.2, demonstrated a strong correlation between the expected and actual findings for the viscosity at 37 °C quadratic model. Fig. 2 displays the response surface plot and response contour plot for Y2.
| Y3 = 25.4118 + 0.125 X1 − 12.375 X2 + 0.25 X3 | (3) |
 | ||
| Fig. 3 QBD model graph for gelation time (A) Perturbation curve, (B) linear correlation plot, (C) response surface plot, (D) contour plot. | ||
Since the model's p-value is less than 0.05 (<0.0001), the ANOVA for the quadratic response surface model of gelation time was determined to be significant. X1 has p-value of 0.8954 showing that factor A had significant effect on the gelation time. While p-values for X2 and X3 were <0.0001 and 0.7928 showing that it has significant effect on the gelation time. The projected R2 (0.8836) and adjusted R2 (0.9155) for the gelation time linear model both showed discrepancies smaller than 0.2, showing a significant correlation between the results of the experiment and those expected.
4.2. Preparation and physiochemical characterization of DOX–CH–PO in situ implant
The in situ gel was prepared by optimizing the parameters. The in situ gel was formulated using poloxamer 407 (20% w/v) dispersed in distilled water which was kept in the refrigerator at 4 °C for 24 hours for completely dissolving. Chitosan (0.5% w/v) was dissolved in a 2% w/v acetic acid solution. Aqueous solution of doxorubicin was added to the chitosan solution with continuous stirring. Finally, at room temperature, this drug solution was added to the poloxamer solution, it provides ease of injection and in situ gelation at the injection site.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretch), and 1412.62 cm−1 (C
O stretch), and 1412.62 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C ring stretch). The peak pattern in the formulation spectrum was precisely the same as that of the pure drug. Considerable surface modification is depicted by a small alteration in the fingerprint region's peak intensity. The formulation had several distinct peaks at approximately 1100 cm−1, which corresponded to the typical peaks of poloxamer 407. The spectra of the in situ implant formulation show all of the doxorubicin's distinctive peaks, indicating that there has been no chemical alteration.
C ring stretch). The peak pattern in the formulation spectrum was precisely the same as that of the pure drug. Considerable surface modification is depicted by a small alteration in the fingerprint region's peak intensity. The formulation had several distinct peaks at approximately 1100 cm−1, which corresponded to the typical peaks of poloxamer 407. The spectra of the in situ implant formulation show all of the doxorubicin's distinctive peaks, indicating that there has been no chemical alteration.
4.3. Pharmaceutical characterization of DOX–CH–PO in situ implant
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 453.3 cPs. The optimized batch showed a viscosity of around 8312.6 ± 114.2 cPs at 37 °C. When chitosan interacts with poloxamer 407 through hydrogen bonding and electrostatic interactions, it enhances the formulation viscosity.41 Upon increasing the temperature, poloxamer 407 forms micelles due to the hydrophobic nature of poly(propylene oxide) blocks, and these micelles aggregate into a gel network at body temperature.42
453.3 cPs. The optimized batch showed a viscosity of around 8312.6 ± 114.2 cPs at 37 °C. When chitosan interacts with poloxamer 407 through hydrogen bonding and electrostatic interactions, it enhances the formulation viscosity.41 Upon increasing the temperature, poloxamer 407 forms micelles due to the hydrophobic nature of poly(propylene oxide) blocks, and these micelles aggregate into a gel network at body temperature.424.4. Biological characterization of DOX–CH–PO in situ implant
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 765.71 and 14
765.71 and 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 160 (ng mL−1 × h) respectively. Therefore, the prepared in situ implant decreases the likelihood of the drug entering the bloodstream which could indicate the drug remains in the tumor for a prolonged time. G. Zibin et al. reported similar results that there was an effective accumulation of DOX in the tumor with reduced drug concentrations in the blood and healthy organs when doxorubicin-loaded zein in situ gel for interstitial chemotherapy.53
160 (ng mL−1 × h) respectively. Therefore, the prepared in situ implant decreases the likelihood of the drug entering the bloodstream which could indicate the drug remains in the tumor for a prolonged time. G. Zibin et al. reported similar results that there was an effective accumulation of DOX in the tumor with reduced drug concentrations in the blood and healthy organs when doxorubicin-loaded zein in situ gel for interstitial chemotherapy.53
| Sr. No. | Pharmacokinetic parameters | Free DOX | DOX–CH–PO ISI |
|---|---|---|---|
| 1 | Cmax (ng mL−1) | 1000 ± 24.69 | 276 ± 21.86 |
| 2 | Tmax (h) | 6 ± 2.45 | 12 ± 1.86 |
| 3 | t1/2 (h) | 22.64 ± 3.78 | 40.26 ± 4.92 |
| 4 | AUC0–t (ng h mL−1) | 31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 765.71 ± 245.32 765.71 ± 245.32 |
14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 160 ± 312.85 160 ± 312.85 |
| 5 | AUC0–∞ (ng h mL−1) | 36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 339.70 ± 296.87 339.70 ± 296.87 |
19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 678.14 ± 321.56 678.14 ± 321.56 |
| 6 | MRT0–∞ (h) | 31.34 ± 4.52 | 59.93 ± 2.81 |
| 7 | CL (L−1 h−1) | 4.12 ± 2.84 | 7.62 ± 1.32 |
5. Conclusion
In the present study, a biodegradable and sustained-release DOX–CH–PO in situ implant was successfully prepared for the treatment of breast cancer. The in situ implant's physicochemical, pharmacological, and biological potential for treating breast cancer has been effectively developed and characterized. The QbD method was used to prepare seventeen formulations (F1–F17) from 33 factorial designs and optimize the amounts of chitosan, poloxamer 407, and stirring time. The results showed that a liquid formulation that gels upon injection at 37 °C in 26 ± 0.2 s was successfully produced using 0.5% w/v chitosan and 20% w/v poloxamer 407 (optimized DOX–CH–PO ISI). The optimized formulation showed good rheological properties and extended drug release. The prepared formulation showed better cytotoxicity due to the synergistic potential of chitosan and poloxamer against the MCF-7 cells compared to the free drug. The DOX–CH–PO ISI exhibits anti-inflammatory characteristics by inhibiting pro-inflammatory cytokines, such as TNF-α, IL-6, and IL-1β, in RAW264.7 cell lines. It has been observed that DOX–CH–PO in situ implant increases the cellular uptake in the MCF-7 cells. The in vivo pharmacokinetic the developed formulation results showed an increase in the plasma half-life, a decrease in the peak plasma concentration, and AUC which may indicate maintaining high concentrations of the drug within the tumor and less exposure of the drug to the bloodstream. Therefore, it is possible to enhance the effectiveness and decrease the systemic toxicity of chemotherapy drugs by using the sustained-release DOX–CH–PO in situ implant which may be a viable option for the treatment of breast cancer. Further, the intrinsic toxicological and repeated dose kinetics profiling is key to predict the clinical outcomes.Data availability
The authors declare that the data supporting the findings of this study entitled “Doxorubicin loaded chitosan–poloxamer in situ implant for the treatment of breast cancer” available within the paper. Should any raw data files be needed in another format, they are available from the corresponding author upon reasonable request. Source data are provided in this paper.Author contributions
Guru Prasanna Sahoo: manuscript writing, data collection and proof reading. Vineet Kumar Rai: data collection, experimental work, compilation, and manuscript writing. Deepak Pradhan: proofreading, software and language editing. Jitu Halder: data analysis, language editing and critical analysis. Tushar Kanti Rajwar: compilation, manuscript writing. Ritu Mahanty: data collection and experimental work. Ivy Saha: data collection, experimentation and statistics. Ajit Mishra: data collection and experimental work. Priyanka Dash: animal experimentation and proof reading. Chandan Dash: software analysis, data collection and proof reading. Jameel Al-Tamimi: data analysis, language editing. Salim Manoharadas: data analysis, language editing and funding support. Biswakanth Kar: animal experiment and data interpretation. Goutam Ghosh: conceptualization of the topics and critical analysis. Goutam Rath: conceptualization of the topic and design of the table of content, supervision, data analysis and paper writing, editing and proof reading.Conflicts of interest
The authors declare no conflict of interestAcknowledgements
The DBT Builder project funded the research with order no (BT/INF/22/SP45078/2022). The authors would also like to thank the Researchers Supporting Project Number (RSPD2024R1082), King Saud University, Riyadh, Saudi Arabia. King Saud University, Riyadh, Saudi Arabia for funding this research work. The authors also acknowledge the financial support of the Department of Biotechnology (DBT), Govt. India.References
- Breast Cancer, World Health Organization (WHO), available from: https://www.who.int/news-room/fact-sheets/detail/breast-cancer Search PubMed.
- S. Nardin, E. Mora, F. M. Varughese, F. D'Avanzo, A. R. Vachanaram and V. Rossi, et al., Breast Cancer Survivorship, Quality of Life, and Late Toxicities, Front. Oncol., 2020, 10, 864 CrossRef PubMed.
- R. De Souza, P. Zahedi, C. J. Allen and M. Piquette-Miller, Polymeric drug delivery systems for localized cancer chemotherapy, Drug Delivery, 2010, 17(6), 365–375 CrossRef CAS PubMed.
- O. G. Scharovsky, L. E. Mainetti and V. R. Rozados, Metronomic Chemotherapy: Changing the Paradigm That More Is Better, Curr. Oncol., 2009, 16(2), 7–15 CrossRef CAS PubMed.
- S. Talebian, J. Foroughi, S. J. Wade, K. L. Vine, A. Dolatshahi-Pirouz and M. Mehrali, et al., Biopolymers for Antitumor Implantable Drug Delivery Systems: Recent Advances and Future Outlook, Adv. Mater., 2018, 30(31), 1706665 CrossRef PubMed.
- S. H. Jang, M. G. Wientjes, D. Lu and J. L. -S. Au, Drug Delivery and Transport to Solid Tumors, Pharm. Res., 2003, 20(9), 1337–1350 CrossRef CAS PubMed.
- J. M. Binkley, S. R. Harris, P. K. Levangie, M. Pearl, J. Guglielmino and V. Kraus, et al., Patient perspectives on breast cancer treatment side effects and the prospective surveillance model for physical rehabilitation for women with breast cancer, Cancer, 2012, 118(S8), 2207–2216 CrossRef PubMed.
- A. I. Riggio, K. E. Varley and A. L. Welm, The lingering mysteries of metastatic recurrence in breast cancer, Br. J. Cancer, 2021, 124(1), 13–26 CrossRef PubMed.
- A. Ahmad, Pathways to Breast Cancer Recurrence, ISRN Oncol., 2013, 1–16 Search PubMed.
- C. Pacheco, A. Baião, T. Ding, W. Cui and B. Sarmento, Recent advances in long-acting drug delivery systems for anticancer drug, Adv. Drug Delivery Rev., 2023, 194, 114724 CrossRef CAS PubMed.
- A. Banerjee, S. Pathak, V. D. Subramanium, G. Dharanivasan, R. Murugesan and R. S. Verma, Strategies for targeted drug delivery in treatment of colon cancer: current trends and future perspectives, Drug Discovery Today, 2017, 22(8), 1224–1232 CrossRef CAS PubMed.
- A. Kumar and J. Pillai. Implantable drug delivery systems, in Nanostructures for the Engineering of Cells, Tissues and Organs. Elsevier; 2018. p. 473–511. Available from: https://linkinghub.elsevier.com/retrieve/pii/B9780128136652000132 Search PubMed.
- J. A. Camargo, A. Sapin, C. Nouvel, D. Daloz, M. Leonard and F. Bonneaux, et al., Injectable PLA-based in situ forming implants for controlled release of Ivermectin a BCS Class II drug: solvent selection based on physico-chemical characterization, Drug Dev. Ind. Pharm., 2013, 39(1), 146–155 CrossRef CAS PubMed.
- S. A. Chew and S. Danti, Biomaterial-Based Implantable Devices for Cancer Therapy, Adv. Healthcare Mater., 2017, 6(2), 1600766 CrossRef PubMed.
- B. D. Weinberg, E. Blanco and J. Gao, Polymer Implants for Intratumoral Drug Delivery and Cancer Therapy, J. Pharm. Sci., 2008, 97(5), 1681–1702 CrossRef CAS PubMed.
- A. A. Exner and G. M. Saidel, Drug-eluting polymer implants in cancer therapy, Expert Opin. Drug Delivery, 2008, 5(7), 775–788 CrossRef CAS PubMed.
- J. G. Hiremath, N. S. Khamar, S. G. Palavalli, C. G. Rudani, R. Aitha and P. Mura, Paclitaxel loaded carrier based biodegradable polymeric implants: preparation and in vitro characterization, Saudi Pharm. J., 2013, 21(1), 85–91 CrossRef PubMed.
- M. Ebrahimnia, S. Alavi, H. Vaezi, M. Karamat-Iradmousa and A. Haeri, Exploring the vast potentials and probable limitations of novel and nanostructured implantable drug delivery systems for cancer treatment, EXCLI J., 2024, 23, 143–179 Search PubMed.
- M. Souri, S. Elahi and M. Soltani, Intratumoral implantable drug delivery system for targeted localized chemotherapy in breast cancer, J. Drug Delivery Sci. Technol., 2024, 94, 105519 CrossRef CAS.
- S. Kempe and K. Mäder, In situ forming implants—an attractive formulation principle for parenteral depot formulations, J. Controlled Release, 2012, 161(2), 668–679 CrossRef CAS PubMed.
- M. Parent, C. Nouvel, M. Koerber, A. Sapin, P. Maincent and A. Boudier, PLGA in situ implants formed by phase inversion: critical physicochemical parameters to modulate drug release, J. Controlled Release, 2013, 172(1), 292–304 CrossRef CAS PubMed.
- Y. Chen, J. H. Lee, M. Meng, N. Cui, C. Y. Dai and Q. Jia, et al., An Overview on Thermosensitive Oral Gel Based on Poloxamer 407, Materials, 2021, 14(16), 4522 CrossRef CAS PubMed.
- M. Almeida, M. Magalhães, F. Veiga and A. Figueiras, Poloxamers, poloxamines and polymeric micelles: definition, structure and therapeutic applications in cancer, J. Polym. Res., 2018, 25(1), 31 CrossRef.
- E. Giuliano, D. Paolino, M. Fresta and D. Cosco, Mucosal Applications of Poloxamer 407-Based Hydrogels: An Overview, Pharmaceutics, 2018, 10(3), 159 CrossRef CAS PubMed.
- N. N. Inamdar and V. Mourya, Chitosan and Low Molecular Weight Chitosan: Biological and Biomedical Applications, in Advanced Biomaterials and Biodevices, ed. Tiwari A. and Nordin A. N., Wiley, 1st edn, 2014, pp. 183–242. available from: https://onlinelibrary.wiley.com/doi/10.1002/9781118774052.ch6 Search PubMed.
- H. S. Adhikari and P. N. Yadav, Anticancer Activity of Chitosan, Chitosan Derivatives, and Their Mechanism of Action, Int. J. Biomater., 2018, 2018, 1–29 CrossRef PubMed.
- C. Qin, Y. Du, L. Xiao, Z. Li and X. Gao, Enzymic preparation of water-soluble chitosan and their antitumor activity, Int. J. Biol. Macromol., 2002, 31(1–3), 111–117 CrossRef CAS PubMed.
- A. Ahsan, M. A. Farooq and A. Parveen, Thermosensitive Chitosan-Based Injectable Hydrogel as an Efficient Anticancer Drug Carrier, ACS Omega, 2020, 5(32), 20450–20460 CrossRef CAS PubMed.
- T. Gratieri, G. M. Gelfuso, E. M. Rocha, V. H. Sarmento, O. De Freitas and R. F. V. Lopez, A poloxamer/chitosan in situ forming gel with prolonged retention time for ocular delivery, Eur. J. Pharm. Biopharm., 2010, 75(2), 186–193 CrossRef CAS PubMed.
- L. Liu, X. Tang, Y. Wang and S. Guo, Smart gelation of chitosan solution in the presence of NaHCO3 for injectable drug delivery system, Int. J. Pharm., 2011, 414(1–2), 6–15 CrossRef CAS PubMed.
- S. Gupta, Carbopol/Chitosan Based pH Triggered In Situ Gelling System for Ocular Delivery of Timolol Maleate, Sci. Pharm., 2010, 78(4), 959–976 CrossRef CAS PubMed.
- E. Kenawy, A. M. Omer, T. M. Tamer, M. A. Elmeligy and M. S. M. Eldin, Fabrication of biodegradable gelatin/chitosan/cinnamaldehyde crosslinked membranes for antibacterial wound dressing applications, Int. J. Biol. Macromol., 2019, 139, 440–448 CrossRef CAS PubMed.
- M. A. Fathalla Z, A. Vangala, M. Longman, K. A. Khaled, A. K. Hussein and O. H. El-Garhy, et al., Poloxamer-based thermoresponsive ketorolac tromethamine in situ gel preparations: Design, characterisation, toxicity and transcorneal permeation studies, Eur. J. Pharm. Biopharm., 2017, 114, 119–134 CrossRef PubMed.
- İ. Erol, N. Üstündağ Okur, D. Orak, H. Sipahi, A. Aydın and Ö. Özer, Tazarotene-loaded in situ gels for potential management of psoriasis: biocompatibility, anti-inflammatory and analgesic effect, Pharm. Dev. Technol., 2020, 25(8), 909–918 CrossRef PubMed.
- B. Xu, L. Yuan, Y. Hu, Z. Xu, J. J. Qin and X. D. Cheng, Synthesis, Characterization, Cellular Uptake, and In Vitro Anticancer Activity of Fullerenol–Doxorubicin Conjugates, Front. Pharmacol, 2021, 11, 598155 CrossRef PubMed.
- R. Jayaganesh, P. Pugalendhi and R. Murali, Effect of citronellol on NF-kB inflammatory signaling molecules in chemical carcinogen-induced mammary cancer in the rat model, J. Biochem. Mol. Toxicol., 2020, 34(3), e22441 CrossRef CAS PubMed.
- H. S. Mahajan and S. Gattani, In situ gels of Metoclopramide Hydrochloride for intranasal delivery: in vitro evaluation and in vivo pharmacokinetic study in rabbits, Drug Delivery, 2010, 17(1), 19–27 CrossRef CAS PubMed.
- H. Abdeltawab, D. Svirskis and M. Sharma, Formulation strategies to modulate drug release from poloxamer based in situ gelling systems, Expert Opin. Drug Delivery, 2020, 17(4), 495–509 CrossRef CAS PubMed.
- Z. Fathalla, W. W. Mustafa, H. Abdelkader, H. Moharram, A. M. Sabry and R. G. Alany, Hybrid thermosensitive-mucoadhesive in situ forming gels for enhanced corneal wound healing effect of L-carnosine, Drug Delivery, 2022, 29(1), 374–385 CrossRef CAS PubMed.
- S. Lee and A. Shanti, Effect of Exogenous pH on Cell Growth of Breast Cancer Cells, Int. J. Mol. Sci., 2021, 22(18), 9910 CrossRef CAS PubMed.
- K. H. Ullah, F. Rasheed, I. Naz, N. Ul Haq, H. Fatima and N. Kanwal, et al., Chitosan Nanoparticles Loaded Poloxamer 407 Gel for Transungual Delivery of Terbinafine HCl, Pharmaceutics, 2022, 14(11), 2353 CrossRef CAS PubMed.
- S. Kajdič, F. Vrečer and P. Kocbek, Preparation of poloxamer-based nanofibers for enhanced dissolution of carvedilol, Eur. J. Pharm. Sci., 2018, 117, 331–340 CrossRef PubMed.
- A. M. Omer, T. M. Tamer, M. A. Hassan, P. Rychter, M. S. Mohy Eldin and N. Koseva, Development of amphoteric alginate/aminated chitosan coated microbeads for oral protein delivery, Int. J. Biol. Macromol., 2016, 92, 362–370 CrossRef CAS PubMed.
- C. Galocha-León, C. Antich, A. Voltes-Martínez, J. A. Marchal, M. Mallandrich and L. Halbaut, et al., Development and characterization of a poloxamer hydrogel composed of human mesenchymal stromal cells (hMSCs) for reepithelization of skin injuries, Int. J. Pharm., 2023, 647, 123535 CrossRef PubMed.
- T. Kojarunchitt, S. Baldursdottir, Y. D. Dong, B. J. Boyd, T. Rades and S. Hook, Modified thermoresponsive poloxamer 407 and chitosan sol–gels as potential sustained-release vaccine delivery systems, Eur. J. Pharm. Biopharm., 2015, 89, 74–81 CrossRef CAS PubMed.
- J. Varshosaz, M. Tabbakhian and Z. Salmani, Designing of a Thermosensitive Chitosan/Poloxamer In Situ Gel for Ocular Delivery of Ciprofloxacin, Open Drug Deliv. J., 2008, 2(1), 61–70 CrossRef CAS.
- Y. I. Cho, S. Park, S. Y. Jeong and H. S. Yoo, In vivo and in vitro anti-cancer activity of thermo-sensitive and photo-crosslinkable doxorubicin hydrogels composed of chitosan–doxorubicin conjugates, Eur. J. Pharm. Biopharm., 2009, 73(1), 59–65 CrossRef CAS PubMed.
- Z. Gu, C. Da Silva, K. Van Der Maaden, F. Ossendorp and L. Cruz, Liposome-Based Drug Delivery Systems in Cancer Immunotherapy, Pharmaceutics, 2020, 12(11), 1054 CrossRef CAS PubMed.
- M. Oliveira, S. Santos, M. Oliveira, A. Torres and M. Barbosa, Chitosan drives anti-inflammatory macrophage polarisation and pro-inflammatory dendritic cell stimulation, Eur. Cells Mater., 2012, 24, 136–153 CrossRef CAS PubMed.
- K. Azuma, T. Osaki, S. Minami and Y. Okamoto, Anticancer and Anti-Inflammatory Properties of Chitin and Chitosan Oligosaccharides, J. Funct. Biomater., 2015, 6(1), 33–49 CrossRef PubMed.
- Y. Zaiki, A. Iskandar and T. W. Wong, Functionalized chitosan for cancer nano drug delivery, Biotechnol. Adv., 2023, 67, 108200 CrossRef CAS PubMed.
- P. Manhas, C. Cokca, R. Sharma, K. Peneva, N. Wangoo and D. Sharma, et al., Chitosan functionalized doxorubicin loaded poly(methacrylamide) based copolymeric nanoparticles for enhanced cellular internalization and in vitro anticancer evaluation, Int. J. Biol. Macromol., 2024, 259, 129242 CrossRef CAS PubMed.
- X. Cao, J. Geng, S. Su, L. Zhang, Q. Xu and L. Zhang, et al., Doxorubicin-Loaded Zein in Situ Gel for Interstitial Chemotherapy, Chem. Pharm. Bull., 2012, 60(10), 1227–1233 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ra06253a |
| This journal is © The Royal Society of Chemistry 2024 |