 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A flexible and scalable synthesis of 4′-thionucleosides†
Callum
Lucas‡
a,
Ethan
Fung‡
 a,
Matthew
Nodwell
a,
Steven
Silverman
b,
Bara
Singh
a,
Matthew
Nodwell
a,
Steven
Silverman
b,
Bara
Singh
 a,
Louis-Charles
Campeau
a,
Louis-Charles
Campeau
 b and
Robert
Britton
b and
Robert
Britton
 *a
*a
aDepartment of Chemistry, Simon Fraser University, Burnaby, British Columbia V5A 1S6, Canada. E-mail: rbritton@sfu.ca
bDepartment of Process Research & Development, Merck & Co., Inc., Rahway, NJ 07065, USA
First published on 27th November 2024
Abstract
4′-Thionucleosides (thNAs) are synthetic nucleoside analogues that have attracted attention as leads for drug discovery in oncology and virology. Here we report a de novo thNA synthesis that relies on a scalable α-fluorination and aldol reaction of α-heteroaryl acetaldehydes followed by a streamlined process involving carbonyl reduction, mesylate formation and a double displacement reaction using NaSH. We demonstrate the multigram preparation of 4′-thio-5-methyluridine and highlight the production of purine and pyrimidine thNAs as well as C2′-modified thNAs.
Introduction
For several decades nucleoside analogues (NAs) have served as a prolific source of antiviral and anticancer therapeutics, and account for more than half of all approved antiviral drugs.1 However, the pressure of new pathogens and emergence of drug resistance has highlighted the need for continued exploration of NA-relevant chemical space to identify compounds with novel mechanisms of action or enhanced resistance-combating properties.2 One promising class of NAs that have been studied since the 1960s3 are 4′-thio NAs (thNAs), where the ring oxygen is replaced with a sulfur atom. This single modification can have a profound impact on biological activity, including pharmacokinetic and pharmacodynamic properties.1,4 Further, due to the increased stability of the C–N anomeric bond, thNAs are generally more resistant to hydrolysis.5 For example, thiarabine (4′-thioaraC (1), Fig. 1),4 a thNA of the sponge metabolite cytarabine, was developed to treat hematological malignancies and solid tumors. Here, replacement of the endocyclic oxygen with sulfur resulted in an improved once daily oral dosing regimen compared to cytarabine, which requires twice-daily intravenous administration.6 The structurally related 2′-deoxyfluoro thNA FF-10502 (2)7 is an anticancer agent with improved potency over the related NA gemcitabine. Additionally, 4′thio-DMDC (3)8 and the C4′ alkyne thNA 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 have demonstrated potent anticancer and anti-HIV activities, respectively. In particular, the C4′-alkyne containing thNA 4 is a nucleoside/nucleotide reverse transcriptase inhibitor (NRTI) that also demonstrated an excellent selectivity index.9 The use of thNAs in oligonucleotide sequences is also of importance, and processes to access 4′-thio locked nucleic acids (LNAs)10 or to carry out nucleobase diversification using biocatalysis11 have advanced efforts in this area.
9 have demonstrated potent anticancer and anti-HIV activities, respectively. In particular, the C4′-alkyne containing thNA 4 is a nucleoside/nucleotide reverse transcriptase inhibitor (NRTI) that also demonstrated an excellent selectivity index.9 The use of thNAs in oligonucleotide sequences is also of importance, and processes to access 4′-thio locked nucleic acids (LNAs)10 or to carry out nucleobase diversification using biocatalysis11 have advanced efforts in this area.
A common approach to thNAs involves production of a protected 4-thioribose (e.g., 6), which can be achieved in as little as 6 steps.12 For example, Miller has shown that 6 can be accessed from the ribose-derived bromo aldehyde 5 on multigram scale by bromide displacement using NaSH.12 This synthesis supported production of 4′-thiouridine (7),12 a precursor to thiarabine (1). An important contribution by Guindon13 demonstrated that thNAs can also be constructed using an acyclic approach where the nucleobase is attached prior to cyclization. For example, the ribose-derived tBu thioether 9 was cyclized under basic conditions to form the 2′-fluoro thNA 10.13
Our groups have previously reported14 a straightforward synthesis of NAs 14 that relies on two key steps: (i) a one-pot α-fluorination and aldol reaction (αFAR), and (ii) an annulative fluoride displacement (AFD) reaction (Fig. 1C). Considering the ease of access to ketofluorohydrins of general structure 13, and precedent for the formation of thioribose analogues via displacement strategies (e.g., Fig. 1B), we sought to extend our NA synthesis platform to the preparation of thNAs. Importantly, this approach would afford orthogonally protected thNAs and should support the synthesis of C2′-modified thNAs (e.g., 1 and 2). Here, we report the development of this process, its application to the synthesis of purine and pyrimidine thNAs and a 4′-seleno NA, and the multigram-scale synthesis of 5-methyl 4′-thiouridine.
Results and discussion
Our initial efforts focused on the use of thymine derivative 17, which was prepared on 100 g scale and is a stable solid that can be stored for months without notable degradation.14 We have shown that the direct reduction of the ketone function in 17 using Me4N·BH(OAc)3 affords 1,3-syn diols with high levels of diastereoselectivity.14 Application to thNA synthesis would require 1,3-anti selectivity in the reduction step owing to the planned invertive cyclization process (i.e., SN2 reaction at C4′). We found this could be readily achieved by first protecting the secondary alcohol function as a TBS ether and subsequently reducing the carbonyl function with L-selectride.15 With the mono-TBS protected 1,3-anti diol in hand, several activation strategies were examined and ultimately mesylation proved to be optimal. Thus, the fluoro mesylate 15 could be reliably prepared in 3 steps from 17 in 75% overall yield following this straightforward process (Scheme 1).We next explored the reaction of fluoromesylate 15 with various sulfur nucleophiles15 with an aim to effect a one-pot double displacement and gain direct access to thNA 16 or generate a masked thiolate group and intercept intermediates related to those described by Guindon13 (e.g., 9, Fig. 1). Surprisingly, common thiol nucleophiles, including benzyl mercaptan (18), potassium thioacetate (19), NaSH (20) and Na2S·9H2O (21), did not react with mesylate 15 in DMF, even at 90 °C (Table 1, entries 1–4). Further heating of these reactions led to hydrolysis of the nucleobase and degradation. However, we were pleased to find that using freshly recrystallized Na2S·9H2O, the desired double displacement occurred readily at 90 °C, giving the thNA 16 in 50% yield (entry 5). Further optimization ultimately identified DMSO as the optimal solvent for this reaction and we found additionally that in DMSO, NaSH was an efficient sulfur nucleophile that reproducibly gave the thNA 16 in ∼60% yield. Thus, following this straightforward sequence, the αFAR product 17 could be converted into thNA 16 in four steps with an overall yield of ∼50%. Importantly, owing to the orthogonal protection of the secondary alcohol functions in 16, this route should support the synthesis of thNA functionalized at C2′ (see below).
| Entry | Thiol | Solvent | Temp. | Yield (%) |
|---|---|---|---|---|
| a Freshly recrystalized 21 (Na2S). | ||||
| 1 | 18 | DMF | 90 °C | 0 |
| 2 | 19 | DMF | 90 °C | 0 |
| 3 | 20 | DMF | 90 °C | 0 |
| 4 | 21 | DMF | 90 °C | 0 |
| 5 | 21 | DMF | 90 °C | 50 |
| 6 | 20 | DMSO | 100 °C | 61 |
Having established a route to the thNA 16, we next evaluated the scope of this thNA synthesis starting with the readily available TBS-protected fluorohydrins 22a–d (Fig. 2). The fluorohydrins can be prepared in 2 or 3 steps from the commercial nucleobase/heterocycle,14,16 though in the case of uracil- and triazole-containing fluorohydrins these were produced as inseparable mixtures of syn- and anti-fluorhydrins as described.12 These diastereomers were separable following TBS protection and mesylate formation (see ESI†). We further demonstrated that this process was compatible with pyrazole and benzoyl-protected adenosine, each of which gave the corresponding thNAs 24a–d in good overall yield. This reaction sequence was further optimized for execution with minimal chromatography and this sequence of steps could be executed as a through process with little impact on the overall yield. Due to challenges in accessing the corresponding cytosine and guanine aldol products (e.g., 22, HAr = cytosine or guanine), synthesis of the corresponding thNAs was not explored.
Unfortunately, reaction of the uracil containing fluoro mesylate 23a with NaSH led to substantial cleavage of the uracil function and degradation, with uracil being released at a similar rate as thNA 24a formation. Thus, using the standard reaction conditions (Table 1, entry 6), the 4′-thiouridine 24a was produced in 33% yield. In an effort to improve on this result, we conducted Design of Experiment (DOE) optimization,17 focusing on the relationship between temperature, concentration, and time (Fig. 2B). Here, we found a correlation between concentration and time, with a maximum yield of 47% at a concentration of ∼0.3 M after 3 hours at 115 °C. Using these optimized conditions, the 4′-thiouridine 24a could be produced in 4 steps and ∼40% overall yield from the TBS-protected keto fluoride 22a. To demonstrate the versatility of this approach, we also reacted the thymine derivative 15 with NaSeH, generated in EtOH by the reduction of Se with NaBH4. As highlighted in Fig. 2C, this reaction gave the 4′-selenonucleoside analogue 25, which is an analogue of the known 4′-selenouridine.18 Notably, selenonucleosides (e.g., 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19) have also attracted attention as anticancer agents.
19) have also attracted attention as anticancer agents.
To assess the scalability of this thNA synthesis, we additionally executed the process starting with 50.0 g of the protected αFAR product 22e (Fig. 3a). Without additional optimization we found that the sequence of reduction and mesylation proceeded in good overall yield, affording 59.0 g of the mesylate 15. From here, reaction with NaSH in DMSO at 100 °C gave 21.0 g of the thNA 16, which was purified by flash column chromatography. Removal of the silyl and acetonide protecting groups by treatment with 4 M HCl in MeOH then afforded 8.0 g of 4′-thio-5-methyluridine 27.20
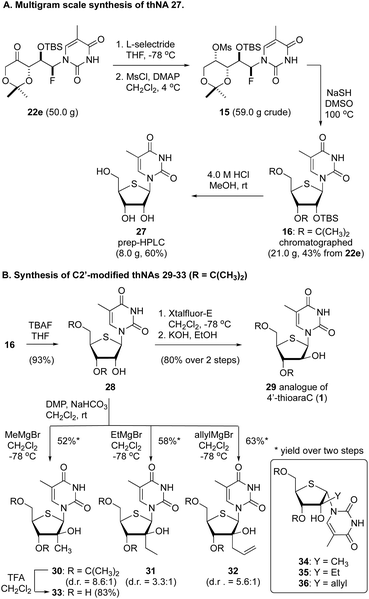 | ||
| Fig. 3 Large scale synthesis of thNA 27 and synthesis of C2′-modified thNAs 29–36. (A) A multigram scale synthesis of the thNAs 16 and 27. (B) Synthesis of C2′-modified thNAs. | ||
Finally, considering that this process affords orthogonally protected thNAs (e.g., 16), we investigated the selective C2′-functionalization of thymine thNA 16. As highlighted in Fig. 3B, removal of the TBS protecting group afforded the 2′-OH thNA 28 in excellent yield. From here, a 2-step sequence involving formation of the anhydro thNA and hydrolysis gave the arabino-configured thNA 29 in good overall yield. Notably, the corresponding triol (i.e., deprotected) has demonstrated activity as low as 0.77 μg mL−1 against HSV-1.21 Additionally, despite concerns regarding anomerization of 2′-keto thNAs,22 we found that oxidation of compound 28 using Dess Martin periodinane buffered with NaHCO3 in CH2Cl2 gave clean conversion to the corresponding 2′-keto derivative. The use of NaHCO3 in this reaction proved critical, and several other standard oxidation conditions failed to provide the 2′-ketone in any reasonable yield. This latter material proved to be unstable on all stationary phases used for chromatographic purifications and thus the crude material was reacted directly with Grignard reagents to afford a small collection of previously unreported C2′-modified thNAs 30–36. In all cases, the arabino-configured stereoisomer was the major product, and the minor product was that derived from epimerization at C1′ prior to reaction with the Grignard reagent (e.g., 34–36, see inset). Similar results have been reported by Matsuda.22 The use of more hindered Grignard reagents (e.g., cPrMgBr or iPrMgBr) resulted in larger amounts of C1′-epimerization. Removal of the acetonide protecting group from 30 using TFA gave the corresponding triol 33 in excellent yield. Notably, Liotta has recently reported related, ribose-configured C2′-modified thNAs.23
Conclusions
In summary, we report a streamlined process for the synthesis of various thNAs that exploits the ready availability of ketofluorohydrin aldol products. Importantly, the resulting thNAs are orthogonally protected, which enables synthesis of C2′-modified thNAs. This overall 7-step sequence was also demonstrated on multi-gram scale in the preparation of 5-methyl-4′-thiouridine (27) suggesting potential utility for larger scale, process research efforts. Importantly, the demonstration that purine and pyrimidine thNAs, 4′-seleno NAs, and C2′-modified thNAs can be readily prepared following straightforward strategies suggests that this new approach should inspire and support medicinal chemistry efforts in this area.Data availability
The experimental procedures, characterization data and 1H and 13C NMR spectroscopic data generated in this study are provided in the ESI.†Author contributions
R. B., C. L. and M. N. designed the study, R. B., C. L. and M. N. developed the synthetic plans and C. L., E. F., M. N. and B. S. optimized and executed the synthesis of all new compounds. S. S. and L.-C. C. supervised the large (multi-gram) scale reactions. R. B. and C. L. prepared the manuscript text. All authors contributed to the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
Financial support from the Natural Sciences and Engineering Research Council (NSERC) of Canada was received by R. B. (Discovery Grant: 2019-06368). R. B. acknowledges support from the Canadian Glycomics Network (Strategic Initiatives Grant CD-81), the Consortium de Recherche Biopharmaceutique (CQDM Quantum Leap Grant), Merck & Co., Inc., Rahway, NJ, USA.Notes and references
-
(a) L. P. Jordheim, D. Durantel, F. Zoulim and C. Dumontet, Advances in the Development of Nucleoside and Nucleotide Analogues for Cancer and Viral Diseases, Nat. Rev. Drug Discovery, 2013, 12(6), 447–464 CrossRef CAS
; (b) M. K. Yates and K. L. Seley-Radtke, The Evolution of Antiviral Nucleoside Analogues: A Review for Chemists and Non-Chemists. Part II: Complex Modifications to the Nucleoside Scaffold, Antiviral Res., 2019, 162, 5–21 CrossRef CAS
; (c) K. L. Seley-Radtke and M. K. Yates, The Evolution of Nucleoside Analogue Antivirals: A Review for Chemists and Non-Chemists. Part 1: Early Structural Modifications to the Nucleoside Scaffold, Antiviral Res., 2018, 154, 66–86 CrossRef CAS PubMed
.
-
(a) N. Tsesmetzis, C. B. J. Paulin, S. G. Rudd and N. Herold, Nucleobase and Nucleoside Analogues: Resistance and Re-Sensitisation at the Level of Pharmacokinetics, Pharmacodynamics and Metabolism, Cancers, 2018, 10(7), 240 CrossRef
; (b) C. M. Galmarini, J. R. Mackey and C. Dumontet, Nucleoside analogues: mechanisms of drug resistance and reversal strategies, Leukemia, 2001, 15(6), 875–890 CrossRef CAS PubMed
.
- E. J. Reist, D. E. Gueffroy and L. Goodman, Synthesis of 4-Thio-D- and -L-ribofuranose and the Corresponding Adenine Nucleosides, J. Am. Chem. Soc., 1964, 86(24), 5658–5663 CrossRef CAS
.
-
(a) W. R. Waud, K. S. Gilbert and J. A. Secrist, Preclinical Antitumor Activity of Thiarabine in Human Leukemia and Lymphoma Xenograft Models, Nucleosides, Nucleotides Nucleic Acids, 2012, 31(9), 647–660 CrossRef CAS PubMed
; (b) P. Gunaga, H. R. Moon, W. J. Choi, D. H. Shin, J. G. Park and L. S. Jeong, Recent advances in 4′-thionucleosides as potential antiviral and antitumor agents, Curr. Med. Chem., 2004, 11(19), 2585–2637 CrossRef CAS PubMed
; (c) Y. Yoshimura, Y. Saito, Y. Natori and H. Wakamatsu, Synthesis of 4′-Thionucleosides as Antitumor and Antiviral Agents, Chem. Pharm. Bull., 2018, 66(2), 139–146 CrossRef CAS
; (d) Y. Yoshimura, M. Endo, S. Miura and S. Sakata, An Alternative Synthesis of the Antineoplastic Nucleoside 4′-ThioFAC and Its Application to the Synthesis of 4′-ThioFAG and 4′-Thiocytarazid, J. Org. Chem., 1999, 64(21), 7912–7920 CrossRef CAS
; (e) M. Yokoyama, Synthesis and Biological Activity of Thionucleosides, Synthesis, 2000, 2000(12), 1637–1655 CrossRef
.
- M. I. Elzagheid, M. Oivanen, R. T. Walker and J. A. Secrist, Kinetics for the Acid-Catalyzed Hydrolysis of Purine and Cytosine 2′-Deoxy-4′-thionucleosides, Nucleosides, 1999, 18(2), 181–186 CrossRef CAS
.
- W. B. Parker, W. R. Waud and J. A. Secrist, 3rd, Thiarabine, 1-(4-Thio-β-D-arabinofuranosyl)cytosine. A Deoxycytidine Analog With Excellent Anticancer Activity, Curr. Med. Chem., 2015, 22(34), 3881–3896 CrossRef CAS PubMed
.
- M. Shinji, K. Chihaya, H. Tamami, S. Kazunori, Y. Takayuki, U. Rena, I. Miki, K. Nobuko, N. Hiroki, K. Hidenobu, M. Takuya, F. Hideyasu, J. P. Linda, S. Yasuhiro and I. Hiroyuki, FF-10502, an Antimetabolite with Novel Activity on Dormant Cells, Is Superior to Gemcitabine for Targeting Pancreatic Cancer Cells, J. Pharmacol. Exp. Ther., 2018, 366(1), 125 CrossRef
.
- S. Miura, M. Tanaka, Y. Yoshimura, H. Satoh, S. Sakata, H. Machida, A. Matsuda and T. Sasaki, In Vitro and in Vivo Antitumor Activity of a Novel Nucleoside, 4′-Thio-2′-deoxy-2′-methylidenecytidine, Biol. Pharm. Bull., 1996, 19(10), 1311–1315 CrossRef CAS
.
-
(a) K. Haraguchi, H. Shimada, K. Kimura, G. Akutsu, H. Tanaka, H. Abe, T. Hamasaki, M. Baba, E. A. Gullen, G. E. Dutschman, Y.-C. Cheng and J. Balzarini, Synthesis of 4′-Ethynyl-2′-deoxy-4′-thioribonucleosides
and Discovery of a Highly Potent and Less Toxic NRTI, ACS Med. Chem. Lett., 2011, 2(9), 692–697 CrossRef CAS PubMed
; (b) K. Haraguchi, H. Shimada, H. Tanaka, T. Hamasaki, M. Baba, E. A. Gullen, G. E. Dutschman and Y.-C. Cheng, Synthesis and Anti-HIV Activity of 4′-Substituted 4′-Thiothymidines: A New Entry Based on Nucleophilic Substitution of the 4′-Acetoxy Group, J. Med. Chem., 2008, 51(6), 1885–1893 CrossRef CAS PubMed
.
- R. Maeda, N. Saito-Tarashima, H. Wakamatsu, Y. Natori, N. Minakawa and Y. Yoshimura, Synthesis and Properties of 4′-ThioLNA/BNA, Org. Lett., 2021, 23(10), 4062–4066 CrossRef CAS
.
- S. Westarp, C. M. M. Benckendorff, J. Motter, V. Röhrs, Y. S. Sanghvi, P. Neubauer, J. Kurreck, A. Kurreck and G. J. Miller, Biocatalytic Nucleobase Diversification of 4′-Thionucleosides and Application of Derived 5-Ethynyl-4′-thiouridine for RNA Synthesis Detection, Angew. Chem., Int. Ed., 2024, 63(33), e202405040 CrossRef CAS PubMed
.
- M. Guinan, N. Huang, C. S. Hawes, M. A. Lima, M. Smith and G. J. Miller, Chemical synthesis of 4′-thio and 4′-sulfinyl pyrimidine nucleoside analogues, Org. Biomol. Chem., 2022, 20(7), 1401–1406 RSC
.
-
(a) D. Chapdelaine, B. Cardinal-David, M. Prevost, M. Gagnon, I. Thumin and Y. Guindon, A stereoselective approach to nucleosides and 4′-thioanalogues from acyclic precursors, J. Am. Chem. Soc., 2009, 131(47), 17242–17245 CrossRef CAS
; (b) S. Dostie, M. Prévost, P. Mochirian, K. Tanveer, N. Andrella, A. Rostami, G. Tambutet and Y. Guindon, Diastereoselective Synthesis of C2′-Fluorinated Nucleoside Analogues Using an Acyclic Approach, J. Org. Chem., 2016, 81(22), 10769–10790 CrossRef CAS
.
-
(a) M. Meanwell, S. M. Silverman, J. Lehmann, B. Adluri, Y. Wang, R. Cohen, L.-C. Campeau and R. Britton, A short de novo synthesis of nucleoside analogs, Science, 2020, 369(6504), 725 CrossRef CAS
; (b) E. K. Davison, D. A. Petrone, M. Meanwell, M. B. Nodwell, S. M. Silverman, L.-C. Campeau and R. Britton, Practical and concise synthesis of nucleoside analogs, Nat. Protoc., 2022, 17(9), 2008–2024 CrossRef CAS
.
- C. Grondal and D. Enders, A Direct Organocatalytic Entry to Selectively Protected Aldopentoses and Derivatives, Adv. Synth. Catal., 2007, 349(4–5), 694–702 CrossRef CAS
.
- C. Huxley, C. Lucas, J. M. M. Bruno, M. J. Anketell, E. K. Davison, G. Muir, M. B. Nodwell, M. Meanwell, S. M. Silverman, R. Britton and L.-C. Campeau, Efficient protocol for the preparation of α-heteroaryl acetaldehydes, Can. J. Chem., 2023, 101(7), 487–490 CrossRef CAS
.
- S. A. Weissman and N. G. Anderson, Design of Experiments (DoE) and Process Optimization. A Review of Recent Publications, Org. Process Res. Dev., 2015, 19(11), 1605–1633 CrossRef CAS
.
- P. K. Sahu, G. Kim, J. Yu, J. Y. Ahn, J. Song, Y. Choi, X. Jin, J.-H. Kim, S. K. Lee, S. Park and L. S. Jeong, Stereoselective Synthesis of 4′-Selenonucleosides via Seleno-Michael Reaction as Potent Antiviral Agents, Org. Lett., 2014, 16(21), 5796–5799 CrossRef CAS
.
- L. S. Jeong, D. K. Tosh, W. J. Choi, S. K. Lee, Y.-J. Kang, S. Choi, J. H. Lee, H. Lee, H. W. Lee and H. O. Kim, Discovery
of a New Template for Anticancer Agents: 2′-deoxy-2′-fluoro-4′-selenoarabinofuranosyl-cytosine (2′-F-4′-Seleno-ara-C), J. Med. Chem., 2009, 52(17), 5303–5306 CrossRef CAS
.
- V. Pejanović, Z. Stokić, B. Stojanović, V. Piperski, M. Popsavin and V. Popsavin, Synthesis and biological evaluation of some novel 4′-Thio-l-ribonucleosides with modified nucleobase moieties, Bioorg. Med. Chem. Lett., 2003, 13(11), 1849–1852 CrossRef
.
- Y. Yoshimura, M. Watanabe, H. Satoh, N. Ashida, K. Ijichi, S. Sakata, H. Machida and A. Matsuda, A Facile, Alternative Synthesis of 4′-Thioarabinonucleosides and Their Biological Activities, J. Med. Chem., 1997, 40(14), 2177–2183 CrossRef CAS
.
- D. Kaga, N. Minakawa and A. Matsuda, Nucleosides and Nucleotides. 232. Synthesis of 2′-C-Methyl-4′-thiocytidine: Unexpected Anomerization of the 2′-Keto-4′-thionucleoside Precursor, Nucleosides, Nucleotides Nucleic Acids, 2005, 24(10–12), 1789–1800 CrossRef CAS
.
- Z. W. Dentmon, T. M. Kaiser and D. C. Liotta, Synthesis and Antiviral Activity of a Series of 2′-C-Methyl-4′-thionucleoside Monophosphate Prodrugs, Molecules, 2020, 25, 5165 CrossRef CAS PubMed
.
Footnotes |
| † Electronic supplementary information (ESI) available: The experimental procedures, characterization data and 1H and 13C NMR spectroscopic data. See DOI: https://doi.org/10.1039/d4sc05679e |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2025 |




