Open Access Article
Open Access ArticleComparison of the effects of perfluoroalkyl and alkyl groups on cellular uptake in short peptides†
Koji Kadotaa,
Ai Kohata‡
 a,
Shinsuke Sando
a,
Shinsuke Sando bc,
Jumpei Morimoto
bc,
Jumpei Morimoto *b,
Kohsuke Aikawa§
*b,
Kohsuke Aikawa§
 *a and
Takashi Okazoead
*a and
Takashi Okazoead
aDepartment of Chemistry and Biotechnology, Graduate School of Engineering, The University of Tokyo, 2-11-16 Yayoi, Bunkyo-ku, Tokyo, 113-0032, Japan
bDepartment of Chemistry and Biotechnology, Graduate School of Engineering, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo, 113-8656, Japan. E-mail: jmorimoto@chembio.t.u-tokyo.ac.jp
cDepartment of Bioengineering, Graduate School of Engineering, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo, 113-8656, Japan
dYokohama Technical Center, AGC Inc., 1-1 Suehiro-cho, Tsurumi-ku, Yokohama 230-0045, Japan
First published on 17th March 2025
Abstract
The differences in the effects of perfluoroalkyl (RF) and alkyl (RH) groups on the cellular uptake of short peptides were evaluated. A facile synthetic method was established to produce Fmoc-protected amino acids bearing RF and RH groups on their side chains. The synthesized Fmoc-protected amino acids were successfully incorporated into peptides using solid-phase peptide synthesis. Peptides with an RF group exhibited higher cellular uptake efficiency compared to peptides with an RH group of the same side-chain length. Intriguingly, the cytotoxicity of the AF647-RF-tripeptide (RF = C8F17) was lower than that of the AF647-RH-tripeptide (RH = C12H25), despite similar cellular uptake efficiencies. An evaluation of the binding affinity of the peptides to liposome membranes suggested that the higher lipophobicity of the RF group, compared to the RH group, contributed to the lower cytotoxicity observed in the peptide with the RF group. These findings indicate that the introduction of an RF group into peptides has considerable potential for developing drug-delivery carriers with enhanced uptake efficiency and low cytotoxicity.
Introduction
The perfluoroalkyl (RF) group is a unique functional group, as RF-containing compounds exhibit both high hydrophobicity and lipophobicity. RF-containing compounds exhibit higher hydrophobicity than their hydrocarbon counterparts with the same carbon chain lengths.1–3 Furthermore, under bulk conditions, perfluoroalkyl compounds form a fluorous phase that is immiscible with both organic and aqueous phases.4,5 While the differences between RF and alkyl (RH) groups, such as oil repellency and phase separation, are commonly observed under bulk conditions, the differences under biologically-relevant low-concentration conditions are not sufficiently understood. One notable biological application of RF groups is their incorporation into dendrimers6,7 and polymers.8,9 Perfluoroalkylation of delivery carriers has been shown to enhance delivery efficiency and biostability while reducing cytotoxicity.10,11 For example, Cheng and coworkers reported that perfluoroalkylation of branched-polyethyleneimine improves biostability and delivery efficiency.12 In addition, they reported that fluorinated dendrimers exhibit enhanced cellular uptake efficiency and reduce the nitrogen-phosphorous (N/P) ratio compared to their non-fluorinated dendrimers.7 Although these studies have compared perfluoroalkylated carriers with their alkylated counterparts, making a precise comparison of the effects of RF and RH groups has been challenging because strict control of the introduction efficiency of RF and RH groups into these carriers is difficult.Peptide-based drug-delivery carriers are useful for precisely evaluating the effect of functional groups because peptides can be synthesized in a sequence-defined manner, allowing strict control over the number and position of functional groups within the sequence. A few studies have evaluated peptides containing RF and RH groups. Cheng and coworkers investigated the cellular uptake efficiency of peptides containing RF and RH groups.13 Similarly, we have previously evaluated short hydrophobic peptides composed of amino acids bearing RF and RH groups.14,15 Although RF groups were shown to enhance the cellular uptake of peptides more effectively than RH groups, the differences between RF and RH groups were not systematically investigated, as the primary focus of these studies was on improving cellular uptake efficiency.
In this study, we first evaluated the differences in cellular uptake as well as cytotoxicity between RF- and RH-containing short peptides. To understand the underlying reasons for these differences observed in cellular assays, we also compared the membrane affinity of tripeptides containing the RF and RH groups. To facilitate the evaluation, we established a facile synthetic method for amino acids and peptides bearing RF groups (Fig. 1A). Our results demonstrated that the RF-containing tripeptides exhibit higher cellular uptake efficiency and lower cytotoxicity than their RH-containing counterparts (Fig. 1B). The RF-containing tripeptide exhibited lower membrane affinity, possibly accounting for the lower cytotoxicity. These findings provide valuable insights into the development of efficient drug-delivery carriers containing RF groups.
Result and discussion
Synthesis of amino acids and peptides bearing a perfluoroalkyl (RF) group
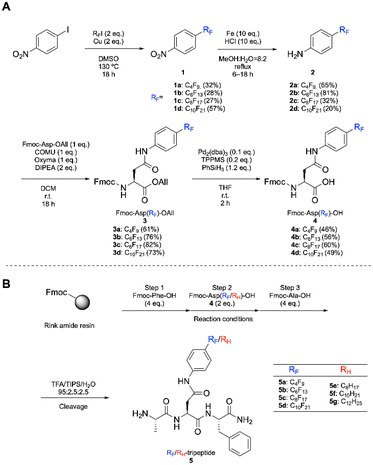
For comparison of the effect of RF and RH groups, Fmoc-protected amino acids bearing RF and RH groups compatible with solid-phase peptide synthesis (SPPS) are useful, as they allow facile synthesis of peptides with these functional groups at desired positions. We previously synthesized RF group-containing amino acids (RF-AAs), which have an RF group on the α-carbon of glycine.14 However, the RF-AAs require multi-step synthesis, have uncontrollable stereochemistry, and exhibit instability under basic conditions because highly acidic α-proton is readily eliminated. Thus, the RF-AAs could not be utilized in the Fmoc-based SPPS. To overcome these limitations, we aimed to develop novel RF-AAs that could be easily synthesized and applied to the Fmoc-based SPPS. The synthetic procedure for amino acids bearing RF groups of various lengths is described in Scheme 1. The synthesis began with the preparation of RF-nitrobenzene (1) via a Cu-mediated coupling reaction between 1-iodo-4-nitrobenzene and perfluoroalkyl iodide (RF–I) according to a conventional procedure.16 The obtained RF-nitrobenzene was reduced using Fe/HCl to yield RF-aniline (2). The resultant RF-aniline was coupled with Fmoc-protected L-aspartic acid allyl ester (Fmoc-Asp-OAll) to afford Fmoc-Asp(RF)-OAll (3). The deprotection of the allyl ester using Pd2(dba)3/PhSiH3 provided the desired Fmoc-Asp(RF)-OH (RF-AAs) (4). The same scheme was adopted to synthesize derivatives bearing RH groups (Scheme S1†). Next, a tripeptide containing RF-AA or RH-AA was synthesized using the standard Fmoc-based SPPS. The peptide sequence was identical to that used in our previous study:14 Ala-X-Phe, where “X” represents the position of the RF-AA or RH-AA. This approach enabled the facile synthesis of RF-AAs in a stereocontrolled manner. Besides, peptides containing RF-AAs can be synthesized without significant side reactions, such as epimerization.Given our previous findings that the introduction of a C8F17 enhances the cellular uptake efficiency of peptides,14 we prepared the tripeptide with RF = C8F17. To investigate the effect of chain length, we also synthesized tripeptides with RF groups of varying lengths, including RF = C4F9, C6F13, and C10F21. For comparison with the alkylated counterparts, we synthesized a tripeptide with RH = C8H17, which has the same chain length as RF = C8F17. Additionally, since the hydrophobicity of a CF2 unit is reported to be approximately 1.5 times higher than that of a CH2 unit,3 we synthesized a tripeptide containing RH = C12H25 to achieve comparable hydrophobicity to the tripeptide with RF = C8F17. To further explore the effect of chain length, we also prepared a tripeptide containing RH = C10H21, which represents an intermediate chain length between C8H17 and C12H25.
Evaluation of the cellular uptake efficiency of the peptides
The cellular uptake efficiencies of the synthesized peptides were initially investigated using flow cytometry. To visualize cellular uptake, a highly hydrophilic fluorophore, Alexa Fluor 647 (AF647), which is inherently not taken up by cells, was conjugated to the N-terminal amine of the peptides (referred to as AF647-RF/RH-tripeptides). AF647-labeled diethylamine (AF647-NEt2) was employed as a negative control (Fig. 2A). HeLa cells were treated with a serum-free culture medium containing 150 nM of the peptide for 1 h. Serum-free conditions were used to eliminate the potential influence of peptide binding to biomolecules. After washing the cells, cellular uptake efficiency was evaluated by measuring and comparing the fluorescence intensities of the cells using a flow cytometer (Fig. 2B and S1†). Cells treated with peptides bearing an RF group showed stronger fluorescence intensities than those treated with the corresponding RH-containing peptides with the same side chain length. For the RH group, peptides bearing shorter side chains (<C8H17) showed negligible cellular uptake (data not shown). In contrast, for the RF group, even the cells treated with the peptides having a short RF group (C4F9) showed a fluorescence signal higher than the cells treated with AF647-NEt2 control. These results suggest that the introduction of RF groups into peptides is more effective than the corresponding RH group in achieving high cellular uptake efficiency.To investigate the effect of amino acid residues other than RF or RH groups on cellular uptake, tripeptides with the general structure Z-Asp(C8F17)-Z were synthesized, where Z represents Phe, Leu, or Pro (Fig. S2 and S3†). The fluorescence intensities of cells treated with the peptides mostly correlated with the hydrophobicity of the peptide sequences, indicating that the hydrophobicity of the peptide plays a critical role in cellular uptake. Based on these results, subsequent evaluations focused primarily on peptides containing C8F17 and C12H25 because they exhibited comparable cellular uptake efficiencies.
Investigation of the cellular internalization mechanism
The mechanism of peptide internalization into cells was investigated using endocytosis inhibitors. First, we examined whether the AF647-RF-tripeptide (RF = C8F17) was internalized via an energy-dependent pathway, such as endocytosis, or through direct penetration across the cell membrane. When the cellular uptake experiment was conducted at 4 °C, the fluorescence intensity of the internalized peptide was significantly reduced, indicating that the internalization process is energy-dependent (Fig. 3A). Next, the specific endocytotic pathways involved in the peptide internalization were investigated using four inhibitors of endocytotic pathways: methyl β-cyclodextrin (MβCD, a lipid raft-mediated endocytosis inhibitor); genistein (GEN, a caveolin-dependent endocytosis inhibitor); 5-(N-ethyl-N-isopropyl)-amiloride (EIPA, a macropinocytosis inhibitor); and chlorpromazine (CPZ, a chlathrin-dependent endocytosis inhibitor). HeLa cells were preincubated with each inhibitor for 30 min prior to the addition of AF647-RF-tripeptide (RF = C8F17). EIPA and CPZ did not inhibit the cellular uptake. In contrast, MβCD and GEN largely inhibited cellular uptake (Fig. 3B). A similar result was observed for AF647-RH-tripeptide (RH = C12F25) (Fig. S4 and S5†). These results indicate that the internalization of AF647-RF-tripeptide (RF = C8F17), as well as AF647-RH-tripeptide (RH = C12F25), occurs primarily via lipid raft-mediated and caveolin-dependent endocytosis.To further understand the internalization process, HeLa cells treated with the peptide were observed using confocal laser scanning microscopy (CLSM). After 1 h of incubation without washing, the peptide was found to be absorbed into the cell membrane (Fig. S6†). To investigate intracellular localization after cellular internalization, cells were incubated with the peptide in the presence of organelle-specific markers. LysoTracker was used to assess whether the peptide underwent a lysosomal pathway, while Golgi-GFP was used to evaluate the potential transport of the tripeptide to the Golgi apparatus, as previous studies have reported that compounds internalized via caveolin- and lipid raft-mediated endocytosis are directed to the Golgi apparatus.17–19 Following 1 h of incubation, residual peptides on the membrane and in the medium were washed away, and the cells were observed under CLSM. Fluorescence signals from the AF647-RF-tripeptide partially colocalized with both LysoTracker and Golgi-GFP signals (Fig. 3C and D). These results suggest that AF647-RF-tripeptide (RF = C8F17) is initially absorbed into the cell membrane, internalizes into cells via endocytosis, and is at least partially transported to the Golgi apparatus. The alkyl counterpart (RH = C12H25) exhibited a similar localization pattern to the RF-containing peptide (Fig. S7†). These results are consistent with previous reports on the behavior of lipophilic molecules, which are internalized via caveolin- and lipid raft-mediated endocytosis and subsequently transported to the Golgi apparatus.17–19
Investigation of the cytotoxicity differences between peptides containing RH and RF groups
Compounds that are efficiently taken up by cells often exhibit cytotoxicity due to their interactions with the cell membrane, which can result in membrane disruption. Therefore, we evaluated the cytotoxicity of the peptides containing RF and RH groups (Fig. 4). The cytotoxicity of the peptides against HeLa cells following 1 h of incubation was evaluated using cell-counting kit-8 (CCK-8). The chain length-dependent cytotoxic effect was observed for the AF647-RF-tripeptide (RF = C6F13, C8F17, and C10F21) (IC50 = 77 ± 7 μM, 41 ± 9 μM, 34 ± 2 μM, respectively) (Fig. S8†). Intriguingly, the cytotoxicity of the AF647-RF-tripeptide (RF = C8F17) (IC50 = 41 ± 9 μM) was lower than that of the AF647-RH-tripeptide (RH = C12H25) (IC50 = 11 ± 3 μM), despite both peptides achieving similar cellular uptake efficiencies. The result indicates that RF-containing peptides are useful for achieving efficient cellular uptake with lower cytotoxicity than RH-containing peptides, consistent with findings reported in previous studies.7,13,20 | ||
| Fig. 4 Cytotoxicity of AF647-RF/RH-tripeptides. Cytotoxicity assay using cell-counting kit-8 (CCK-8); 5 × 103 of HeLa cells were treated with the AF647-RF/RH-tripeptides for 1 h. | ||
Evaluation of the interaction of peptide with lipid membrane
To elucidate the potential reasons for the differences in cellular uptake efficiency and cytotoxicity between peptides containing RF and RH groups, we investigated the interaction of the AF647-RF-tripeptide (RF = C8F17) with a model lipid membrane. Giant unilamellar vesicles (GUVs) composed of 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC) were prepared according to the previously described method.21 The AF647-RF-tripeptide (150 nM; RF = C8F17) was added to the liposome suspension, and its interaction with the membrane was observed under CLSM (Fig. 5A, top). Red fluorescence corresponding to the tripeptide was observed on the surface of the liposomal membrane, indicating that the peptide absorbs into the membrane. This result aligns with earlier CLSM studies on cells, where the peptide was initially observed to adsorb onto the cell membrane. Similar behavior of adsorption on the liposome was also observed for the AF647-RH-tripeptide (RH = C12H25) (Fig. S9†). In contrast, no fluorescence was observed when the control AF647-NEt2 was added to the liposome suspension (Fig. 5A, bottom). These results indicate that the peptides interact with membrane lipids through the hydrophobic peptide moieties rather than electrostatic interaction between the anionic fluorescent group and the lipid bilayer.To compare the relative affinities of the AF647-RF-tripeptide and AF647-RH-tripeptide to the liposomal membrane, we measured the zeta potential of the liposomes following the peptide treatment (Fig. 5B, S10, and Table S1†).22 An increase in the chain length of RF and RH groups corresponded to a more negative zeta potential. This observation demonstrated that the peptides with longer RF and RH groups are more strongly absorbed into the liposomal membrane. This result suggests that the higher cellular uptake efficiency of peptides with longer RF/RH groups is attributable to the higher affinity of the peptides to the cell membrane. Interestingly, AF647-RH-tripeptide (RH = C12H25) exhibited stronger binding to the liposome than AF647-RF-tripeptide (RF = C8F17), despite both peptides exhibiting similar cellular uptake efficiencies.
The weaker interaction of peptides bearing RF groups with lipids, as compared to those with RH group, was further suggested by their retention times on an octadecylsilyl (ODS) column. The retention times of the tripeptides without the N-terminal AF647 were measured using a high-performance liquid chromatography (HPLC) system, and the water–octanol partition coefficients (Log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P) were calculated from the obtained values (Fig. 5C, S11, S12, Tables S2 and S3†). The C8F17-containing tripeptide exhibited a lower Log
P) were calculated from the obtained values (Fig. 5C, S11, S12, Tables S2 and S3†). The C8F17-containing tripeptide exhibited a lower Log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P value than the C12H25-containing tripeptide despite the two peptides having similar cellular uptake efficiencies. Similarly, the C6F13-containing tripeptide exhibited a lower Log
P value than the C12H25-containing tripeptide despite the two peptides having similar cellular uptake efficiencies. Similarly, the C6F13-containing tripeptide exhibited a lower Log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P value than the C10H21-containing tripeptide, and the C4F9-containing tripeptide exhibited a lower Log
P value than the C10H21-containing tripeptide, and the C4F9-containing tripeptide exhibited a lower Log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P value than the C8H17-containing tripeptide, although these two pairs exhibited similar cellular uptake efficiencies. The lower Log
P value than the C8H17-containing tripeptide, although these two pairs exhibited similar cellular uptake efficiencies. The lower Log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P values of RF-containing tripeptides relative to RH-containing tripeptides with similar cellular uptake efficiencies suggest a weaker interaction of the RF groups with the alkyl chain of the ODS column due to the lipophobicity of the RF group.
P values of RF-containing tripeptides relative to RH-containing tripeptides with similar cellular uptake efficiencies suggest a weaker interaction of the RF groups with the alkyl chain of the ODS column due to the lipophobicity of the RF group.
These combined results suggest that when tripeptides with RF and RH groups exhibiting similar cellular uptake efficiencies are compared, tripeptides with RF groups exhibit lower lipophilicity or higher lipophobicity. The lower lipophilicity of the RF group may lead to a shorter retention time on the cell membrane, thereby reducing the extent of cell membrane disruption caused by the hydrophobic groups. This could explain the lower cytotoxicity observed for the AF647-RF-tripeptide compared to the AF647-RH-tripeptide.
Conclusions
In this study, we aimed to achieve a systematic comparison of the effects of RF and RH groups on the cellular uptake efficiency of peptides. The development of a facile synthetic method for Fmoc-protected amino acids bearing RF and RH groups with various chain lengths enabled a detailed comparison of their properties.The comparison of RF-containing tripeptides and RH-containing tripeptides showed that the RF-containing tripeptides exhibit higher cellular uptake efficiency than their RH-containing counterparts with the same chain length. Intriguingly, when an RF-containing tripeptide and an RH-containing tripeptide with similar cellular uptake efficiencies are compared, the RF-containing tripeptide exhibited lower cytotoxicity. While previous studies have also reported that RF groups can exhibit lower cytotoxicity than the corresponding RH groups,12,23 the underlying reasons for the difference have remained unclear. In this study, liposome binding experiments suggested that when peptides with RF and RH groups of similar cellular uptake efficiencies are compared, the RF-containing peptides exhibit lower lipophilicity, which may account for the lower cytotoxicity.
The effect of RF modification depends on the peptide sequence and how the peptide is modified by the RF group. For example, our previous study showed that the stereochemistry of the RF group in the peptide influences the size of the nanoparticles formed by the peptides, which in turn has a large influence on cellular uptake efficiency. Furthermore, the RF-modified peptides with high cellular uptake efficiency were found to form nanoparticles with diameters of approximately 100 nm, which were internalized via caveolae-dependent endocytosis and micropinocytosis.14 On the other hand, DLS measurement showed that the C8F17-modified peptide in this study formed nanoparticles with larger diameters (180 ± 20 nm) (Fig. S13†) than our previously reported peptide, and the peptide was internalized into the cells via lipid raft-mediated and caveolae-dependent endocytosis. Further investigation on the effect of RF modifications on peptide cellular uptake is required to more comprehensively understand the effect of the RF modification. The facile synthetic method for RF-modified peptides established in this study would facilitate the study.
Similar to peptides, oligonucleotides are sequence-defined oligomers that can be systematically modified. A few studies have compared the effects of RF- and RH-group modifications on the cellular uptake and cytotoxicity of nucleotides.24,25 These studies on sequence-defined oligomers, including peptides and oligonucleotides, provide valuable insights into the differences between RF- and RH-group modifications.
Although some per- and poly-fluoroalkylated compounds are under regulation because they lead to biological accumulation and environmental pollution,26 the RF group has considerable potential in the development of efficient drug-delivery carriers. Appropriate utilization of the RF group would also be useful in various biological applications such as 19F MRI27 and Raman imaging.28
Data availability
The data used and analyzed during the development of this work is available in the ESI† files accompanying this document.Author contributions
K. K. conducted the experiments, analyzed the data, and wrote the original draft of the manuscript. K. A. directed the project. A. K., J. M., and S. S. provided advice and discussed the data. T. O. provided advice and the original concept. All the authors contributed to the review and editing of the manuscript.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
We thank Prof. T. Aida at the University of Tokyo for the use of DLS and CLSM instruments. This work was supported by a JSPS KAKENHI Grant-in-Aid for Scientific Research (C) (20K05460) to K. A. and AGC Inc.Notes and references
- V. M. Sadtler, F. Giulieri, M. P. Krafft and J. G. Riess, Chem.–A Eur. J., 1998, 4, 1952–1956 CrossRef CAS.
- E. Moriyama, J. Lee, Y. Moroi, Y. Abe and T. Takahashi, Langmuir, 2005, 21, 13–18 CrossRef CAS PubMed.
- M. C. Z. Kasuya, S. Nakano, R. Katayama, K. Hatanaka and J. Fluor, Chem, 2011, 132, 202–206 CAS.
- R. Pollice and P. Chen, J. Am. Chem. Soc., 2019, 141, 3489–3506 CrossRef CAS PubMed.
- M. Cametti, B. Crousse, P. Metrangolo, R. Milani and G. Resnati, Chem. Soc. Rev., 2012, 41, 31–42 RSC.
- M. Wang and Y. Cheng, Acta Biomater., 2016, 46, 204–210 CrossRef CAS.
- M. Wang, H. Liu, L. Li and Y. Cheng, Nat. Commun., 2014, 5, 1–8 Search PubMed.
- G. Yan, J. Wang, P. Zhang, L. Hu, X. Wang, G. Yang, S. Fu, X. Cheng and R. Tang, Polym. Chem., 2017, 8, 2063–2073 RSC.
- G. Li, Q. Lei, F. Wang, D. Deng, S. Wang, L. Tian, W. Shen, Y. Cheng, Z. Liu and S. Wu, Small, 2019, 15, 1900936 CrossRef.
- C. Ge, J. Yang, S. Duan, Y. Liu, F. Meng and L. Yin, Nano Lett., 2020, 20, 1738–1746 CrossRef CAS PubMed.
- A. Stefanek, K. Łęczycka-Wilk, S. Czarnocka-Śniadała, W. Frąckowiak, J. Graffstein, A. Ryżko, A. Nowak and T. Ciach, Colloids Surf., B, 2021, 200, 111603 CrossRef CAS PubMed.
- Z. Zhang, W. Shen, J. Ling, Y. Yan, J. Hu and Y. Cheng, Nat. Commun., 2018, 9, 1377 CrossRef.
- G. Rong, C. Wang, L. Chen, Y. Yan and Y. Cheng, Sci. Adv., 2020, 6, eaaz1774 CrossRef CAS PubMed.
- K. Kadota, T. Mikami, A. Kohata, J. Morimoto, S. Sando, K. Aikawa and T. Okazoe, ChemBioChem, 2023, 24, e202300374 CrossRef CAS PubMed.
- T. Ono, K. Aikawa, T. Okazoe, J. Morimoto and S. Sando, Org. Biomol. Chem., 2021, 19, 9386–9389 RSC.
- V. C. R. Mcloughlin and J. Thrower, Tetrahedron, 1969, 25, 5921–5940 CrossRef CAS.
- A. Chakraborty and N. R. Jana, J. Phys. Chem. Lett., 2015, 6, 3688–3697 CrossRef CAS PubMed.
- B. Nichols, J. Cell Sci., 2003, 116, 4707–4714 CrossRef CAS.
- P. U. Le and I. R. Nabi, J. Cell Sci., 2003, 116, 1059–1071 CrossRef CAS.
- Z. Yuan, X. Guo, M. Wei, Y. Xu, Z. Fang, Y. Feng and W.-E. Yuan, NPG Asia Mater., 2020, 12, 34 CrossRef CAS.
- K. Tsumoto, Y. Hayashi, J. Tabata and M. Tomita, Colloids Surf., A, 2018, 546, 74–82 CrossRef CAS.
- N. J. M. Fitzgerald, A. Wargenau, C. Sorenson, J. Pedersen, N. Tufenkji, P. J. Novak and M. F. Simcik, Environ. Sci. Technol., 2018, 52, 10433–10440 CrossRef CAS PubMed.
- W. Shen, H. Wang, Y. Ling-hu, J. Lv, H. Chang and Y. Cheng, J. Mater. Chem. B, 2016, 4, 6468–6474 RSC.
- G. Godeau, H. Arnion, C. Brun, C. Staedel and P. Barthélémy, Med. Chem. Commun., 2010, 1, 76–78 RSC.
- M. Narita, A. Kohata, T. Kageyama, H. Watanabe, K. Aikawa, D. Kawaguchi, K. Morihiro, A. Okamoto and T. Okazoe, ChemBioChem, 2024, 25, e202400436 CrossRef CAS.
- M. G. Evich, M. J. B. Davis, J. P. McCord, B. Acrey, J. A. Awkerman, D. R. U. Knappe, A. B. Lindstrom, T. F. Speth, C. Tebes-Stevens, M. J. Strynar, Z. Wang, E. J. Weber, W. M. Henderson and J. W. Washington, Science, 2022, 375, eabg9065 CrossRef CAS.
- I. Tirotta, A. Mastropietro, C. Cordiglieri, L. Gazzera, F. Baggi, G. Baselli, M. Grazia Bruzzone, I. Zucca, G. Cavallo, G. Terraneo, F. Baldelli Bombelli, P. Metrangolo and G. Resnati, J. Am. Chem. Soc., 2014, 136, 8524–8527 CrossRef CAS.
- C. Chirizzi, C. Morasso, A. A. Caldarone, M. Tommasini, F. Corsi, L. Chaabane, R. Vanna, F. B. Bombelli and P. Metrangolo, J. Am. Chem. Soc., 2021, 143, 12253–12260 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5ra00304k |
| ‡ Current address: School of Life Science and Technology, Institute of Science Tokyo, 4259 Nagatsuta-cho, Yokohama-shi, Kanagawa, 226-8501, Japan. |
| § Current address: School of Medicine, Nihon University, 30-1 Oyaguchi-Kamicho, Itabashi-ku, Tokyo, 173-8610, Japan, E-mail: E-mail: aikawa.kousuke@nihon-u.ac.jp |
| This journal is © The Royal Society of Chemistry 2025 |