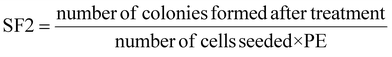Novel doxycycline gold nanoparticles via green synthesis using PEO-PPO block copolymers for enhanced radiosensitization of melanoma†
Agostina
Cammarata
 ab,
Julieta
Marino
ab,
Julieta
Marino
 c,
Mariel N.
Atia
c,
Mariel N.
Atia
 de,
Hebe
Durán
de,
Hebe
Durán
 def and
Romina J.
Glisoni
def and
Romina J.
Glisoni
 *ab
*ab
aUniversidad de Buenos Aires (UBA), Facultad de Farmacia y Bioquímica, Departamento de Microbiología, Inmunología, Biotecnología y Genética, Cátedra de Biotecnología, Junín 956, C1113AAD Buenos Aires, Argentina
bCONICET – Universidad de Buenos Aires, Facultad de Farmacia y Bioquímica, Instituto de Nanobiotecnología (NANOBIOTEC), Buenos Aires, Argentina. E-mail: rglisoni@ffyb.uba.ar; romy.glisoni@gmail.com
cUniversidad de Buenos Aires (UBA), Facultad de Farmacia y Bioquímica, Instituto de Química y Fisicoquímica Biológicas “Prof. Alejandro C. Paladini” (IQUIFIB), Buenos Aires, Argentina
dComisión Nacional de Energía Atómica (CNEA), Gerencia de Investigación y Aplicaciones, Subgerencia de Tecnología y Aplicaciones de Aceleradores, San Martín, Buenos Aires, Argentina
eInstituto de Nanociencia y Nanotecnología (INN-CNEA-CONICET), San Martín, Buenos Aires, Argentina
fUniversidad Nacional de San Martín, Escuela de Ciencia y Tecnología, San Martín, Buenos Aires, Argentina
First published on 17th April 2025
Abstract
This study focuses on a green and sustainable nanoplatform for the delivery of therapeutic agents, based on gold nanoparticles (AuNPs) synthesized using PEO-PPO block copolymers (F127, F68, P85, and their F127:P85 combination) as dual-function reducing and stabilizing agents. This eco-friendly approach eliminates the need for toxic chemical reductants, adheres to green chemistry principles, and yields highly stable, biocompatible nanosystems. The resulting polymer-stabilized AuNPs were associated with doxycycline (DOXY), a mitochondrial biogenesis inhibitor with radiosensitizing properties, and characterized using UV-Vis spectroscopy, dynamic light scattering (DLS), transmission electron microscopy (TEM), and X-ray fluorescence (XRF). The nanoparticles exhibited high colloidal stability, with tunable hydrodynamic diameters modulated by the copolymer composition. In vitro studies on A-375 and IIB-MEL-J melanoma cell lines revealed that DOXY-associated AuNPs, combined with gamma radiation (2 Gy, 137Cs), significantly enhanced radiosensitivity, reducing both cell viability and clonogenic survival. The physicochemical features of the nanosystems, particularly particle size and surface composition, influenced cellular uptake and therapeutic response. Notably, AuNPs stabilized with F127:P85 copolymer combination (∼19 nm) outperformed those with F127 (∼30 nm), despite displaying slightly higher polydispersity. Compared to Turkevich AuNPs, our copolymer-coated nanosystems demonstrated superior colloidal stability and cellular internalization. These findings highlight the potential of green-synthesized AuNPs as multifunctional, biocompatible platforms for therapeutic delivery, supporting the development of effective and environmentally responsible multimodal cancer therapies. Moreover, the simplicity, scalability, and cost-effectiveness of the synthesis process support its potential for future translational applications.
1. Introduction
Cancer treatment research has seen rapid advances to address the growing demand for precision therapeutics. Conventional strategies for treating solid tumors, including surgery, radiation therapy, and chemotherapy, often face challenges such as suboptimal efficacy and significant side effects in clinical settings.1–3 Malignant melanoma, the most aggressive form of skin cancer, epitomizes these challenges due to its potential for rapid metastasis. Despite advances in treatments like targeted therapy4 and immune checkpoint blockade,5,6 their benefits are not always sustained.7 Patients with advanced malignant melanoma often experience heterogeneous responses and poor prognoses due to extensive intra- and intertumoral heterogeneity caused by its high mutation rate.8,9 As the leading cause of skin cancer-related deaths,10 malignant melanoma highlights the urgent need for innovative therapeutic strategies to improve patient outcomes.Nanotechnology, as an innovative treatment modality, shows promising potential for enhancing anticancer efficacy and future clinical applications.11,12 Several nanoparticle-based formulations have been approved by the Food and Drug Administration (FDA) for chronic disease treatment, cancer therapies, and numerous nanotechnology-based theranostics are currently undergoing clinical trials.12–14
Gold nanoparticles (AuNPs) stand out for their biocompatibility, colloidal stability, and unique optical properties, making them attractive candidates for drug delivery applications.13–18 Their preferential accumulation in tumor tissues through the Enhanced Permeability and Retention (EPR) effect19–23 and their capacity for active targeting via surface functionalization,24–26 further enhance their therapeutic potential.
One of the major challenges in radiotherapy is maintaining a balance between radiotoxicity and treatment efficacy, as conventional radiotherapy protocols often lead to significant side effects due to the irradiation of normal tissues.25 To address this issue, radiosensitizers have long been explored as a strategy to enhance tumor sensitivity to radiation. In combination with technological advances aimed at improving the tumor-to-normal tissue dose ratio, these agents can increase radiotherapy efficiency while allowing for lower radiation doses, thereby reducing side effects or sensitizing radioresistant cells.25,26 Some FDA-approved chemotherapy agents are selected for their ability to amplify the effects of radiotherapy, depending on the type and location of the tumor, and are used as radiosensitizers. For example, cisplatin improves radiation sensitivity by interfering with tumor DNA repair, especially in head and neck cancers; gemcitabine enhances radiation-induced damage by inhibiting DNA synthesis; and 5-fluorouracil (5-FU) is frequently combined with radiotherapy to treat gastrointestinal cancers and others.25,26 Anthracyclines like doxorubicin also exhibit radiosensitizing properties by intercalating DNA and generating free radicals that enhance oxidative damage. However, their clinical use is often limited by severe side effects such as cumulative cardiotoxicity, highlighting the need for safer and more selective radiosensitizers.27
Recent advancements in nanomaterials have facilitated the development of novel and effective radiosensitizers.28–30 In particular, metallic and metal oxide nanoparticles have attracted significant interest due to their large surface area, inherent radiosensitizing properties, and functionalization potential, making them promising candidates for enhancing radiotherapy efficacy.29–31
AuNPs have been shown to enhance the radiation dose delivered to biological targets, primarily due to the high absorption coefficient of gold atoms, which result from their elevated atomic number (Z) and physical density.32–34 These properties significantly increase the likelihood of photoelectric effects, while Compton scattering contributes to secondary electron production.34–36 Moreover, the physical properties of gold nanoparticles allow them to be used as imaging agents. As novel radiosensitizing agents, AuNPs hold great potential for enhancing the efficacy of radiation therapy while simultaneously enabling real-time imaging, making them promising candidates for the development of integrated nanotheranostic platforms for cancer diagnosis and treatment.
However, traditional synthesis methods, such as electrochemical,37 two-phase,38 or seed-mediated approaches,39,40 frequently involve toxic solvents or surfactants and often require post-synthesis stabilization, which can limit their applicability in biomedicine. In contrast, green synthesis strategies based on biological agents, plant extracts, or synthetic polymers offer more environmentally friendly alternatives.11 These methods reduce hazardous waste generation, streamline the production process, and enhance the biocompatibility of the resulting nanomaterials. Among them, water-soluble block copolymers have emerged as versatile platforms that can act simultaneously as reducing and stabilizing agents, enabling the one-step, organic solvent-free synthesis of stable AuNPs.41–47 This approach allows the production of stable aqueous AuNPs dispersions while avoiding harmful chemicals, in line with green chemistry principles, and holds great promise for biomedical applications. Moreover, the use of block copolymers offers additional advantages in terms of cost-effectiveness, scalability, and compatibility with standard pharmaceutical manufacturing processes, further supporting their translational potential.41–47
Polymeric micelles (PMs) are a class of amphiphilic core–shell nanoparticles that form through the self-assembly of polymeric amphiphiles when their concentration exceeds the Critical Micelle Concentration (CMC).48,49 PMs have emerged as highly promising platforms for nano-drug delivery systems (nano-DDS) and have attracted significant attention, resulting in a substantial body of intellectual property.50–53
Among the most studied self-assembling biomaterials are the thermo-responsive poly(ethylene oxide)-poly(propylene oxide) (PEO-PPO) block copolymers, extensively investigated as efficient drug nanocarriers.54–56 Certain PEO-PPO derivatives have even received FDA approval for use in pharmaceutical products and medical devices due to their excellent cell compatibility. Commercially available as poloxamers (Pluronic®), linear PEO-PPOs are offered in various molecular weights and hydrophilic-lipophilic balances.54
Our research group has conducted comprehensive studies on both pristine and modified PEO-PPOs with different active ligands, investigating their potential for drug encapsulation, release, and targeted delivery.56–65 Additionally, several studies of our group,66,67 have explored the ability of PEO-PPOs to inhibit efflux transporters such as P-glycoprotein (P-gp), which are crucial in the development of the multidrug resistance (MDR) phenotype.68,69
Doxycycline (DOXY), an antibacterial agent approved by the FDA in the 1960s,70 exerts its antibacterial effects by inhibiting protein synthesis in bacteria. This mechanism involves binding aminoacyl-tRNAs at the A-site of the 30S ribosomal subunit, preventing elongation during translation.71 Due to the evolutionary relationship between mitochondrial and bacterial ribosomes, DOXY also disrupts mitochondrial protein synthesis.72–74 This side effect has been repurposed in oncology to target mitochondrial biogenesis in cancer cells.75,76 Although adverse effects of DOXY during clinical use are rare,77 studies have highlighted the advantages of incorporating DOXY into nanoformulations. For instance, DOXY-polymeric nanoparticles have been developed to enhance antibacterial efficacy against specific bacteria or to reduce toxicity.78,79 In cancer therapy, various DOXY-based nanoparticles have been designed to improve tumor penetration,80 amplify anticancer activity,81 and optimize drug delivery and effectiveness.82–84 Thus, combining mitochondria-targeting nanoformulations with radiotherapy would be highly significant to achieve effective tumor control under lower radiation doses.
This work explores the ability of PEO-PPO block copolymers to form AuNPs via one-step green synthesis, resulting in the formation of AuNPs-PMs complexes (cAuNPs-PMs). The block copolymers act as in situ reducing and stabilizing agents. As a control, hybrid AuNPs/PMs blends (bAuNPs/PMs) were prepared by self-assembling preformed AuNPs (synthetized via the Turkevich method) with block copolymers in aqueous dispersions. F127, F68 and the P-glycoprotein inhibitor P85 were used in the formulations. The incorporation of DOXY resulted in cAuNPs-PMs/DOXY complexes and bAuNPs/PMs/DOXY blends, which were evaluated for their potential to enhance radiotherapy efficacy in melanoma treatment. The nanosystems were fully characterized by UV-Vis spectroscopy, DLS, TEM, and XRF. In addition, the cellular internalization capacity of both conventional and copolymer-stabilized AuNPs systems containing DOXY was assessed in melanoma cell lines. Their radiosensitizing effects were also evaluated through in vitro cytotoxicity and clonogenic survival assays, marking, to our knowledge, the first study to explore the combined radiosensitizing potential of AuNPs and DOXY. These investigations underscore the relevance of physicochemical parameters such as particle size and surface composition in modulating biological performance and therapeutic response. Altogether, this work highlights the potential of green-synthesized AuNPs as multifunctional and scalable platforms for the development of effective, biocompatible, and environmentally responsible multimodal therapies against melanoma.
2. Materials and methods
2.1. Materials
All chemicals and reagents were used as received, without further purification. Pluronic® F127 (Mw ∼ 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 g mol−1; PEO content = 70 wt%; hydrophilic–lipophilic balance, HLB = 22), Pluronic® F68 (Mw ∼ 8400 g mol−1; PEO content = 80 wt%; HLB = 29), and Pluronic® P85 (Mw ∼ 4600 g mol−1; PEO content = 50 wt%; HLB = 16) were kindly provided by BASF Corporation (Buenos Aires, Argentina). These copolymers were selected to evaluate the effects of molecular weight (Mw), PEO/PPO block lengths, and PEO/PPO ratio on the green synthesis of AuNPs.
600 g mol−1; PEO content = 70 wt%; hydrophilic–lipophilic balance, HLB = 22), Pluronic® F68 (Mw ∼ 8400 g mol−1; PEO content = 80 wt%; HLB = 29), and Pluronic® P85 (Mw ∼ 4600 g mol−1; PEO content = 50 wt%; HLB = 16) were kindly provided by BASF Corporation (Buenos Aires, Argentina). These copolymers were selected to evaluate the effects of molecular weight (Mw), PEO/PPO block lengths, and PEO/PPO ratio on the green synthesis of AuNPs.
Chloroauric acid (gold(III) chloride hydrate, HAuCl4·H2O, Mw = 339.8 g mol−1, anhydrous basis, 99.995% trace metals basis, density = 3.9 g mL−1 at 25 °C) and trisodium citrate dihydrate (Mw = 294.1 g mol−1, ACS reagent ≥99%) were purchased from Sigma-Aldrich (Research AG S.A., Argentina). Doxycycline hydrochloride (DOXY, C22H24N2O8·HCl, Mw = 480.9 g mol−1) was obtained from Droguería Saporiti (S.A.C.I.F.I.A., Buenos Aires, Argentina). Cell culture media were sourced from GIBCO and fetal bovine serum (FBS) from NatoCor (Córdoba, Argentina).
2.2. Synthesis and functionalization of AuNPs
2.3. Characterization
Following association with DOXY, UV-Vis spectroscopy was also employed as the first analytical step to characterize the resulting nanostructures. The spectra were analyzed for shifts or changes in the SPR band, providing initial evidence of DOXY adsorption onto the nanoparticle surface. These spectral changes offered preliminary confirmation of surface modification and helped assess the interaction between DOXY and the nanocarriers. The observed spectral changes provided qualitative and quantitative information regarding the size, shape, and optical characteristics of the nanoparticles. Additionally, the analysis offered insights into nanoparticle stabilization within the polymeric systems studied and the dynamics of their formation.
For reference, measurements of Dh were performed on polystyrene latex nanosphere standards (NIST 3020A and 3400A, Thermo Scientific), with nominal average sizes of 20 and 400 nm, respectively, under identical experimental conditions. These standards were used to validate the accuracy and reliability of the DLS measurements. According to their certificates, NIST 3020A has a mean diameter of 21–25 nm (PCS, by Intensity), while the NIST 3400A has a narrow size distribution of 398–430 nm (PCS, by Intensity).
For sample preparation, three types of nanosystems were analyzed: naked AuNPs, cAuNPs-PMs complexes, and bAuNPs/PMs blends, each formulated with and without DOXY. All samples contained 250 μM gold(III) chloride, while DOXY-containing formulations additionally included 250 μM DOXY. Complexes and blends were prepared using 10% w/v of each copolymer. The components were mixed in Milli-Q water (5 mL) and pre-stabilized at 37 °C for 1 hour to ensure homogeneity. Subsequently, 10 μL of the prepared solution was deposited onto ultrathin carbon-coated grids treated with a hydrophilic acrylic resin and incubated for 1 additional hour at 37 °C. Excess solution was carefully removed with filter paper, and the grids were stained with a drop of 2% phosphotungstic acid (PTA) in Milli-Q water for 60 seconds. After staining, the grids were air-dried and examined under the TEM. The use of PTA enhanced the contrast by selectively staining the background, allowing for better visualization of the nanoparticles against the substrate. This approach ensured high-resolution imaging and reliable morphological characterization.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 v/v) medium. The murine fibroblast cell line NIH/3T3 (ATCC CRL-1658) was maintained in Dulbecco's modified Eagle's medium (DMEM). All culture media were supplemented with 10% (v/v) fetal bovine serum (FBS), 2 mM L-glutamine, 50 U mL−1 penicillin, 50 μg mL−1 streptomycin, and 4 mM sodium bicarbonate. Cells were grown in a controlled humidified incubator at 37 °C with 5% CO2 under standard culture conditions.
50 v/v) medium. The murine fibroblast cell line NIH/3T3 (ATCC CRL-1658) was maintained in Dulbecco's modified Eagle's medium (DMEM). All culture media were supplemented with 10% (v/v) fetal bovine serum (FBS), 2 mM L-glutamine, 50 U mL−1 penicillin, 50 μg mL−1 streptomycin, and 4 mM sodium bicarbonate. Cells were grown in a controlled humidified incubator at 37 °C with 5% CO2 under standard culture conditions.
Briefly, cells were washed twice with phosphate-buffered saline (PBS) and then incubated at 37 °C with a solution of 3.25 mM p-nitrophenol-N-acetyl-β-D-glucosaminide dissolved in 50 mM citrate buffer, pH 5, 0.25% Triton X-100. After 45 min for A-375 and IIB-MEL-J cells and 4 h for NIH/3T3 cells, the color reaction was developed and enzyme activity was blocked with 50 mM glycine buffer, pH 10.4, containing 5 mM EDTA. Absorbance values were measured at 405 nm in a Biotrack II Microplate Reader (GE Healthcare, Piscataway, NY).
2.3.6.1. X-Ray fluorescence (XRF) spectroscopy. The intracellular gold concentration in A-375 cells was quantified using X-ray fluorescence (XRF) spectroscopy (S2 PICOFOX, X-Ray Fluorescence Laboratory, Constituyentes Atomic Center, CNEA). Cells were treated with cAuNPs-F127/DOXY and cAuNPs-F127:P85/DOXY complexes (25 μM chloroauric acid as the Au precursor, 1% w/v copolymer, and 25 μM DOXY), naked AuNPs (25 μM), free DOXY (25 μM), or left untreated as a control.
XRF detects characteristic X-ray emissions following atomic electron ejection by ionizing radiation, enabling precise quantification of intracellular gold content. Results are expressed as elemental gold (Au0) concentration, facilitating direct comparison of uptake between cAuNPs-F127/DOXY and cAuNPs-F127:P85/DOXY complexes and control naked Turkevich AuNPs.
2.3.6.2. Fluorescence-based analysis of DOXY uptake. To quantify intracellular DOXY uptake, A-375 cells were treated with free DOXY (25 μM), cAuNPs-F127/DOXY, or cAuNPs-F127:P85/DOXY (each containing 25 μM chloroauric acid, 1% w/v copolymer and 25 μM DOXY), or left untreated. After 24 hours, cells were harvested by scraping, centrifuged, and resuspended in lysis buffer (PBS and 0.1% Triton-X100).
A series of standard DOXY solutions (0–50 μM) were prepared in lysis buffer to generate a calibration curve by fitting fluorescence intensity as a function of concentration using a linear regression model (R2 = 0.9921). Fluorescence measurements were performed using an Infinite 200 PRO TECAN plate reader with an excitation wavelength of 350 nm and an emission wavelength of 580 nm, enabling precise quantification of intracellular DOXY levels in A-375 cells.
2.3.7.1. Irradiation protocol. A-375 and IIB-MEL-J cells were incubated for 24 h with AuNPs/DOXY complexes and blends (1% w/v copolymer, 25 μM chloroauric acid as the Au precursor, and 25 μM DOXY), naked Turkevich AuNPs (25 μM), free DOXY (25 μM), or left untreated. Before irradiation, the culture medium was replaced with fresh medium. Cells were then exposed to gamma irradiation (2 Gy) using a 137Cs source (IBL-437C Irradiator, CIS BioInternational, CEBIRSA, Argentina) or sham irradiated (0 Gy). To ensure uniform radiation exposure, the drum containing the cell culture plates rotated 180° during irradiation.
2.3.7.2. Colony formation assay. Immediately after irradiation, cells were harvested by trypsinization and seeded at predetermined densities to ensure a countable number of colonies. Non-irradiated and irradiated cells were plated homogeneously in 6-well plates at densities of 500 and 1000 cells per well, respectively, and incubated in a humidified atmosphere (5% CO2, 37 °C) for 8–10 days.
Colonies were then fixed with a methanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acetic acid (3
acetic acid (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) solution, stained with crystal violet (0.5% w/v in 0.25% v/v methanol), and counted, considering as colonies those groups containing more than 50 cells. The surviving fraction at 2 Gy (SF2) was calculated as follows:90
1 v/v) solution, stained with crystal violet (0.5% w/v in 0.25% v/v methanol), and counted, considering as colonies those groups containing more than 50 cells. The surviving fraction at 2 Gy (SF2) was calculated as follows:90
2.4. Statistical and data analysis
All data were analyzed and presented using GraphPad Prism (version 8.02, GraphPad Software, La Jolla, California, USA, https://www.graphpad.com). Statistical analyses were performed using one-way or two-way ANOVA, as appropriate. Differences were considered statistically significant for p-values < 0.05 (p < 0.05).3. Results and discussion
3.1. Research rationale
The rising global incidence of melanoma underscores the urgent need for innovative therapeutic strategies to overcome the limitations of current treatments. Nanotechnology-based approaches, particularly those employing AuNPs, have gained considerable interest due to their biocompatibility, tunable surface properties, and potential for precise drug delivery.This study explores the use of PEO-PPO block copolymers (Fig. S1†), as dual-function agents in the green synthesis of AuNPs. These copolymers not only act as reducing agents, facilitating the conversion of AuCl4− ions into colloidal gold (Au0), but also stabilize the resulting nanoparticles, providing a sustainable non-toxic approach for AuNPs production.
Unlike conventional nano-radiosensitizers, our strategy introduces a novel concept by integrating green-synthesized AuNPs with polymeric clusters that adsorb drugs onto their surface. In this system, DOXY acts as a mitochondrial biogenesis inhibitor (Fig. S2†), a mechanism that may be particularly effective against quiescent, treatment-resistant melanoma cells. This dual approach not only enhances drug delivery and therapeutic efficacy but also opens new avenues for melanoma treatment.
3.2. Synthesis and characterization of cAuNPs-PMs and bAuNPs/PMs: a green approach
The green synthesis of cAuNPs-PMs using PEO-PPO copolymers highlights the dual functionality of these amphiphilic materials, which not only reduce but also stabilize AuNPs (Fig. 1A). During the process, the hydrophilic PEO segments reduce gold ions (AuCl4−, derived from HAuCl4·H2O) to colloidal gold (Au0), initiating the nucleation of gold clusters. Simultaneously, the hydrophobic PPO segments adsorb onto the growing gold clusters, preventing aggregation and ensuring nanoparticle stability. This dual mechanism facilitates controlled nanoparticle growth, yielding well-defined colloidal AuNPs with enhanced stability.The schematic representation in Fig. 1A illustrates this process in detail: gold ions are progressively reduced by the PEO blocks, releasing chloride ligands, while the PPO blocks stabilize the growing clusters and promote further gold reduction at their surface. Upon synthesis completion, DOXY is incorporated by adsorption onto the nanoparticle surface at an initial concentration of 250 μM. For specific assays, such as in vitro evaluations, dilutions were performed to a final concentration of 25 μM to assess both incorporation and therapeutic effects.
To further validate the effectiveness of this green synthesis approach, we evaluated the concentration-dependent effect of copolymers on nanoparticle stability and uniformity. As shown in Fig. 2, increasing the copolymer concentrations (0.5 to 10% w/v) enhanced the surface plasmon resonance (SPR) band intensity at 520 nm. This trend was consistent for all three tested copolymers – F127, F68, and P85 – regardless of their Mw (see Section 2.1.), indicating improved particle dispersion and stability (Fig. 2A, B and C). These findings underscore the robustness and versatility of PEO-PPO copolymers in the synthesis and stabilization of AuNPs.
In parallel, bAuNPs/PMs blends were prepared by mixing pre-synthesized AuNPs (obtained via the Turkevich method) with hydrated PEO-PPO block copolymers, forming polymeric micelles (PMs) (Fig. 1B). The interaction between AuNPs and PMs results in stable colloidal mixtures, where the micelles act as both carriers and stabilizers for the nanoparticles. Unlike the green synthesis approach, the SPR band intensity of bAuNPs/PMs remained consistent regardless of copolymer concentration or Mw (Fig. 3). This result suggests that the pre-formed AuNPs were successfully encapsulated within the micelles, demonstrating a concentration and Mw independent encapsulation process that was consistent across all three copolymers (Fig. 3A–C).
The addition of DOXY preserved the characteristic spectra of both the cAuNPs-PMs complexes and the bAuNPs/PMs blends, with slight shifts observed (Fig. S3†). These shifts correlated with the particle sizes found for each formulation (Tables 1 and 2). In contrast, a color change to violet was detected in bare AuNPs synthesized via the Turkevich method in the presence of DOXY (Fig. S3†).
| Free PMs and naked Turkevich AuNPs | D h (nm) (±S.D) | PDI (±S.D.) | Z-Pot (mV) (±S.D.) | |||
|---|---|---|---|---|---|---|
| Peak 1 | % intensity (±S.D.) | Peak 2 | % intensity (±S.D.) | |||
| F127 | 23.7 (0.7) | 100.0 (0.0) | — | — | 0.079 (0.019) | −4.3 (1.0) |
| F127/DOXY | 33.7 (2.3) | 100.0 (0.0) | — | — | 0.227 (0.025) | −4.0 (0.8) |
| F68 | 6.5 (0.4) | 79.5 (3.8) | 409.5 (63.9) | 20.5 (3.8) | 0.229 (0.307) | −9.0 (0.3) |
| F68/DOXY | 5.4 (0.1) | 63.1 (3.2) | 191.6 (31.6) | 36.9 (3.2) | 0.335 (0.093) | −8.0 (0.5) |
| P85 | 22.8 (2.6) | 100.0 (0.0) | — | — | 0.286 (0.023) | −3.0 (1.0) |
| P85/DOXY | 18.9 (0.6) | 100.0 (0.0) | — | — | 0.291 (0.015) | −4.0 (0.5) |
| F127:P85 | 24.6 (1.3) | 100.0 (0.0) | — | — | 0.071 (0.019) | −6.5 (1.0) |
| F127:P85/DOXY | 25.4 (0.7) | 100.0 (0.0) | — | — | 0.075 (0.019) | −5.0 (0.8) |
| Naked AuNPs | 29.7 (3.6) | 100.0 (0.0) | — | — | 0.327 (0.114) | −20.9 (4.3) |
| Naked AuNPs/DOXY | — | — | 84.9 (3.1) | 93.5 (0.6) | 0.305 (0.024) | −8.1 (4.3) |
| cAuNPs-PMs and bAuNPs/PMs | D h (nm) (±S.D) | PDI (±S.D.) | Z-Pot (mV) (±S.D.) | |||
|---|---|---|---|---|---|---|
| Peak 1 | % intensity (±S.D.) | Peak 2 | % intensity (±S.D.) | |||
| Peak 1: smaller size population. Peak 2: larger size population. | ||||||
| cAuNPs-F127 | 33.6 (2.8) | 100.0 (0.0) | — | — | 0.219 (0.003) | −3.0 (0.7) |
| cAuNPs-F127/DOXY | 30.2 (0.4) | 100.0 (0.0) | — | — | 0.221 (0.018) | −3.1 (0.7) |
| bAuNPs/F127 | 24.2 (1.3) | 100.0 (0.0) | — | — | 0.187 (0.067) | −1.2 (0.2) |
| bAuNPs/F127/DOXY | 25.0 (3.0) | 100.0 (0.0) | — | — | 0.220 (0.029) | −8.6 (2.3) |
| cAuNPs-F68 | 10.2 (2.6) | 7.9 (0.9) | 102.4 (4.7) | 92.1 (0.9) | 0.320 (0.165) | −10.4 (1.3) |
| cAuNPs-F68/DOXY | 9.1 (1.2) | 7.0 (0.8) | 95.9 (1.3) | 93.0 (0.8) | 0.207 (0.039) | −1.2 (0.1) |
| bAuNPs/F68 | 6.4 (0.4) | 80.5 (2.1) | 47.2 (10.2) | 19.5 (2.1) | 0.251 (0.039) | −14.2 (2.0) |
| bAuNPs/F68/DOXY | 6.2 (0.2) | 38.8 (1.9) | 58.2 (12.0) | 61.2 (1.9) | 0.207 (0.039) | −7.5 (2.3) |
| cAuNPs-P85 | 20.5 (1.7) | 3.1 (2.6) | 123.5 (4.3) | 96.9 (2.6) | 0.288 (0.007) | −5.8 (0.5) |
| cAuNPs-P85/DOXY | 44.0 (10.5) | 18.8 (3.8) | 214.4 (21.8) | 81.2 (3.8) | 0.465 (0.037) | −7.1 (0.1) |
| bAuNPs/P85 | 30.6 (2.3) | 100.0 (0.0) | — | — | 0.229 (0.010) | −5.0 (1.5) |
| bAuNPs/P85/DOXY | 37.4 (3.9) | 100.0 (0.0) | — | — | 0.359 (0.018) | −9.7 (1.8) |
| cAuNPs-F127:P85 | 21.6 (0.4) | 41.7 (8.5) | 89.4 (9.9) | 58.3 (8.5) | 0.367 (0.012) | −2.8 (0.4) |
| cAuNPs-F127:P85/DOXY | 19.4 (1.9) | 18.8 (3.7) | 99.0 (15.0) | 81.2 (3.7) | 0.459 (0.076) | −5.1 (0.4) |
| bAuNPs/F127:P85 | 27.2 (0.4) | 100.0 (0.0) | — | — | 0.138 (0.026) | −9.6 (1.8) |
| bAuNPs/F127:P85/DOXY | 26.4 (0.6) | 100.0 (0.0) | — | — | 0.119 (0.002) | −10.6 (1.8) |
| Free PMs and naked Turkevich AuNPs | D h (nm) (±S.D) | PDI (±S.D.) | |||
| Peak 1 | % intensity (±S.D.) | Peak 2 | % intensity (±S.D.) | ||
| F127 | 23.9 (2.1) | 100.0 (0.0) | — | — | 0.310 (0.017) |
| F127/DOXY | 22.5 (1.8) | 100.0 (0.0) | — | — | 0.392 (0.028) |
| F68 | 5.1 (0.1) | 84.6 (2.6) | 507.5 (62.3) | 15.4 (2.6) | 0.232 (0.047) |
| F68/DOXY | 5.5 (0.2) | 77.6 (3.4) | 424.7 (44.9) | 22.4 (3.4) | 0.265 (0.041) |
| P85 | 12.2 (0.5) | 100.0 (0.0) | — | — | 0.233 (0.035) |
| P85/DOXY | 12.7 (0.6) | 100.0 (0.0) | — | — | 0.196 (0.049) |
| F127:P85 | 15.1 (0.7) | 100.0 (0.0) | — | — | 0.230 (0.108) |
| F127:P85/DOXY | 16.9 (0.5) | 100.0 (0.0) | — | — | 0.227 (0.057) |
| Naked AuNPs | 31.5 (3.2) | 100.0 (0.0) | — | — | 0.278 (0.104) |
| Naked AuNPs/DOXY | — | — | 172.1 (11.1) | 100.0 (0.0) | 0.343 (0.034) |
| cAuNPs-PMs and bAuNPs/PMs | D h (nm) (±S.D) | PDI (±S.D.) | |||
|---|---|---|---|---|---|
| Peak 1 | % intensity (±S.D.) | Peak 2 | % intensity (±S.D.) | ||
| Peak 1: smaller size population. Peak 2: larger size population. | |||||
| cAuNPs-F127 | 19.9 (7.5) | 16.5 (7.6) | 452.1 (3.3) | 83.5 (7.6) | 0.622 (0.107) |
| cAuNPs-F127/DOXY | 20.1 (0.6) | 15.8 (2.1) | 338.1 (44.8) | 83.2 (2.1) | 0.708 (0.169) |
| bAuNPs/F127 | 16.8 (3.0) | 21.6 (0.5) | 519.0 (91.4) | 78.4 (0.5) | 0.950 (0.050) |
| bAuNPs/F127/DOXY | 15.5 (0.8) | 28.4 (2.3) | 506.6 (13.5) | 71.6 (2.3) | 0.994 (0.011) |
| cAuNPs-F68 | — | — | 341.7 (38.7) | 100.0 (0.0) | 0.278 (0.076) |
| cAuNPs-F68/DOXY | — | — | 264.0 (15.7) | 100.0 (0.0) | 0.293 (0.092) |
| bAuNPs/F68 | 4.9 (0.1) | 11.3 (2.8) | 235.9 (6.2) | 88.7 (2.8) | 0.633 (0.066) |
| bAuNPs/F68/DOXY | 5.0 (0.4) | 14.3 (5.1) | 167.3 (5.2) | 85.7 (5.1) | 0.448 (0.187) |
| cAuNPs-P85 | 15.6 (1.8) | 11.0 (0.8) | 181.2 (8.3) | 89.0 (0.8) | 0.556 (0.023) |
| cAuNPs-P85/DOXY | 16.0 (1.5) | 10.6 (1.0) | 192.8 (12.3) | 89.4 (1.0) | 0.545 (0.030) |
| bAuNPs/P85 | 13.7 (0.7) | 67.5 (2.8) | 116.0 (10.6) | 32.5 (2.8) | 0.457 (0.045) |
| bAuNPs/P85/DOXY | 13.4 (0.9) | 67.4 (3.2) | 94.3 (9.6) | 32.6 (3.2) | 0.366 (0.131) |
| cAuNPs-F127:P85 | 101.2 (18.3) | 6.5 (4.0) | 993.3 (102.2) | 93.5 (4.0) | 0.434 (0.130) |
| cAuNPs-F127:P85/DOXY | 50.7 (5.4) | 8.7 (1.2) | 495.1 (17.6) | 91.3 (1.2) | 0.493 (0.036) |
| bAuNPs/F127:P85 | 16.7 (1.9) | 19.2 (2.4) | 676.6 (80.0) | 80.8 (2.4) | 0.943 (0.090) |
| bAuNPs/F127:P85/DOXY | 17.6 (1.7) | 40.9 (1.2) | 320.7 (30.1) | 59.1 (1.2) | 0.870 (0.075) |
3.3. Size, morphology, and stability
DLS measurements revealed that pristine micelles composed of F127, P85, and the F127:P85 combination (90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) at 37 °C exhibited hydrodynamic diameters (Dh) ranging from 23 to 25 nm at 1% w/v (Table 1) and from 12–24 nm at 10% w/v (Table 2), in agreement with previous reports from our group.56–63 In contrast, F68 displayed a bimodal size distribution at both concentrations, with a small-size population attributed to unimers (5–7 nm) and a larger population corresponding to micellar aggregates (410–508 nm) (Tables 1 and 2). NIST polystyrene latex nanoparticle standards (3020A and 3400A) were also analyzed as references, yielding Dh values of 24 nm and 418 nm, respectively, consistent with the nominal diameters specified by the manufacturer.
10) at 37 °C exhibited hydrodynamic diameters (Dh) ranging from 23 to 25 nm at 1% w/v (Table 1) and from 12–24 nm at 10% w/v (Table 2), in agreement with previous reports from our group.56–63 In contrast, F68 displayed a bimodal size distribution at both concentrations, with a small-size population attributed to unimers (5–7 nm) and a larger population corresponding to micellar aggregates (410–508 nm) (Tables 1 and 2). NIST polystyrene latex nanoparticle standards (3020A and 3400A) were also analyzed as references, yielding Dh values of 24 nm and 418 nm, respectively, consistent with the nominal diameters specified by the manufacturer.
Upon incorporation of DOXY, the Dh values of F127/DOXY, P85/DOXY, and F127:P85/DOXY at 1% w/v increased up to 34 nm, while sizes at 10% w/v ranged from 13 to 23 nm. These results indicate that micellar integrity and stability was preserved upon drug adsorption, maintaining size distributions compatible with systemic administration. Conversely, F68/DOXY exhibited a reduction in the size of the aggregate population relative to free F68, with Dh values of 192 nm at 1% w/v and 425 nm at 10% w/v (Tables 1 and 2), suggesting that DOXY incorporation may contribute to the stabilization of this formulation.
AuNPs synthesized via the Turkevich method exhibited initial Dh values of approximately 30–32 nm at both 25 and 250 μM gold concentrations. However, upon DOXY incorporation, their sizes increased markedly to 85 and 172 nm, respectively, indicating a strong tendency toward aggregation (Tables 1 and 2). This aggregation was further corroborated by a reduction in Z-Pot from −21 mV to −8 mV, suggesting destabilization of AuNPs due to DOXY-induced surface interactions (Table 1). The observed red shift in the UV-Vis absorbance spectra and the color change from reddish to violet provide additional evidence of aggregation (Fig. S3†). The shift in the surface plasmon resonance (SPR) peak further supports the occurrence of DOXY-mediated interactions leading to particle clustering (Fig. S3†). Notably, this destabilizing behavior was only observed in bare AuNPs synthesized via the Turkevich method and not in the AuNPs formulated with PEO-PPO copolymers (Fig. S3†). In the latter systems, DOXY incorporation did not induce aggregation, and the nanosystems remained colloidally stable.
This difference highlights the role of PEO-PPO copolymers not only as reducing and stabilizing agents in the green synthesis process, but also as key elements that confer AuNPs/drug compatibility. The copolymers provide a steric and protective interface that prevents unfavorable interactions between DOXY and the gold surface, enabling efficient drug incorporation while preserving nanoparticle integrity. These findings underscore the importance of copolymer stabilization in ensuring both the physicochemical stability of the nanosystem and its suitability for subsequent biological evaluations.
In contrast, cAuNPs-PMs complexes exhibited significantly smaller Dh values, ranging from 10 to 34 nm at 1% w/v (Table 1), suggesting the formation of more compact and stable structures. At higher copolymer concentrations (10% w/v), the Dh values increased to a range of 17 to 342 nm, with a second, larger population reaching up to 993 nm observed at 250 μM gold (Table 2). This suggests that elevated copolymer concentrations may favor AuNPs clustering.
Additionally, particle size distribution varied depending on the formulation. For instance, cAuNPs-F127 (1% w/v) displayed a mean Dh of 34 nm with a PDI of 0.219, while its DOXY-adsorbed counterpart (cAuNPs-F127/DOXY) retained similar size (30 nm) and PDI (0.221), indicating good colloidal stability after drug incorporation. Similarly, bAuNPs/F127:P85 blends exhibited a smaller size of 27 nm, and their DOXY-loaded forms maintained low PDI values (0.119–0.138), highlighting excellent preservation of nanoparticle uniformity upon drug addition.
Finally, all formulations maintained stable Dh and PDI values for at least 20 days (data not shown), further confirming the long-term stability and robustness of the nanosystems.
The PDI values obtained in our study align with the DLS-derived size measurements and primarily reflect the influence of copolymer concentration rather than the specific copolymer type. All cAuNPs-PMs complexes without DOXY, prepared using 1% w/v copolymer concentration, whether F127, F68, P85, or the F127:P85 combination, exhibited relatively low and consistent PDI values (ranging from 0.219 to 0.367), indicative of monodisperse AuNPs populations with minimal aggregation (Table 1).
For cAuNPs-PMs complexes incorporating DOXY (25 μM), the PDI values ranged from 0.207 to 0.465, demonstrating that DOXY incorporation did not significantly impact particle size distribution (Table 1).
In contrast, increasing the copolymer concentration to 10% w/v resulted in a marked rise in PDI values, reaching up to 0.9. This increase is consistent with broader particle size distributions and the emergence of aggregated subpopulations (Peak 2, Table 2). These secondary populations were particularly evident in cAuNPs-PMs complexes and bAuNPs/PMs blends formulated with F127 (with and without DOXY) (Table 2), whereas they were absent in the corresponding systems at 1% w/v, where only a single, well-defined peak was observed (Table 1). This absence accounts for the lower PDI values under those conditions and supports the formation of more homogeneous nanosystems at lower copolymer concentrations.
Interestingly, the 1% w/v formulations used for in vitro assays (Table 1) were obtained by diluting the 10% w/v preparations (Table 2), resulting in narrower size distributions compared to those synthetized directly at 1% w/v (data not shown). This improved performance likely stems from the enhanced reducing capacity of PEO-PPO copolymers at higher concentrations during AuNPs formation, followed by a more controlled dilution step post-synthesis. Our results suggest that elevated copolymer concentrations promote the formation of larger and more heterogeneous assemblies, possibly due to increased viscosity and micellar crowding, which can hinder proper gold nanoparticles formation and favor aggregation (Table 2).
While structural differences between F127, F68 and P85 (e.g., molecular weight, HLB, PPO/PEO ratio and experimental CMC) can influence micelle stability and drug association, our data indicate that these factors do not significantly impact size and PDI under the tested conditions. Instead, the concentration of the copolymer plays a more decisive role in controlling the uniformity of gold nanoparticle size. Overall, these findings highlight the importance of optimizing copolymer concentration during formulation, as it directly affects nanoparticle homogeneity, a key parameter for batch-to-batch reproducibility and predictable in vivo performance.
Z-Pot values remained negative across most formulations, ranging from −1 mV to −21 mV, indicating that the particles remained stable and resisted aggregation under physiological conditions (Table 1). For instance, the bAuNPs/F127 blend formulation (1% w/v) exhibited a Z-Pot of −1 mV, while bAuNPs/F127/DOXY blend showed a more negative value of −9 mV (Table 1). In contrast, naked AuNPs synthesized via the Turkevich method exhibited a Z-Pot of −21 mV, which decreased to −8 mV upon DOXY incorporation, highlighting the strong aggregation tendency of these nanoparticles in the presence of DOXY (Table 1).
It is important to note that the PEO-PPO block copolymers naturally exhibit Z-Pot values that are neutral to slightly negative, as previously described by our group.56–63 These values do not indicate nanosystem instability, as they remain constant over time, preserving the original particle size. This behavior is attributed to the nature of the copolymer, which lacks surface charges, rather than intrinsic tendency toward aggregation.
These findings were further corroborated by TEM images, which confirmed the spherical morphology of the nanoparticles. In the case of naked AuNPs in the presence of DOXY, extensive aggregation was observed, with nanoparticles forming chain-like clusters and losing their spherical shape (Fig. 4), a result consistent with the changes observed in UV-Vis, SPR and DLS measurements. In contrast, some degree of compaction was evident in the AuNPs complexes and blends with DOXY, the nanoparticles largely retained their spherical morphology, with only mild aggregation (Fig. 4). This preservation of shape in the DOXY-adsorbed AuNPs, relative to the unloaded systems, further supports the stabilizing role of the copolymer matrix.
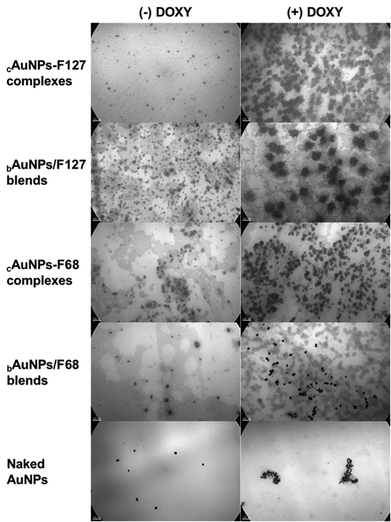 | ||
Fig. 4 TEM images of cAuNPs-PMs complexes and bAuNPs/PMs blends, both without (−) and with (+) DOXY, using F127 and F68 at 10% w/v. Magnification: 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000×; scale bar: 100 nm. 000×; scale bar: 100 nm. | ||
It is worth noting that the star-shaped or flattened structures observed in some DOXY-loaded AuNPs complexes and blends (Fig. 4) may arise from sample preparation artifacts, particularly those associated with the drying process and interactions with the carbon-coated TEM grid.59–63 These effects are frequently reported for polymer-stabilized nanosystems, especially when high copolymer concentrations are employed, as in this case (10% w/v copolymer), and typically reflect the morphology of the sample in its dried state, rather than its native conformation in dispersion.59–63 Under hydrated conditions and lower concentrations (1% w/v copolymer), the nanoparticles maintain a spherical shape with a well-defined hydrodynamic diameter that includes the solvation layer, as evidenced by the DLS measurements (Table 1), which indicate a monodisperse size distribution.
3.4. Biological effects of cAuNPs-PMs/DOXY and bAuNPs/PMs/DOXY: cytotoxicity and selectivity
Cytotoxicity assays revealed that both cAuNPs-PMs/DOXY complexes and bAuNPs/PMs/DOXY blends induced a concentration-dependent decrease in cell viability in melanoma cell lines A-375 and IIB-MEL-J (Fig. 5). Notably, bAuNPs/F127:P85/DOXY blends reduced cell viability to 24% in at 50 μM (Fig. 5A). In contrast, this dose-dependent effect was less pronounced for free DOXY, suggesting that its cytotoxic activity in melanoma cells may be limited or may require higher concentrations to achieve a comparable effect (Fig. 5A and B). This difference can be attributed to the enhanced cellular uptake of DOXY when associated with AuNPs, which prolongs retention and enhances cellular entry. Additionally, the presence of copolymer PEO-PPO coating likely improves drug internalization, leading to greater intracellular accumulation compared to the diffusion-limited uptake of free DOXY.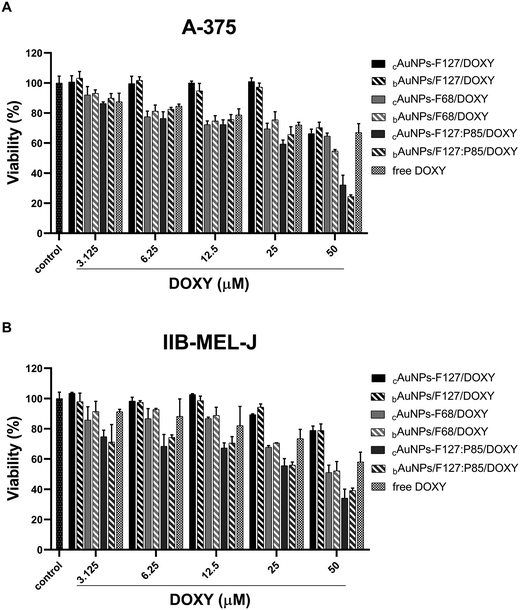 | ||
| Fig. 5 Cytotoxicity of free DOXY and DOXY-combined AuNPs complexes and blends in (A) A-375 and (B) IIB-MEL-J cells. | ||
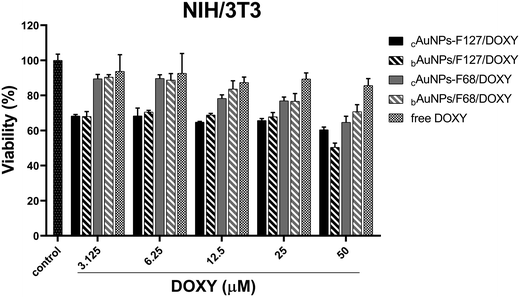
This selective cytotoxicity highlights the potential of these nanosystems as targeted therapeutic agents for melanoma, while minimizing off-target toxicity in normal cells. The moderate viability observed in NIH/3T3 cells further confirms that the nanosystems containing DOXY (up to 25 μM) do not induce significant intrinsic cytotoxic effects, reinforcing their biocompatibility and favorable safety profile (Fig. 6). The lowest cell viability recorded for bAuNPs/PMs blends with DOXY (50 μM) in normal cells was 50% (bAuNPs/F127/DOXY) (Fig. 6). This finding justifies the selection of 25 μM DOXY (with >70% viability) for radiosensitization assays, ensuring minimal cytotoxic effects on normal cells.
Malignant cells were more susceptible to the combined action of DOXY and AuNPs-based delivery, especially at elevated concentrations (Fig. 5 and 6), possibly due to enhanced internalization and accumulation, disruption of mitochondrial function, or amplification of oxidative stress pathways. In this sense, it is important to mention that melanoma cells have higher basal levels of reactive species of oxygen (ROS) than normal cells.91
Moreover, naked AuNPs did not show any toxic effects at any of the studied concentrations in A-375, IIB-MEL-J, and NIH/3T3 cells (Fig. 7A), further confirming the biocompatibility of the nanoplatform components. Free F127 and F68 copolymers up to 1% w/v, exhibited minimal cytotoxicity in both malignant and normal cell lines (Fig. S4A and B†). A key finding in this study is the role of the F127:P85 combination in enhancing nanosystem stability and mitigating the cytotoxic observed when using P85 alone (Fig. S4C and D†). Although P85 has been extensively studied for its ability to inhibit the P-glycoprotein (P-gp) efflux pump,68,69 our results show that its standalone use increased toxicity in both melanoma and normal cells (Fig. S4C†). However, co-formulation P85 with F127 in a 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 (F127:P85) ratio alleviated this effect, enabling the incorporation of P85 into our nanosystems without compromising cell viability (Fig. S4D†). This is especially relevant for overcoming multidrug resistance (MDR) in melanoma, as P85-mediated inhibition of P-gp can enhance intracellular drug accumulation. These findings align with previous studies and emphatize the importance of optimizing copolymer characteristics for drug delivery.62,63,66,67
10 (F127:P85) ratio alleviated this effect, enabling the incorporation of P85 into our nanosystems without compromising cell viability (Fig. S4D†). This is especially relevant for overcoming multidrug resistance (MDR) in melanoma, as P85-mediated inhibition of P-gp can enhance intracellular drug accumulation. These findings align with previous studies and emphatize the importance of optimizing copolymer characteristics for drug delivery.62,63,66,67
The marked reduction in melanoma cell viability is primarily attributed to DOXY, a known anticancer agent with documented antiproliferative effects. Interestingly, free DOXY alone led to a significant decrease in cell viability only at very high concentrations (above 100 μM, Fig. 7B). However, its incorporation into the nanosystem significantly enhanced its antitumoral effect (Fig. 5), likely due to improved cellular internalization and retention. The nanosystems serve as effective drug carriers, potentially boosting DOXY delivery and bioavailability, which may lead to improved therapeutic outcomes. Importantly, the adsorption of DOXY onto these nanosystems may prolong drug retention, protecting it from premature degradation, and ensure sustained intracellular release. This mechanism could explain the enhanced cytotoxic observed in melanoma cells treated with the nanosystem formulations compared to free DOXY (Fig. 5).
3.5. Intracellular uptake and radiosensitization effects of cAuNPs-PMs/DOXY and bAuNPs/PMs/DOXY
To further investigate the intracellular behavior of these nanosystems, we employed X-ray fluorescence (XRF) spectroscopy to quantify gold uptake and fluorescence spectroscopy to assess DOXY accumulation in A-375 melanoma cells. The treatments included free DOXY (25 μM) and naked Turkevich AuNPs (25 μM) as controls, along with two nanosystem complexes containing 25 μM chloroauric acid, 1% w/v copolymer, and 25 μM DOXY: (i) cAuNPs-F127/DOXY and (ii) cAuNPs-F127:P85/DOXY. These formulations were selected based on their promising characteristics and potential differences in cellular uptake mechanisms. Specifically, PEO-PPO-stabilized AuNPs may enhance internalization due to their amphiphilic nature and interaction with the cell membrane, whereas naked AuNPs possibly rely on passive diffusion. Additionally, the incorporation of P85 provides an alternative mechanism for cellular entry. Finally, the association of DOXY with mitochondria targeting capabilities may further influence uptake dynamics, potentially altering intracellular accumulation and distribution.Cellular internalization studies revealed significant differences depending on the surface coating, drug association, and physicochemical characteristics of the nanosystems. XRF results showed comparable intracellular gold concentrations for the cAuNPs-F127/DOXY and cAuNPs-F127:P85/DOXY complexes formulations (0.234 and 0.236 mg L−1, respectively; Table 3), indicating that the addition of P85 did not significantly alter AuNPs internalization under these conditions. However, naked AuNPs synthesized by the Turkevich method, with similar size (∼30 nm) but a more negative Z-Pot (−21 mV) (Table 1), exhibited a markedly lower intracellular gold content (0.172 mg L−1, Table 3). This suggests that coating with PEO-PPO polymers, along with DOXY association, enhances cellular internalization compared to unmodified Turkevich AuNPs, likely due to improved colloidal stability, surface masking of negative charge, or active drug-mediated uptake.
| Formulations | Au element (Au0) Conc./(mg L−1) |
|---|---|
| Control (untreated) | 0 |
| Free DOXY | 0 |
| Naked Turkevich AuNPs | 0.172 |
| cAuNPs-F127/DOXY | 0.234 |
| cAuNPs-F127:P85/DOXY | 0.236 |
Fluorescence spectroscopy confirmed that cAuNPs-PMs complexes formulations significantly improved DOXY delivery to A-375 cells compared to free DOXY, which showed only 1% uptake (Fig. 8). Specifically, cAuNPs-F127/DOXY, with an average Dh of 30 nm (PDI ∼ 0.2) and Z-Pot value of −3 mV (Table 1), achieved 84% internalization of DOXY (Fig. 8). In comparison, cAuNPs-F127:P85/DOXY, with a smaller population size (19 nm) and slightly more negative Z-Pot (−5 mV), showed 97% DOXY internalization, despite a higher PDI (PDI > 0.4, Table 1). These differences suggest that the reduced nanoparticle size and the distinctive composition, potentially favored by the F127:P85 combination, enhance cellular uptake, even at the expense of increased polydispersity.
The use of P85 as a co-stabilizer may also modulate cell-nanoparticle interactions through mechanisms such as P-gp inhibition, favoring drug retention. Although this effect was not evident in XRF measurements of gold content, it may explain the higher DOXY internalization observed in the presence of P85. Collectively, these findings underscore the importance of nanosystem design (particularly size, surface charge, and polymer composition) for optimizing cellular delivery and potential therapeutic efficacy.
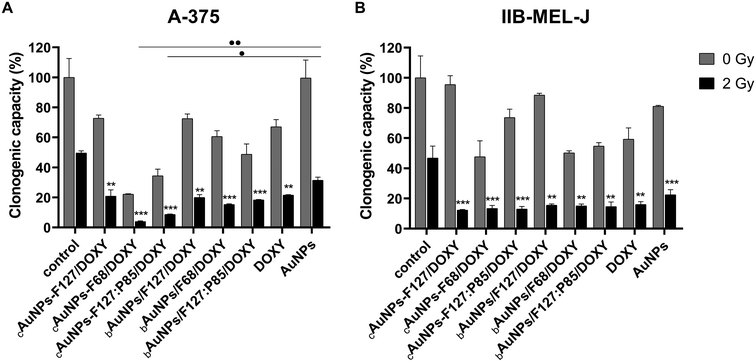
The combination of cAuNPs-PMs/DOXY complexes and bAuNPs/PMs/DOXY blends with radiation (2 Gy) significantly enhanced cytotoxicity. Pre-incubation with these nanosystems reduced the clonogenic capacity of melanoma cells, improving the overall effectiveness of radiotherapy (Fig. 9).
In A-375 cells, cAuNPs-F68/DOXY and cAuNPs-F127:P85/DOXY complexes were the most effective in reducing clonogenic capacity (Fig. 9A). These formulations not only showed a significant difference compared to the irradiated (2 Gy) untreated control but also compared to irradiated naked AuNPs synthesized via Turkevich. This suggests that AuNPs exhibit a greater radiosensitizing effect when associated with PEO-PPO copolymers.
In IIB-MEL-J cells, cAuNPs-PMs/DOXY complexes generally had a stronger impact on clonogenic capacity compared to bAuNPs/PMs/DOXY blends (Fig. 9B), suggesting that these formulations may interact differently with cells and radiation.
When analyzing the surviving fraction (SF2), all treatments reduced cell survival at 2 Gy compared to radiation alone (Fig. 10), reinforcing the radiosensitizing potential of these nanosystems. Although the differences were not statistically significant in all cases, cAuNPs-PMs/DOXY complexes showed a stronger effect than free DOXY or naked AuNPs. Differential radiosensitization effects were observed between the two melanoma cell lines. In A-375 cells, cAuNPs-F68/DOXY complexes reduced the surviving fraction more effectively than naked AuNPs or free DOXY (Fig. 10A). However, in IIB-MEL-J cells, cAuNPs-F127/DOXY complexes exhibited the greatest impact on survival (Fig. 10B). These results suggest that the nanosystem composition, alongside the intrinsic characteristics of each melanoma cell line, significantly influences treatment efficacy.
Although the inclusion of P85 in the F127:P85 formulation led to a noticeable increase in DOXY uptake (Fig. 8), it did not result in a significant enhancement of radiosensitization compared to F127 alone (Fig. 9 and 10). While P85 may contribute to drug internalization, this effect was not reflected in a corresponding increase in radiosensitizing activity. The lack of difference could be attributed to the specific experimental model or conditions employed in this study.
The enhanced radiation effects observed can be attributed to several complementary mechanisms. Firstly, AuNPs exhibit a high atomic number (Z = 79), which increases local energy deposition upon gamma radiation exposure via the photoelectric effect and Compton scattering.31 This leads to the generation of low-energy secondary electrons (e.g., Auger electrons and photoelectrons), which induce significant oxidative stress and cause localized DNA double-strand breaks. Secondly, AuNPs can catalyze the radiolysis of water, further enhancing the production of ROS, which are known to damage cellular components including nucleic acids, proteins, and membranes.92 Thirdly, the incorporation of DOXY in the nanosystems may synergistically contribute to radiosensitization by interfering with mitochondrial function, impairing oxidative phosphorylation, and promoting ROS accumulation.76 DOXY has also been reported to inhibit matrix metalloproteinases (MMPs) and disrupt cell cycle progression, thereby increasing cellular vulnerability to radiation-induced damage.83 Lastly, the use of PEO-PPO copolymers in the formulation can modulate membrane permeability and nanoparticle–cell interactions, potentially facilitating endosomal escape and enabling deeper cytoplasmic or mitochondrial delivery of the therapeutic payload.54 Taken together, these mechanisms act in concert to reduce clonogenic survival and enhance radiation-induced cytotoxicity.
The selective cytotoxicity of these nanosystems toward melanoma cells, while sparing normal fibroblasts, further underscores their therapeutic potential. The lack of significant toxicity in non-tumorigenic NIH/3T3 cells suggests that these formulations could be integrated into radiotherapy regimens with minimal off-target effects, thereby improving the therapeutic window.
In conclusion, these findings highlight the potential of AuNPs-PMs based nanosystems as effective multimodal platforms for both chemotherapy and radiotherapy, enhancing melanoma treatment. Further research is needed to uncover the molecular mechanisms behind the enhanced radiosensitivity and to validate the therapeutic benefits in vivo.
4. Conclusions and perspectives
This study presents a sustainable, green approach for the synthesis of colloidal gold nanoparticles (AuNPs) using PEO-PPO block copolymers, which function as both reducing and stabilizing agents. Unlike traditional synthesis methods that often require organic solvents, reducing agents, or hazardous reagents, this environmentally friendly technique relies solely on aqueous media and FDA-approved biocompatible copolymers, minimizing environmental impact and cytotoxicity. The AuNPs produced exhibit exceptional stability, biocompatibility, and a superior capacity for adsorbing therapeutic agents like DOXY, making them promising candidates for melanoma treatment.A significant finding of this work is the enhanced intracellular accumulation of DOXY in the AuNPs formulations, with the incorporation of P85 playing a crucial and differential role in promoting drug uptake, likely by inhibiting P-glycoprotein (P-gp) activity, a key factor in overcoming multidrug resistance. XRF analysis confirmed that gold uptake in the cAuNPs-F127/DOXY and cAuNPs-F127:P85/DOXY formulations was comparable, emphasizing that copolymer composition does not substantially impact AuNPs internalization. Notably, both formulations exhibited greater gold uptake than Turkevich-synthesized AuNPs, highlighting the advantages of the green synthesis approach in improving nanoparticle internalization and formulation stability. In line with this, fluorescence spectroscopy demonstrated that cAuNPs-PMs complexes significantly improved DOXY uptake in melanoma cells, with the F127:P85-based formulation reaching the highest internalization rate (97%), underscoring the role of nanostructure composition and size in enhancing cellular delivery efficiency.
In addition to their role as drug delivery vehicles, these AuNPs-based nanosystems also demonstrated a significant radiosensitizing effect, enhancing melanoma cell sensitivity to radiation-induced damage. The reduction in clonogenic survival following nanoparticle pre-incubation indicates their ability to amplify radiation absorption, as a result of the high atomic number of gold that enhances the photoelectric effect and secondary electron generation, leading to increased ROS production, DNA damage, and mitotic cell death. Moreover, DOXY disrupts mitochondrial function, further sensitizing melanoma cells to radiotherapy.
These findings underscore the dual functionality of AuNPs in cancer therapy: as effective drug carriers and as enhancers of radiotherapy efficacy. The integration of chemotherapy and radiotherapy in a single nanosystem offers a promising solution to the ongoing challenges of drug resistance, poor pharmacokinetics, and inefficient tumor penetration.
Furthermore, this study highlights the potential of green nanotechnology, not only for its environmental and regulatory benefits but also for its tangible therapeutic advantages. The use of PEO-PPO block copolymers facilitates a cost-effective, scalable synthesis process that can be easily translated to industrial-scale production using standard pharmaceutical equipment.
Looking ahead, future studies should explore the application of this nanotechnology platform in other cancer types, especially those resistant to conventional chemotherapy and radiotherapy. Refining nanoparticle properties such as size, surface charge, and drug-loading capacity will further optimize therapeutic outcomes. Additionally, integrating combination therapies like immunotherapy or gene therapy could offer even greater therapeutic potential. The inclusion of imaging techniques within these systems could lead to the development of advanced theranostic nanoformulations, enabling real-time monitoring of drug delivery and therapeutic response.
In conclusion, this study provides the groundwork for the development of multimodal cancer therapies that integrate nanotechnology and radiotherapy. The promising results suggest that these nanosystems hold great potential to revolutionize melanoma treatment by enabling more personalized, effective therapeutic strategies.
Author contributions
Conceptualization, A.C., J.M., H.D. and R.J.G.; methodology, A.C., J.M., M.N.A., H.D., and R.J.G.; software, A.C., J.M., H.D., and R.J.G.; validation, A.C., J.M., H.D., and R.J.G.; formal analysis, A.C., J.M., H.D., and R.J.G.; investigation, A.C., J.M., M.N.A., H.D., and R.J.G.; resources, A.C., J.M., M.N.A., H.D., and R.J.G.; data curation, A.C., and R.J.G.; writing – original draft preparation, A.C., J.M., H.D., and R.J.G.; writing – review and editing, A.C., J.M., H.D., and R.J.G.; visualization, A.C., J.M., M.N.A., H.D., and R.J.G.; supervision, J.M., H.D., and R.J.G.; project administration, J.M., H.D., and R.J.G.; funding acquisition, A.C., J.M., H.D., and R.J.G. All authors have read and agreed to the published version of the manuscript.Data availability
Data for this article are available at Repositorio Institucional CONICET Digital https://datosdeinvestigacion.conicet.gov.ar/. Complete data are available upon request from the authors..
Conflicts of interest
There are no conflicts to declare regarding the publication of this article.Acknowledgements
A. C. gratefully acknowledges the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) for her doctoral fellowship. M. N. A. and H. D. are members of the Comisión Nacional de Energía Atómica. J. M., H. D., and R. J. G. are staff members of CONICET. We sincerely thank Dr Lina Formica (UNITEFA, Universidad Nacional de Córdoba, Argentina) for her invaluable assistance with Z-potential measurements using DLS, as well as CEBIRSA S.A. (Argentina) for providing the gamma irradiation service. This work was partially supported by CONICET (PIP 11220170100098CO), the Universidad de Buenos Aires (UBA-CyT 20020170200301BA and UBA-CyT 20020190200182BA), and the Agencia Nacional de Promoción de la Investigación, el Desarrollo Tecnológico y la Innovación (PICT 2019-03331).References
- S. Chen, S. Long, Y. Liu, S. Wang, Q. Hu, L. Fu and D. Luo, Front. Oncol., 2024, 14, 1432869 CrossRef CAS PubMed
.
- Y. Chen, Y. Deng, Y. Li, Y. Qin, Z. Zhou, H. Yang and Y. Sun, ACS Appl. Mater. Interfaces, 2024, 16, 21546–21556 CrossRef CAS PubMed
.
- D. Y. Sun, Y. J. Hu, X. Li, J. Peng, Z. J. Dai and S. Wang, Int. Immunopharmacol., 2025, 144, 113392 CrossRef CAS PubMed
.
- P. B. Chapman, A. Hauschild, C. Robert, J. B. Haanen, P. Ascierto, J. Larkin, R. Dummer, C. Garbe, A. Testori, M. Maio, D. Hogg, P. Lorigan, C. Lebbe, T. Jouary, D. Schadendorf, A. Ribas, S. J. O'Day, J. A. Sosman, J. M. Kirkwood, A. M. M. Eggermont, B. Dreno, K. Nolop, J. Li, B. Nelson, J. Hou, R. J. Lee, K. T. Flaherty and G. A. McArthur, N. Engl. J. Med., 2011, 364, 2507–2516 CrossRef CAS PubMed
.
- F. S. Hodi, S. J. O'Day, D. F. McDermott, R. W. Weber, J. A. Sosman, J. B. Haanen, R. Gonzalez, C. Robert, D. Schadendorf, J. C. Hassel, W. Akerley, A. J. M. van den Eertwegh, J. Lutzky, P. Lorigan, J. M. Vaubel, G. P. Linette, D. Hogg, C. H. Ottensmeier, C. Lebbé, C. Peschel, I. Quirt, J. I. Clark, J. D. Wolchok, J. S. Weber, J. Tian, M. J. Yellin, G. M. Nichol, A. Hoos and W. J. Urba, N. Engl. J. Med., 2010, 363, 711–723 CrossRef CAS PubMed
.
- S. L. Topalian, F. S. Hodi, J. R. Brahmer, S. N. Gettinger, D. C. Smith, D. F. McDermott, J. D. Powderly, R. D. Carvajal, J. A. Sosman, M. B. Atkins, P. D. Leming, D. R. Spigel, S. J. Antonia, L. Horn, C. G. Drake, D. M. Pardoll, L. Chen, W. H. Sharfman, R. A. Anders, J. M. Taube, T. L. McMiller, H. Xu, A. J. Korman, M. Jure-Kunkel, S. Agrawal, D. McDonald, G. D. Kollia, A. Gupta, J. M. Wigginton and M. Sznol, N. Engl. J. Med., 2012, 366, 2443–2454 CrossRef CAS PubMed
.
- P. A. Prieto, J. C. Yang, R. M. Sherry, M. S. Hughes, U. S. Kammula, D. E. White, C. L. Levy, S. A. Rosenberg and G. Q. Phan, Clin. Cancer Res., 2012, 18, 2039–2047 CrossRef CAS PubMed
.
- A. Reuben, C. N. Spencer, P. A. Prieto, V. Gopalakrishnan, S. M. Reddy, J. P. Miller, X. Mao, M. P. De Macedo, J. Chen, X. Song, H. Jiang, P.-L. Chen, H. C. Beird, H. R. Garber, W. Roh, K. Wani, E. Chen, C. Haymaker, M.-A. Forget, L. D. Little, C. Gumbs, R. L. Thornton, C. W. Hudgens, W.-S. Chen, J. Austin-Breneman, R. S. Sloane, L. Nezi, A. P. Cogdill, C. Bernatchez, J. Roszik, P. Hwu, S. E. Woodman, L. Chin, H. Tawbi, M. A. Davies, J. E. Gershenwald, R. N. Amaria, I. C. Glitza, A. Diab, S. P. Patel, J. Hu, J. E. Lee, E. A. Grimm, M. T. Tetzlaff, A. J. Lazar, I. I. Wistuba, K. Clise-Dwyer, B. W. Carter, J. Zhang, P. A. Futreal, P. Sharma, J. P. Allison, Z. A. Cooper and J. A. Wargo, Npj Genomic Med., 2017, 2, 1–11 CrossRef CAS PubMed
.
- M. S. Lawrence, P. Stojanov, P. Polak, G. V. Kryukov, K. Cibulskis, A. Sivachenko, S. L. Carter, C. Stewart, C. H. Mermel, S. A. Roberts, A. Kiezun, P. S. Hammerman, A. McKenna, Y. Drier, L. Zou, A. H. Ramos, T. J. Pugh, N. Stransky, E. Helman, J. Kim, C. Sougnez, L. Ambrogio, E. Nickerson, E. Shefler, M. L. Cortés, D. Auclair, G. Saksena, D. Voet, M. Noble, D. DiCara, P. Lin, L. Lichtenstein, D. I. Heiman, T. Fennell, M. Imielinski, B. Hernandez, E. Hodis, S. Baca, A. M. Dulak, J. Lohr, D.-A. Landau, C. J. Wu, J. Melendez-Zajgla, A. Hidalgo-Miranda, A. Koren, S. A. McCarroll, J. Mora, R. S. Lee, B. Crompton, R. Onofrio, M. Parkin, W. Winckler, K. Ardlie, S. B. Gabriel, C. W. M. Roberts, J. A. Biegel, K. Stegmaier, A. J. Bass, L. A. Garraway, M. Meyerson, T. R. Golub, D. A. Gordenin, S. Sunyaev, E. S. Lander and G. Getz, Nature, 2013, 499, 214–218 CrossRef CAS PubMed
.
- R. I. Hartman and J. Y. Lin, Hematol./Oncol. Clin. North Am., 2019, 33, 25–38 CrossRef PubMed
.
- V. C. Thipe, A. R. Karikachery, P. Çakılkaya, U. Farooq, H. H. Genedy, N. Kaeokhamloed, D.-H. Phan, R. Rezwan, G. Tezcan, E. Roger and K. V. Katti, J. Drug Delivery Sci. Technol., 2022, 70, 103256 CrossRef CAS
.
- D. Bobo, K. J. Robinson, J. Islam, K. J. Thurecht and S. R. Corrie, Pharm. Res., 2016, 33, 2373–2387 CrossRef CAS PubMed
.
- T. Wu, X. Duan, C. Hu, C. Wu, X. Chen, J. Huang, J. Liu and S. Cui, Artif. Cells, Nanomed., Biotechnol., 2019, 47, 512–523 CrossRef CAS PubMed
.
- M. Yafout, A. Ousaid, Y. Khayati and I. S. El Otmani, Sci. Afr., 2021, 11, e00685 CAS
.
- Q. Gao, J. Zhang, J. Gao, Z. Zhang, H. Zhu and D. Wang, Front. Bioeng. Biotechnol., 2021, 9, 647905 CrossRef PubMed
.
- R. Kannan, A. Zambre, N. Chanda, R. Kulkarni, R. Shukla, K. Katti, A. Upendran, C. Cutler, E. Boote and K. V. Katti, Wiley Interdiscip. Rev.:Nanomed. Nanobiotechnol., 2012, 4, 42–51 CAS
.
- N. Chanda, P. Kan, L. D. Watkinson, R. Shukla, A. Zambre, T. L. Carmack, H. Engelbrecht, J. R. Lever, K. Katti, G. M. Fent, S. W. Casteel, C. J. Smith, W. H. Miller, S. Jurisson, E. Boote, J. D. Robertson, C. Cutler, M. Dobrovolskaia, R. Kannan and K. V. Katti, Nanomedicine, 2010, 6, 201–209 CrossRef CAS PubMed
.
- R. Zhao, J. Xiang, B. Wang, L. Chen and S. Tan, Bioinorg. Chem. Appl., 2022, 2022, 2444516 CrossRef PubMed
.
- A. Nel, E. Ruoslahti and H. Meng, ACS Nano, 2017, 11, 9567–9569 CrossRef CAS PubMed
.
- H. Maeda, J. Wu, T. Sawa, Y. Matsumura and K. Hori, J. Controlled Release, 2000, 65, 271–284 CrossRef CAS PubMed
.
- L. C. Kennedy, A. S. Bear, J. K. Young, N. A. Lewinski, J. Kim, A. E. Foster and R. A. Drezek, Nanoscale Res. Lett., 2011, 6, 283 CrossRef PubMed
.
- S. D. Perrault, C. Walkey, T. Jennings, H. C. Fischer and W. C. W. Chan, Nano Lett., 2009, 9, 1909–1915 CrossRef CAS PubMed
.
- J. W. Nichols and Y. H. Bae, J. Controlled Release, 2014, 190, 451–464 CrossRef CAS PubMed
.
- Y. Lu and P. S. Low, Adv. Drug Delivery Rev., 2002, 54, 675–693 CrossRef CAS PubMed
.
- J. D. Rybka, Rep. Pract. Oncol. Radiother., 2019, 24, 152–157 CrossRef PubMed
.
- N. Goswami, Z. Luo, X. Yuan, D. T. Leong and J. Xie, Mater. Horiz., 2017, 4, 817–831 RSC
.
- A. Xu, F. Deng, Y. Chen, Y. Kong, L. Pan, Q. Liao, Z. Rao, L. Xie, C. Yao, S. Li, X. Zeng, X. Zhu, H. Liu, N. Gao, L. Xue, F. Chen, G. Xu, D. Wei, X. Zhou, Z. Li and X. Sheng, Biomed. Pharmacother., 2020, 130, 110525 CrossRef CAS PubMed
.
- W. Liu, B. Chen, H. Zheng, Y. Xing, G. Chen, P. Zhou, L. Qian and Y. Min, Pharmaceutics, 2021, 13, 1757 CrossRef CAS PubMed
.
- I. L. Ibañez, C. Notcovich, P. N. Catalano, M. G. Bellino and H. Durán, Cancer Lett., 2015, 359, 9–19 CrossRef PubMed
.
- C. Grissi, M. Taverna Porro, M. Perona, M. Atia, L. Negrin, M. S. Moreno, J. Sacanell, M. S. Olivera, M. del Grosso, H. Durán and I. L. Ibañez, Radiat. Environ. Biophys., 2023, 62, 357–369 CrossRef CAS PubMed
.
- J. F. Hainfeld, F. A. Dilmanian, Z. Zhong, D. N. Slatkin, J. A. Kalef-Ezra and H. M. Smilowitz, Phys. Med. Biol., 2010, 55, 3045 CrossRef CAS PubMed
.
- W. N. Rahman, N. Bishara, T. Ackerly, C. F. He, P. Jackson, C. Wong, R. Davidson and M. Geso, Nanomedicine, 2009, 5, 136–142 CrossRef CAS PubMed
.
- D. B. Chithrani, S. Jelveh, F. Jalali, M. van Prooijen, C. Allen, R. G. Bristow, R. P. Hill and D. A. Jaffray, Radiat. Res., 2010, 173, 719–728 CAS
.
- K. T. Butterworth, J. A. Coulter, S. Jain, J. Forker, S. J. McMahon, G. Schettino, K. M. Prise, F. J. Currell and D. G. Hirst, Nanotechnology, 2010, 21, 295101 CrossRef CAS PubMed
.
- C. Cunningham, M. de Kock, M. Engelbrecht, X. Miles, J. Slabbert and C. Vandevoorde, Front. Public Health, 2021, 9, 699822 CrossRef PubMed
.
- K. T. Butterworth, S. J. McMahon, F. J. Currell and K. M. Prise, Nanoscale, 2012, 4, 4830–4838 RSC
.
- W. Ye, J. Yan, Q. Ye and F. Zhou, J. Phys. Chem. C, 2010, 114, 15617–15624 CrossRef CAS
.
- M. Brust, M. Walker, D. Bethell, D. J. Schiffrin and R. Whyman, J. Chem. Soc., Chem. Commun., 1994, 801–802 RSC
.
- N. R. Jana, L. Gearheart and C. J. Murphy, Adv. Mater., 2001, 13, 1389–1393 CrossRef CAS
.
- B. Nikoobakht and M. A. El-Sayed, Chem. Mater., 2003, 15, 1957–1962 CrossRef CAS
.
- L. Longenberger and G. Mills, J. Phys. Chem., 1995, 99, 475–478 CrossRef CAS
.
- T. Sakai and P. Alexandridis, Langmuir, 2005, 21, 8019–8025 CrossRef CAS PubMed
.
- T. Sakai and P. Alexandridis, Nanotechnology, 2005, 16, S344–S353 CrossRef PubMed
.
- T. Sakai and P. Alexandridis, J. Phys. Chem. B, 2005, 109, 7766–7777 CrossRef CAS PubMed
.
- Q. Shou, C. Guo, L. Yang, L. Jia, C. Liu and H. Liu, J. Colloid Interface Sci., 2011, 363, 481–489 CrossRef CAS PubMed
.
- M. Sokolsky-Papkov and A. Kabanov, Polymers, 2019, 11, 1553 CrossRef CAS PubMed
.
- N. R. S. Sibuyi, V. C. Thipe, K. Panjtan-Amiri, M. Meyer and K. V. Katti, Nanobiomedicine, 2021, 8, 1849543521995310 CrossRef PubMed
.
- K. Kataoka, A. Harada and Y. Nagasaki, Adv. Drug Delivery Rev., 2001, 47, 113–131 CrossRef CAS PubMed
.
-
C. Alvarez-Lorenzo and A. Concheiro, in Smart Materials for Drug Delivery, ed. C. Alvarez-Lorenzo and A. Concheiro, The Royal Society of Chemistry, Cambridge, 2013, vol. 1, pp. 1–32 Search PubMed
.
- L. Bromberg, J. Controlled Release, 2008, 128, 99–112 CrossRef CAS PubMed
.
- X.-B. Xiong, Z. Binkhathlan, O. Molavi and A. Lavasanifar, Acta Biomater., 2012, 8, 2017–2033 CrossRef CAS PubMed
.
- G. Gaucher, P. Satturwar, M.-C. Jones, A. Furtos and J.-C. Leroux, Eur. J. Pharm. Biopharm., 2010, 76, 147–158 CrossRef CAS PubMed
.
-
R. Glisoni, in Encapsulation and Structural Modifications of Biotherapeutics, Springer, Berlin, Heidelberg, 2024, pp. 1–15 Search PubMed
.
- E. V. Batrakova and A. V. Kabanov, J. Controlled Release, 2008, 130, 98–106 CrossRef CAS PubMed
.
- J. Gonzalez-Lopez, C. Alvarez-Lorenzo, P. Taboada, A. Sosnik, I. Sandez-Macho and A. Concheiro, Langmuir, 2008, 24, 10688–10697 CrossRef CAS PubMed
.
- R. J. Glisoni and A. Sosnik, J. Nanosci. Nanotechnol., 2014, 14, 4670–4682 CrossRef CAS PubMed
.
- M. L. Cuestas, R. J. Glisoni, V. L. Mathet and A. Sosnik, J. Nanopart. Res., 2013, 15, 1389 CrossRef
.
- R. J. Glisoni and A. Sosnik, Macromol. Biosci., 2014, 14, 1639–1651 CrossRef CAS PubMed
.
- N. Lecot, R. Glisoni, N. Oddone, J. Benech, M. Fernández, J. P. Gambini, P. Cabral and A. Sosnik, Adv. Ther., 2020, 4, 2000010 CrossRef
.
- N. Lecot, G. Rodríguez, V. Stancov, M. Fernández, M. González, R. J. Glisoni, P. Cabral and H. Cerecetto, Braz. J. Pharm. Sci., 2022, 58, e191055 CrossRef CAS
.
- N. Lecot, B. Dávila, C. Sánchez, M. Fernández, M. Gonzalez, P. Cabral, H. Cerecetto and R. J. Glisoni, Polymers, 2022, 14, 71 CrossRef CAS PubMed
.
- N. Lecot, M. Fernández-Lomónaco, H. Cerecetto, J. P. Gambini, P. Cabral and R. J. Glisoni, RSC Pharm., 2024, 1, 57–67 RSC
.
- R. Castelli, M. Ibarra, R. Faccio, I. Miraballes, M. Fernández, A. Moglioni, P. Cabral, H. Cerecetto, R. J. Glisoni and V. Calzada, Pharmaceuticals, 2022, 15, 15 CrossRef CAS PubMed
.
- P. G. Márquez, F. Wolman and R. J. Glisoni, NanoTrends, 2024, 8, 100058 Search PubMed
.
- M. C. García Vior, J. Marino, L. P. Roguin, A. Sosnik and J. Awruch, Photochem. Photobiol., 2013, 89, 492–500 CrossRef PubMed
.
- M. L. Cuestas, A. Sosnik and V. L. Mathet, Mol. Pharm., 2011, 8, 1152–1164 CrossRef CAS PubMed
.
- M. L. Cuestas, A. I. Castillo, A. Sosnik and V. L. Mathet, Bioorg. Med. Chem. Lett., 2012, 22, 6577–6579 CrossRef CAS PubMed
.
- C. Alvarez-Lorenzo, A. Rey-Rico, J. Brea, M. I. Loza, A. Concheiro and A. Sosnik, Nanomedicine, 2010, 5, 1371–1383 CrossRef CAS PubMed
.
- A. Sosnik, Adv. Drug Delivery Rev., 2013, 65, 1828–1851 CrossRef CAS PubMed
.
- M. O. Griffin, G. Ceballos and F. J. Villarreal, Pharmacol. Res., 2011, 63, 102–107 CrossRef CAS PubMed
.
- R. Lamb, H. Harrison, J. Hulit, D. L. Smith, M. P. Lisanti and F. Sotgia, Oncotarget, 2014, 5, 11029–11037 CrossRef PubMed
.
- K. Riesbeck, A. Bredberg and A. Forsgren, Antimicrob. Agents Chemother., 1990, 34, 167–169 CrossRef CAS PubMed
.
- N. Moullan, L. Mouchiroud, X. Wang, D. Ryu, E. G. Williams, A. Mottis, V. Jovaisaite, M. V. Frochaux, P. M. Quiros, B. Deplancke, R. H. Houtkooper and J. Auwerx, Cell Rep., 2015, 10, 1681–1691 CrossRef CAS PubMed
.
- E. M. D. Francesco, G. Bonuccelli, M. Maggiolini, F. Sotgia and M. P. Lisanti, Oncotarget, 2017, 8, 67269–67286 CrossRef PubMed
.
- R. Lamb, M. Fiorillo, A. Chadwick, B. Ozsvari, K. J. Reeves, D. L. Smith, R. B. Clarke, S. J. Howell, A. R. Cappello, U. E. Martinez-Outschoorn, M. Peiris-Pagès, F. Sotgia and M. P. Lisanti, Oncotarget, 2015, 6, 14005–14025 CrossRef PubMed
.
- S. N. Dijk, M. Protasoni, M. Elpidorou, A. M. Kroon and J.-W. Taanman, Sci. Rep., 2020, 10, 4363 CrossRef CAS PubMed
.
- B. Sloan and N. Scheinfeld, Expert Opin. Drug Saf., 2008, 7, 571–577 CrossRef CAS PubMed
.
- M. Toledano-Osorio, J. P. Babu, R. Osorio, A. L. Medina-Castillo, F. García-Godoy and M. Toledano, Materials, 2018, 11, 1013 CrossRef PubMed
.
- M. Toledano, M. Toledano-Osorio, M. D. Navarro-Hortal, A. Varela-López, R. Osorio and J. L. Quiles, Antioxidants, 2019, 8, 550 CrossRef CAS PubMed
.
- Ç. Yücel, Z. Değim and Ş. Yilmaz, Biomed. Pharmacother., 2013, 67, 459–467 CrossRef PubMed
.
- R. Ramírez-Agudelo, K. Scheuermann, A. Gala-García, A. P. F. Monteiro, A. D. Pinzón-García, M. E. Cortés and R. D. Sinisterra, Mater. Sci. Eng., C, 2018, 83, 25–34 CrossRef PubMed
.
- N. F. Cover, S. Lai-Yuen, A. Parsons and A. Kumar, Int. J. Nanomed., 2012, 7, 2411–2419 Search PubMed
.
- V. Parasaram, N. Nosoudi, R. J. LeClair, A. Binks and N. Vyavahare, Pulm. Pharmacol. Ther., 2016, 39, 64–73 CrossRef CAS PubMed
.
- A. R. Gardouh, M. A. Attia, E. T. Enan, A. M. Elbahaie, R. A. Fouad, M. El-Shafey, A. M. Youssef, S. Y. Alomar, Z. A.-E. Ali, S. A. Zaitone and M. K. E. Qushawy, Molecules, 2020, 25, 3230 CrossRef CAS PubMed
.
- J. Turkevich, P. C. Stevenson and J. Hillier, Discuss. Faraday Soc., 1951, 11, 55–75 RSC
.
- T. Rodrigues and L. S. Ferraz, Biochem. Pharmacol., 2020, 182, 114282 CrossRef CAS PubMed
.
- P. Ghosh, C. Vidal, S. Dey and L. Zhang, Int. J. Mol. Sci., 2015, 21, 3363 CrossRef PubMed
.
- L. Guerra, J. Mordoh, I. Slavutsky, I. Larripa and E. E. Medrano, Pigm. Cell Res., 1989, 2, 504–509 CrossRef CAS PubMed
.
- U. Landegren, J. Immunol. Methods, 1984, 67, 379–388 CrossRef CAS PubMed
.
- N. A. P. Franken, H. M. Rodermond, J. Stap, J. Haveman and C. van Bree, Nat. Protoc., 2006, 1, 2315–2319 CrossRef CAS PubMed
.
- E. Aplak, C. von Montfort, L. Haasler, D. Stucki, B. Steckel, A. S. Reichert, W. Stahl and P. Brenneisen, PLoS One, 2020, 15, e0227926 CrossRef CAS PubMed
.
- V. Shcherbakov, S. A. Denisov and M. Mostafavi, RSC Adv., 2023, 13, 8557–8563 RSC
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5bm00253b |
| This journal is © The Royal Society of Chemistry 2025 |