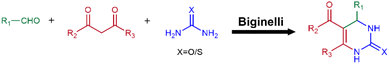Hydrogels constructed by multicomponent reactions
Siyu
Pan
a,
Chongyu
Zhu
 *b and
Lei
Tao
*b and
Lei
Tao
 *a
*a
aThe Key Laboratory of Bioorganic Phosphorus Chemistry & Chemical Biology (Ministry of Education), Department of Chemistry, Tsinghua University, Beijing, 100084, P. R. China. E-mail: leitao@mail.tsinghua.edu.cn
bCollege of Chemistry and Chemical Engineering, Donghua University, Shanghai 201620, China. E-mail: czhu@dhu.edu.cn
First published on 25th November 2024
Abstract
Hydrogels have been widely used in many fields. To meet the application requirements of different fields, hydrogels with different functions have been developed through efficient organic reactions. Multicomponent reactions (MCRs) have been used as distinct tools in hydrogel preparation, owing to their high efficiency, mild reaction conditions and tolerance for various functional groups. In this mini-review, we present a comprehensive overview of the roles of MCRs as crosslinking reactions and as functional group introducers in hydrogel construction. Finally, we will discuss the future perspectives of MCRs in exploring (multi)functional hydrogels.
Introduction
Hydrogels are soft materials with high water content, which are composed of crosslinked hydrophilic polymer networks.1,2 According to the types of crosslinking, hydrogels can be prepared through covalent bonds, dynamic covalent bonds and physical interactions. These hydrogels have been widely used in various fields.3 For example, the unique structures of hydrogels are similar to the extracellular matrix and are beneficial for cell metabolism and tissue growth. Thus, hydrogels have been used for cell culture, tissue regeneration and biosensing in the biomedical area.4–8 In agriculture fields, hydrogels have been used to encapsulate pesticides for controllable release to enhance the utilization efficiency of pesticides and minimize environmental impact.9–12 Over the years, by including different functional groups on gelators, researchers have explored new hydrogels to further improve the performance and broaden the application scope of hydrogels.Multicomponent reactions (MCRs) are a class of distinctive organic reactions that use more than two reactants to generate a single complex product in a one-pot manner. MCRs are modular, atom economical and highly efficient; most of them use easily accessible substrates and can be performed under mild conditions with water as the only byproduct. Notably, one of the advantages of MCRs is the ability to use a diverse range of solvents, including water, which is quite important for hydrogel synthesis. Besides, the byproduct (i.e. water) hardly affects the reaction process, which is superior to other bond-forming and functionalization reactions (e.g. amide condensation or ester synthesis) that require removal of byproducts to proceed reactions. Since Meier's introduction of the Passerini reaction into polymer chemistry, more and more MCRs have been considered as useful tools to develop elegant polymers.13 These MCRs include the Passerini,14,15 Ugi,16,17 Kabachnik–Fields (KF),18,19 Hantzsch20,21 and Biginelli reactions,22,23 as well as metal-catalyzed24,25 and alkyne-based MCRs.26,27 These MCRs have been sufficiently studied for hundreds of years, including their scope of synthesis, reaction characteristics and experimental methods.28–32 MCRs can effectively introduce two or more functional groups into polymers in just one step, which enriches the functionality, properties, topology and diversity of polymers.33,34 MCRs offer new opportunities to develop functional hydrogels with improved performances. Initially, MCRs were mainly used as coupling reactions to construct hydrogels. Recently, MCRs have been used in forming functional hydrogels by providing desired functional components and crosslinkers.
In this mini-review, we focus on hydrogels constructed through MCRs and summarize the various roles that MCRs play in hydrogel formation, including as crosslinking reactions and as functional group introducers. Finally, we will discuss the future perspectives of MCRs in exploring (multi)functional hydrogels for different applications.
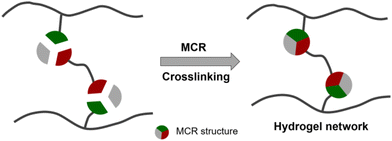
The role as crosslinking reactions
In the early stages, MCRs have been used as crosslinking reactions of polymers to prepare hydrogels. These polymers are mainly natural polysaccharides including carboxymethyl cellulose (CMC), hyaluronic acid (HA), alginate and gelatin. They are attractive candidates for building biomedical hydrogels, owing to their non-toxicity, non-immunogenicity, biocompatibility, and often biodegradability.35 Moreover, many polysaccharides contain reactive sites such as carboxyl and amino groups. Thus, by incorporating difunctional groups and other components, MCRs can crosslink polymer chains to generate hydrogels (Fig. 1).The Passerini reaction
The Passerini reaction is a three-component reaction that involves an aldehyde, a carboxylic acid and an isocyanide to yield an α-(acyloxy)-amide as a product (Fig. 2a). When used for forming a hydrogel, the Passerini reaction can crosslink the carboxyl groups in polysaccharides with further added bifunctional reactants, such as glutaraldehyde.36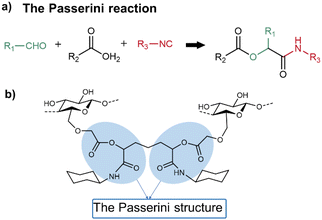 | ||
| Fig. 2 (a) The Passerini reaction. (b) Possible linkages formed by a Passerini crosslinking reaction using CMC as the polysaccharide component. | ||
Nooy and co-workers reported a series of ionic polysaccharide hydrogels via the Passerini reaction at ambient temperature.37 As a typical example, the polysaccharide component and isocyanide component were CMC and cyclohexyl isocyanide, respectively. Glutaraldehyde was used to link two α-(acyloxy)-amides (Fig. 2b). The degree of crosslinking and network structure of the hydrogels were analysed by 13C CP-MAS and 15N CP-MAS spectroscopy. In combination with solid-state NMR measurements and elemental analysis, the efficiency of the reactions was calculated to be about 80% of theoretical values, showing the high efficiency of MCRs to form hydrogels.
The Ugi reaction
The Ugi reaction is another efficient MCR that involves four components including an aldehyde, a carboxylic acid, an isocyanide and a primary amine to generate an α-(acylamine)-amide (Fig. 3a). Besides glutaraldehyde, di-isocyanides, 1,5-diaminopentane and L-lysine ethyl ester can also be used as difunctional components.38 The Ugi reaction can be employed to prepare polysaccharide-based hydrogels by forming dipeptide-like crosslinkers.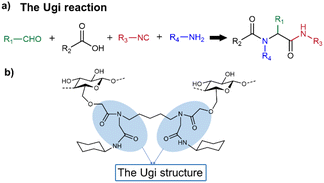 | ||
| Fig. 3 (a) The Ugi reaction. (b) Possible linkages formed by a Ugi crosslinking reaction using CMC as the polysaccharide component. | ||
Nooy and co-workers prepared polysaccharide hydrogels via the Ugi reaction.37 The polysaccharide component and isocyanide component were CMC and cyclohexyl isocyanide, respectively. Then, formaldehyde and 1,5-diaminopentane were involved to form amide-like linkages (Fig. 3b). According to the comparison in this research, amide bonds were more stable than ester bonds across a wide range of conditions and time scales. Thus, the hydrogels produced by the Ugi reaction were more robust than those generated by the Passerini reaction. This may be one of the reasons why the Ugi reaction has been used more often than the Passerini reaction for hydrogel construction. Shulepov and co-workers used the Ugi reaction to generate CMC-based sub-micron microgels that can be used as emulsifiers.39 In this research, CMC emulsion was first obtained to form physical microgels; then, formaldehyde, 1,4-bis(3-isocyanopropyl) piperazine and 4-picolylamine (or benzylamine, furfuryl amine and isopropyl amine) were introduced to obtain crosslinked CMC microgels via the Ugi reaction (Fig. 4). Both physical and crosslinked microgels exhibited pronounced emulsifying properties at low concentrations (circa 2 g L−1). Compared with the metastable physical microgels, the cross-linked microgels were more stable and could be directly used to obtain solutions with prominent surface activity.
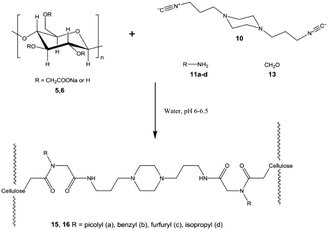 | ||
| Fig. 4 Model Ugi reaction with diisocyanide 10 to form a CMC network. Reprinted with permission from ref. 34. Copyright 2016, Springer Nature. | ||
Apart from CMC, HA is another excellent carboxyl-containing candidate to form a hydrogel.40 Crescenzi and co-workers developed a HA-based hydrogel by the Ugi condensation reaction using lysine ethyl ester as the crosslinker diamine (Fig. 5).41 Formaldehyde and cyclohexyl isocyanide were applied as the other two components for the Ugi reaction. This hydrogel exhibited excellent physical properties and swelling behaviour, showing great potential to be used in biomedical applications such as controlled drug delivery, cell scaffolding and tissue engineering. In addition, the deacetylation process of HA introduces amine groups into its backbone, making it an ideal reactant for the Ugi reaction and a bifunctional crosslinker for hydrogels. The same group prepared a partially deacetylated hyaluronan (deHA)-based hydrogel via the Ugi reaction.42 The degree of crosslinking and the structure of the network were assessed through 13C CP-MAS NMR, indicating the smooth process of the Ugi reaction to crosslink deHA chains.
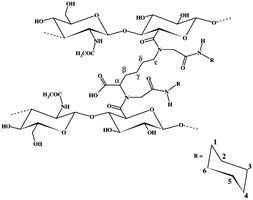 | ||
| Fig. 5 Proposed structure of the network formed by HA via the Ugi reaction with formaldehyde, cyclohexyl isocyanide and lysine with carbon atom assignments. Reprinted with permission from ref. 36. Copyright 2003, Elsevier Science Ltd. | ||
Besides hydrogel construction, the degradation process of HA-based hydrogels was also investigated by researchers. Maleki and co-authors monitored the formation and destruction of HA-based hydrogels generated by the Ugi reaction in the presence of different chemical crosslinker agents.43 Rheology and dynamic light scattering were applied to study the influence of pH values on the gelation process and the stability of hydrogels. The results showed that ester linkages and glycosidic bonds were sensitive to hydrolysis, while the amide linkages in the product of the Ugi reaction were resistant to hydrolysis.
Alginate is a nontoxic, nonimmunogenic and biodegradable anionic polymer that can form hydrogels with divalent cations, such as Ca2+, through electrostatic interactions. It can also form alginic acid gel with slowly hydrolysing D-glucono-δ-lactone via intermolecular hydrogen bonds.44 Apart from physical hydrogels, chemical hydrogels based on alginate have been widely investigated.45 In this context, the Ugi reaction is a useful crosslinking reaction. Bu and co-workers reported an alginate-based chemical hydrogel via the Ugi reaction.46 They chose 1,5-diaminopentane as the bifunctional crosslinker agent. The hydrogels were successfully prepared with the help of formaldehyde and cyclohexyl isocyanide. They also monitored the rheological properties of semi-dilute aqueous alginate solution during the Ugi reaction; the influence of crosslinker concentration on the physical properties and gelation time of hydrogels was also studied. In particular, the authors focused on the influence of pH values on the formation of alginate-based hydrogels and discussed the impact of pH values on the kinetics of the Ugi reaction.47 Their results showed that the acidic conditions are favourable for the Ugi reaction.
Beyond the physicochemical properties of hydrogels prepared by the Ugi reaction, researchers have also investigated their potential in biomedical applications. The 3D network with high water content endows hydrogels with the potential to serve as drug carriers. For example, Javanbakht and co-workers used citric acid-based graphene quantum dots to crosslink gelatin through the Ugi reaction.48 The resulting hydrogel could selectively release doxorubicin (DOX) under tumour tissue conditions (pH 4.5) and exhibited notable cytotoxicity against breast cancer cell lines.
Researchers have exploited other gelators. For example, Li and co-workers developed a new polymer to form biomedical hydrogels (Fig. 6).49 Briefly, they modified the chain ends of poly(ethylene glycol) (PEG) with carboxylic groups, and then simultaneously incorporated phenylboronic acid groups and benzaldehyde groups at the chain ends via the Ugi reaction. This polymer formed a double-dynamic-network hydrogel through reactions between phenylboronic acid groups and poly(vinyl alcohol) (PVA), as well as between benzaldehyde groups and glycol chitosan (Fig. 6a). The hydrogel was self-healing due to the dynamic linkages (imine and borate ester) and showed low cytotoxicity (Fig. 6b). The authors also studied the advantages of this hydrogel for controllable release of DOX in vivo. When encapsulating DOX for tumour therapy, the tumour size under the treatment of DOX laden gels was much smaller than those under the treatments of the controls (DOX-situ and DOX-vein) (Fig. 6c–e). Therefore, this gel achieved controllable release of drugs and exhibited better antitumor effects than traditional methods. This double-network hydrogel crosslinked by a Ugi-modified polymer expanded the gelator scope and demonstrated great potential for biomedical applications.
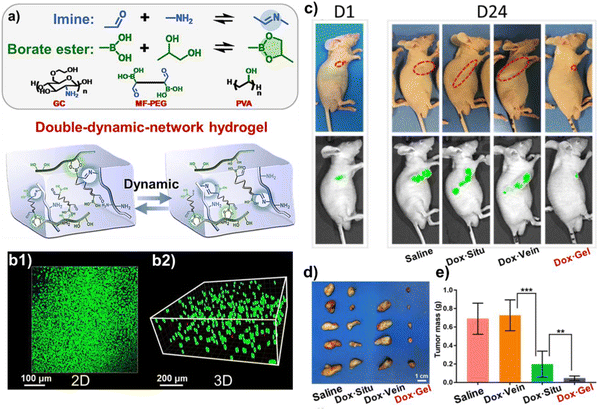 | ||
| Fig. 6 (a) Schematic illustration of the double-dynamic-network hydrogel with imine and borate ester linkages. (b1 and b2) Cell viability after a 24 h culture with the hydrogel on the top (b1) and inside the hydrogel (b2). (c) Optical and fluorescence images of the mice after different treatments on day 1 and day 24. Saline: a saline solution injected in situ at the tumor bed; DOX-situ: a DOX solution via direct injection at the tumor bed; DOX-vein: a DOX solution via intravenous administration; and DOX-gel: DOX-loaded hydrogel. (d) Images of the tumors after different treatments. (e) Mass of the tumors after different treatments; data are represented as mean ± SD, n = 5, **p < 0.01, and ***p < 0.001. Reprinted with permission from ref. 44. Copyright 2019, American Chemical Society. | ||
In summary, MCRs are efficient coupling reactions that can proceed under mild conditions. Polysaccharides are biocompatible polymers that contain functional groups such as carboxylic and amine groups. Thus, the Passerini and Ugi reactions provide a straightforward pathway to crosslink these polysaccharide chains to form hydrogels. Generally, the four-component Ugi reaction can introduce more reactants and generate more stable products than the Passerini reaction. Therefore, the Ugi reaction has broader applications than the Passerini reaction to produce hydrogels. Initially, polysaccharides are the main gelators and most research studies focus on structure verification, physical properties and influencing factors of hydrogels. Later on, with the deepening understanding of MCRs, other gelators have been developed via MCRs and the resulting hydrogels have been exploited as drug carriers, which demonstrates the value of MCR-crosslinked hydrogels.
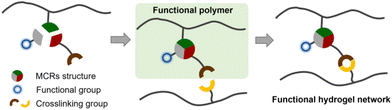
The role as functional group introducers
MCRs involve three or more reactants, and some MCRs are regarded as green click reactions compatible with various functional groups. Thus, they can simultaneously introduce different functional groups to develop new (multi)functional polymers, which opens up a new avenue to explore (multi)functional hydrogels (Fig. 7).The Passerini reaction
Incorporating photo-sensitive groups via MCRs and crosslinking through click reactions provides a facile synthetic approach to UV-degradable hydrogels. Li and co-workers used the Passerini reaction to design a UV-degradable gelator that contained both double bonds and ortho-nitrobenzyl ester groups.50 Then, this gelator was mixed with 8-arm PEG-thiol to yield a hydrogel through a thiol–ene click reaction. When exposed to UV light, ortho-nitrobenzyl ester absorbed the energy to break the chemical bond, resulting in the destruction of the hydrogel. This method was easily performed and greatly simplified the preparation of UV-degradable hydrogels.Truong and co-workers took further steps to develop photo-responsive hydrogels for selective release of different cell types.51 Briefly, they modified PEG with photolabile and azide groups by the Passerini reaction and therefore prepared a library of photolabile PEG linkers (Fig. 8a). Then, azide–alkyne cycloaddition was performed to generate photodegradable hydrogels. The hydrogels containing ortho-nitrobenzyl (P2) encapsulated L929 cells, while the hydrogels containing 7-(diethylamino) coumarin (P8) encapsulated human mesenchymal stem cells (hMSC). Fig. 8b shows the great cell viability of different cells in P2/P8 composite gels, indicating that the chromophores and cross-linking process of both hydrogels were cyto-compatible. Furthermore, by selecting appropriate light wavelengths (365 nm for P2 and 470 nm for P8) to irradiate the composite gel, specific components of the gel rapidly degraded while leaving the other components unaffected, which enabled the complete separation and release of desired cells. After being collected and transferred to tissue culture polystyrene (TCPS) plates for 3 days of cultivation, both released L929 cells and hMSCs exhibited high cell viability compared to cells without encapsulation and release (Fig. 8c and d). This methodology shed light on the preparation of light-responsive materials for spatiotemporal delivery of therapeutics, advanced treatment of diseases, and tissue regeneration.
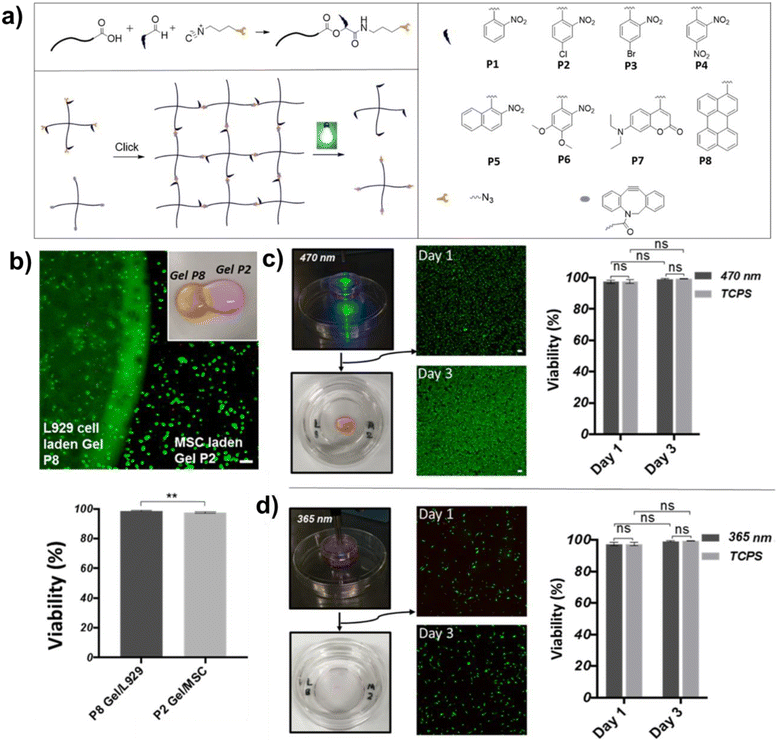 | ||
| Fig. 8 (a) Preparation of the photolabile PEG linkers and the resulting networks. (b) Image of composite gel from P2 and P8 linkers crosslinked with PEG together with the fluorescent live dead staining image of cell laden gel at the interface, and a graph showing the viability of the encapsulated L929 cells and hMSC (live cell: green and dead cell: red; scale bar = 100 μm). (c) Complete degradation of hydrogel P8 under blue light irradiation (470 nm) after 5 min. The released L929 cells showed high viability as well as normal cellular activity after 3 days post-release. (d) Subsequent degradation of the hMCS laden hydrogel P2 by UV light (365 nm). The collected hMSCs also displayed high cell viability and normal cellular activity compared to cells without encapsulation and release (live cell: green and dead cell: red; scale bar = 100 μm; ns = not significant). Reprinted with permission from ref. 46. Copyright 2017, American Chemical Society. | ||
The Ugi reaction
The Ugi reaction has been used to facilely prepare a novel antibacterial polymer with crosslinking groups. For instance, Zeng and his co-workers designed and synthesized a multifunctional PEG derivative via the Ugi reaction that contained both phenylboronic acid and phenolic groups (Fig. 9a).52 The phenylboronic acid group crosslinked PVA through a dynamic borate ester bond to form a self-healing hydrogel, while the phenolic groups played the antibacterial role (Fig. 9b). This self-healing hydrogel showed antibacterial capability towards both E. coli and S. aureus. In particular, this hydrogel achieved an inhibitory effect on E. coli similar to that of 50 times the antibiotic minimal concentration. The introduction of the antibacterial and crosslinking groups into the polymer structure by the Ugi reaction avoided tedious processes. Besides, including antibacterial groups in hydrogel networks has imparted potential long-term antibacterial effects to hydrogels.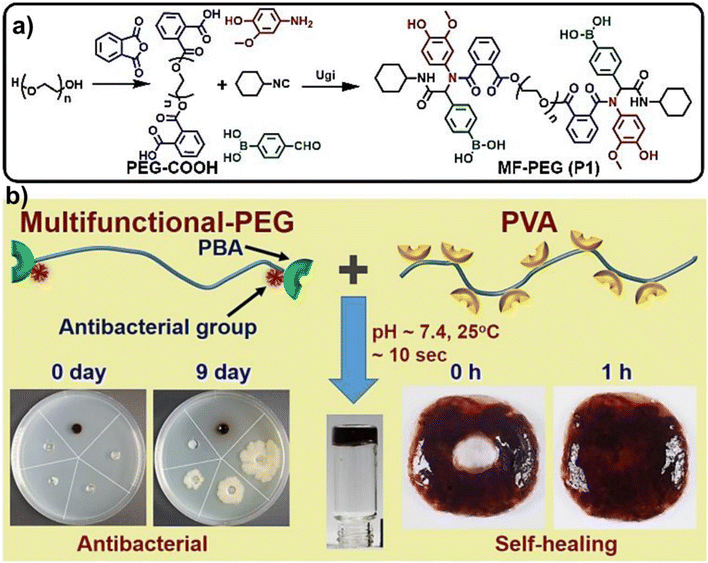 | ||
| Fig. 9 (a) Synthesis of multifunctional PEG via the Ugi reaction. (b) The schematic diagram of the antibacterial and self-healing hydrogel. Reprinted with permission from ref. 47. Copyright 2020, American Chemical Society. | ||
Hydrophobic groups, such as long alkane chains, can be easily introduced into polymers via MCRs. Thus, the resulting hydrogels are able to encapsulate hydrophobic small molecules for sustainable release. For instance, Zhang and co-workers designed an amphiphilic alginate derivative by the Ugi reaction.53 Then, a hydrogel was formed by adding Ca2+ and was loaded with the hydrophobic pesticide acetamiprid. The controllable release of acetamiprid enhanced soil aggregation and minimized the mobility of pesticides in the agricultural system. Besides single-network hydrogels, Wang and co-workers developed stretchable double-network nanocomposite hydrogels to achieve controlled release of pesticides.54 The hydrophobic modification of sodium alginate was carried out by the Ugi reaction, so that it can trap λ-cyhalothrin through hydrophobic interactions. Then, the hydrogels were formed through a physical network generated by electrostatic interactions between modified alginate and Ca2+ and a chemical network generated by polyacrylamide. The incorporation of montmorillonite provided hydrogels with both sustained release behaviour and outstanding mechanical properties. This composite hydrogel has the potential to serve as a carrier for insoluble pesticides, which enhances the utilization efficiency of pesticides and reduces environmental pollution. These results demonstrate that functional hydrogels prepared via MCRs have great potential for agricultural applications.
The KF reaction
The tricomponent KF reaction generates an α-aminophosphonate structure through common reactants (aldehydes, amines and phosphites). The hydrolysis product of α-aminophosphonate is α-aminophosphonic acid that is a chelating group of many metal ions (Fig. 10).55 He and co-workers designed a multifunctional polymer containing phenylboronic acid and α-aminophosphonic acid groups through a one-step KF reaction and free radical polymerization, followed by an effective hydrolysis reaction (P1) (Fig. 11a).56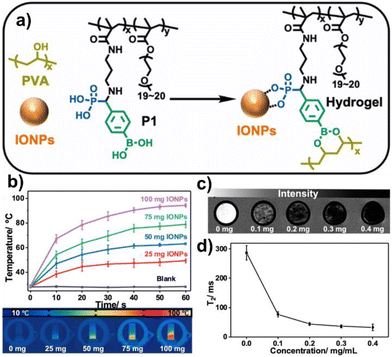 | ||
| Fig. 11 (a) Preparation of P1 and the P1-IONP complex. (b) Temperature of P1-PVA hydrogels containing different amounts of IONPs vs. time in an alternating magnetic field (f = 285 kHz and H = 201.2 Oe). Data are presented as mean ± SD, n = 5. (c) MRI images of P1-PVA hydrogels containing different amounts of IONPs. (d) Spin–spin relaxation time (known as T2) values of different hydrogels; data are collected from the pixels in a 21 mm2 area and presented as mean ± SD. Reprinted with permission from ref. 51. Copyright 2022, American Chemical Society. | ||
This polymer effectively dispersed iron oxide nanoparticles (IONPs) owing to the robust interactions between the α-aminophosphonic acid groups and the surface of the IONPs. Then, a magnetic self-healing hydrogel was formed through dynamic borate ester linkages after the introduction of PVA. This hydrogel showed great biosafety both in vitro and in vivo. The addition of IONPs also allows the hydrogel for magnetic manipulation. Furthermore, the hydrogel exhibited thermal behaviour in alternating magnetic fields and potential as a contrast agent for magnetic resonance imaging (MRI) (Fig. 11b–d).
Besides, Tian and his co-workers used the same polymer containing phenylboronic acid and α-aminophosphonic acid groups to bind positively-charged cis-dichlorodiammineplatinum(II) (CDDP) and form a self-healing hydrogel with PVA.57 This hydrogel showed prolonged release of CDDP and increased the cyto-safety of CDDP by reducing its release rate, exhibiting potential as a 3D cell culture matrix and an injectable carrier to deliver positively-charged small drugs for chronic diseases.
The Biginelli reaction
The Biginelli reaction is a tricomponent reaction that uses common reactants (aldehyde, β-ketoester and (thio)urea) to effectively produce 3,4-dihydropyrimidin-2(1H)-(thi)ones (DHPM(T)) derivatives (Fig. 12).The products of the Biginelli reaction have been known as potential calcium antagonists, mitotic inhibitors, antioxidants and bacterial inhibitors.58–60 Thus, antioxidant hydrogels can be formed through the Biginelli reaction. Yuan and co-workers used the Biginelli reaction to prepare a polymer containing ferrocene groups, which provided antioxidant properties.61 Then, phenylboronic acid groups were introduced via efficient nucleophilic substitution. The phenylboronic acid groups were crosslinked with PVA to build a self-healing hydrogel. This antioxidant hydrogel not only exhibited low cytotoxicity and biocompatibility, but also promoted wound healing, demonstrating its potential for wound treatment.
Besides, Yuan and co-workers used the Biginelli reaction to modify a cellulose acetoacetate (CAA) hydrogel film with fluorescent groups (Fig. 13).62 This hydrogel film provided photoluminescence, high transparency and UV-shielding properties, showing potential to be used in various fields including eco-friendly packaging.
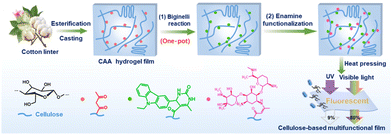 | ||
| Fig. 13 The schematic illustration of the preparation of a cellulose-based multifunctional hydrogel film through a multistep chemical modification process. Reprinted with permission from ref. 62. Copyright 2024, Elsevier Science Ltd. | ||
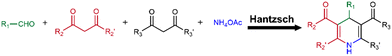
The Hantzsch reaction
The Hantzsch reaction is a classical MCR that has been widely studied since its introduction in 1881.63 It is a four-component reaction involving aldehyde, 1,3-diketone, β-ketoester and NH4OAc to generate 1,4-dihydropyridine (1,4-DHP) derivatives that have potential antioxidant, antibacterial, anti-inflammatory and antihypertensive properties (Fig. 14).64–66 Thus, the Hantzsch reaction is useful to explore antioxidant hydrogels with synergistic antioxidation between 1,4-DHP rings and additional antioxidant groups.Pan and co-workers developed an antioxidant polymer by modifying PVA with an antioxidant natural compound vanillin through the Hantzsch reaction (PVA-vanillin).67 A PVA derivative containing phenyl groups instead of vanillin was also prepared through the Hantzsch reaction and used as a control (PVA-ph, Fig. 15a). Then, the radical-scavenging ability of PVA-vanillin was investigated using 2,2′-azinobis(3-ethylbenzothiazoline-6-sulfonate) (ABTS˙+). The antioxidant ability can be measured by the extent of decolorization (Fig. 15b1). Within 30s after the solutions of PVA derivatives and ABTS˙+ were mixed, PVA-vanillin completely faded the blue colour of the ABTS˙+ solution, while the colour of PVA-ph/ABTS˙+ solution changed from blue to pale blue (Fig. 15b2). After 30 min, the PVA-ph/ABTS˙+ solution had become colourless, but the other two controls remained their blue colour (Fig. 15b3). The visual results agreed well with the quantitative data (Fig. 15c). These results demonstrated that the inherently antioxidant ability of 1,4-DHP moieties remained after being introduced into PVA chains; by using antioxidant reactants in the Hantzsch reaction, the radical-scavenging ability of PVA derivatives can be enhanced.
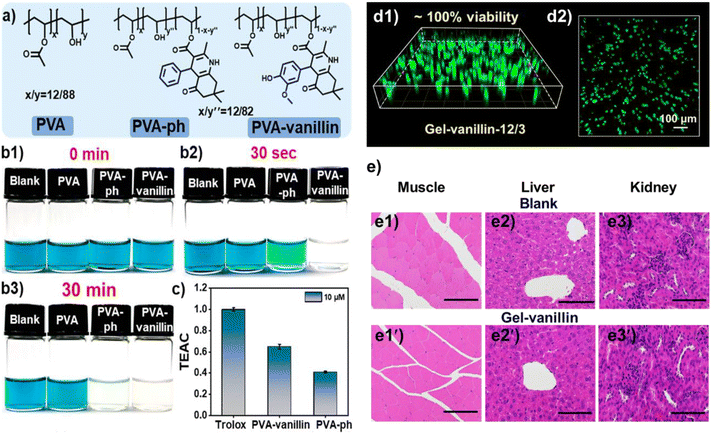 | ||
| Fig. 15 (a) Polymers used in the radical-scavenging test. (b1–b3) Color change of ABTS˙+ solutions in the presence of PVA, PVA-ph, and PVA-vanillin. (c) Trolox equivalent antioxidant capacity (TEAC) values of PVA derivatives. Trolox served as a standard compound (1.0). (d1) FDA/PI staining of cells in Gel-vanillin; (d2) cell observation of Gel-vanillin from the top. (e) Pathological analyses using H&E staining (400×); sections of muscle tissues around the gel (e1 and e1′), liver (e2 and e2′); and kidneys (e3 and e3′) from healthy mice (blank, e1–e3) and mice injected with gels (Gel-vanillin, e1′–e3′). Data are presented as mean ± SD, n = 6. Reprinted with permission from ref. 62. Copyright 2023, American Chemical Society. | ||
Then, native PVA and the PVA-vanillin were crosslinked with a phenylboronic acid-containing polymer (PB) that was also prepared using the Hantzsch reaction to form self-healing hydrogels through borate ester linkages (Gel-PVA and Gel-vanillin). In the ABTS˙+ test, both gels faded the blue colour of ABTS˙+ because of the native antioxidant capability of PB. However, Gel-vanillin quenched ABTS˙+ faster than Gel-PVA, which was attributed to the antioxidant PVA-vanillin. Besides, Gel-vanillin showed low cytotoxicity (Fig. 15d); negligible differences in the sections of muscle tissue (Fig. 15e1 and e1′), liver (Fig. 15e2 and e2′) and kidneys (Fig. 15e3 and e3′) were observed between the healthy mice and the mice injected with the gel. Thus, this hydrogel showed great biosafety both in vitro and in vivo, exhibiting its potential as an implantable biomaterial.
In this part, MCRs are not only used as efficient coupling reactions for hydrogel preparation, but also introduce functional groups for various applications. To do this, MCRs incorporate functional groups with crosslinking groups in one step to develop multifunctional polymers, either at the chain ends or along the chains. Then, the resulting functional hydrogels can be formed. Meanwhile, as the products of the Biginelli and Hantzsch reactions are promising antioxidants, functional hydrogels with enhanced antioxidant ability can be generated by incorporating other antioxidant components. Furthermore, self-healing and double-network hydrogels developed via MCRs are expected to meet the diverse needs of biomedical and agricultural applications.
Conclusions and perspectives
Hydrogels are soft materials that have been widely used in various fields, such as agricultural and biomedical. To meet the application requirements of different fields, hydrogels with different functions have been developed through efficient organic reactions. MCRs provide great convenience for hydrogel preparation, owing to their mild reaction conditions, high efficiency, environmentally friendly byproducts and most importantly, the simultaneous introduction of two or more functional groups. Thus, the physicochemical properties of hydrogels are customizable by selecting different reactants. The hydrogels prepared by MCRs can be crosslinked through covalent bonds, dynamic covalent bonds and physical interactions; thus, different types of hydrogels can be developed via MCRs according to different requirements.MCRs are documented as efficient coupling reactions. Thus, MCRs are commonly used as crosslinking reactions for hydrogel preparation. In the early stages, carboxylated polysaccharides are the main gelators. MCRs, such as the Passerini and Ugi reactions, crosslink polysaccharide chains to form a hydrogel. The primary focus of many research studies is on investigating the gelation and degradation processes of hydrogels, as well as their physicochemical properties. Later on, researchers exploited new polymeric crosslinkers based on synthetic polymers such as PEG, which expanded the library of gelators.
Subsequently, researchers set about developing the functionality of hydrogels to expand their applications. MCRs have been employed to create new polymers that incorporate both crosslinking and functional groups. These crosslinking groups generated stable covalent networks and dynamic networks via imine and borate ester bonds that endow hydrogels with self-healing properties. By simultaneously incorporating functional groups, the resulting hydrogels exhibit photo-degradable, antibacterial, antioxidant or photoluminescent properties, making them highly suitable for biomedical and agricultural applications. Moreover, the products of some MCRs have inherent bioactivities, which can be used as functional groups directly. For instance, the products of the Biginelli and Hantzsch reactions are recognized as antioxidants. Therefore, other antioxidant reactants can be included in these reactions to prepare functional polymers; the resulting hydrogel can achieve enhanced antioxidant ability owing to the synergistic effect of additional antioxidant groups and intrinsic structures formed by MCRs. The hydrogels with improved performance may promote wound healing, taking a step further in solving practical issues in biomedical fields.
In future works, the characteristics and advantages of common MCRs should be fully exploited. For example, the Ugi reaction, which involves four components, provides an opportunity to develop hydrogels that combine three or more functions. Meanwhile, beyond antioxidant ability, the products of the Biginelli and Hantzsch reactions also exhibit excellent UV-shielding properties. Therefore, it is recommended to prepare anti-UV hydrogels using these two reactions with the help of other anti-UV reactants. Besides, isocyanide is toxic and may remain after the hydrogel synthesis via the Passerini and Ugi reactions. Therefore, in order to meet the application requirements in biotechnological fields, these hydrogels should be soaked in water for a long time to remove residual isocyanides. Additionally, the library of functional gelators can be expanded through the development of synthetic strategies. Functional polymers can be synthesized from monomers via free radical polymerization or polycondensation; they can also be derived from existing polymers by post-polymerization modification. Incorporating MCRs with other efficient organic reactions also provides opportunities for developing new multifunctional hydrogels. In all, MCRs are valuable tools for preparing multifunctional polymers and related hydrogels. In the future, more MCRs are expected to be applied to construct hydrogels for broader applications such as human healthcare, the food industry and environmental treatment.
Author contributions
S. P. drafted the manuscript. C. Z. and L. T. reviewed and revised the manuscript.Data availability
Data availability is not applicable to this article as no new data were created or analyzed in this study.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was supported by the National Natural Science Foundation of China (21971141), the Dushi special project of Tsinghua University (2023Z11DSZ001), and “the Fundamental Research Funds for the Central Universities” from Donghua University (No. 2232024D-13).References
- P. Li, W. Sun, J. Li, J.-P. Chen, X. Wang, Z. Mei, G. Jin, Y. Lei, R. Xin, M. Yang, J. Xu, X. Pan, C. Song, X.-Y. Deng, X. Lei, K. Liu, X. Wang, Y. Zheng, J. Zhu, S. Lv, Z. Zhang, X. Dai and T. Lei, Science, 2024, 384, 557–563 CrossRef CAS PubMed.
- H. Yuk, J. Wu and X. Zhao, Nat. Rev. Mater., 2022, 7, 935–952 CrossRef CAS.
- X. Zhao, X. Chen, H. Yuk, S. Lin, X. Liu and G. Parada, Chem. Rev., 2021, 121, 4309–4372 CrossRef CAS PubMed.
- J. L. Drury and D. J. Mooney, Biomaterials, 2003, 24, 4337–4351 CrossRef CAS PubMed.
- Q. Feng, K. Wei, S. Lin, Z. Xu, Y. Sun, P. Shi, G. Li and L. Bian, Biomaterials, 2016, 101, 217–228 CrossRef CAS PubMed.
- Y. Huang, L. Mu, X. Zhao, Y. Han and B. Guo, ACS Nano, 2022, 16, 13022–13036 CrossRef CAS PubMed.
- L. Dong, A. K. Agarwal, D. J. Beebe and H. Jiang, Nature, 2006, 442, 551–554 CrossRef CAS PubMed.
- T. Q. Trung, S. Ramasundaram, B. U. Hwang and N. E. Lee, Adv. Mater., 2016, 28, 502–509 CrossRef CAS PubMed.
- M. Al-Jabari, R. A. Ghyadah and R. Alokely, J. Environ. Manage., 2019, 239, 255–261 CrossRef CAS PubMed.
- Khushbu, S. G. Warkar and A. Kumar, Polymer, 2019, 182, 121823 CrossRef CAS.
- E. Malka, A. Dombrovsky and S. Margel, ACS Agric. Sci. Technol., 2022, 2, 430–436 CrossRef CAS.
- A. Bora, D. Sarmah and N. Karak, Int. J. Biol. Macromol., 2023, 253, 126555 CrossRef CAS PubMed.
- O. Kreye, T. Tóth and M. A. R. Meier, J. Am. Chem. Soc., 2011, 133, 1790–1792 CrossRef CAS PubMed.
- A. Hirama, L.-C. Chou and R. Kakuchi, Polym. J., 2021, 53, 1175–1185 CrossRef CAS.
- X.-X. Deng, L. Li, Z.-L. Li, A. Lv, F.-S. Du and Z.-C. Li, ACS Macro Lett., 2012, 1, 1300–1303 CrossRef CAS PubMed.
- X. Zhang, S. Wang, J. Liu, Z. Xie, S. Luan, C. Xiao, Y. Tao and X. Wang, ACS Macro Lett., 2016, 5, 1049–1054 CrossRef CAS PubMed.
- O. Kreye, O. Turunc, A. Sehlinger, J. Rackwitz and M. A. Meier, Chem. – Eur. J., 2012, 18, 5767–5776 CrossRef CAS PubMed.
- R. Kakuchi and P. Theato, ACS Macro Lett., 2014, 3, 329–332 CrossRef CAS PubMed.
- J. Dou, J. Chen, Q. Huang, H. Huang, L. Mao, F. Deng, Y. Wen, X. Zhu, X. Zhang and Y. Wei, J. Environ. Chem. Eng., 2020, 8, 103634 CrossRef CAS.
- G. Liu, Y. Zeng, T. Lv, T. Mao, Y. Wei, S. Jia, Y. Gou and L. Tao, Nat. Commun., 2020, 11, 6214 CrossRef CAS PubMed.
- G. Liu, Z. Xu, X. Dai, Y. Zeng, Y. Wei, X. He, L.-T. Yan and L. Tao, J. Am. Chem. Soc., 2021, 143, 17250–17260 CrossRef CAS PubMed.
- C. Zhu, B. Yang, Y. Zhao, C. Fu, L. Tao and Y. Wei, Polym. Chem., 2013, 4, 5395–5400 RSC.
- H. Wu, L. Yang and L. Tao, Polym. Chem., 2017, 8, 5679–5687 RSC.
- H. Kim, K.-T. Bang, I. Choi, J.-K. Lee and T.-L. Choi, J. Am. Chem. Soc., 2016, 138, 8612–8622 CrossRef CAS PubMed.
- I. H. Lee, K. T. Bang, H. S. Yang and T. L. Choi, Macromol. Rapid Commun., 2022, 43, 2100642 CrossRef CAS PubMed.
- L. V. Kayser, M. Vollmer, M. Welnhofer, H. Krikcziokat, K. Meerholz and B. A. Arndtsen, J. Am. Chem. Soc., 2016, 138, 10516–10521 CrossRef CAS PubMed.
- B. Wei, W. Li, Z. Zhao, A. Qin, R. Hu and B. Z. Tang, J. Am. Chem. Soc., 2017, 139, 5075–5084 CrossRef CAS PubMed.
- L. Banfi, A. Basso, C. Lambruschini, L. Moni and R. Riva, Chem. Sci., 2021, 12, 15445–15472 RSC.
- S. E. Hooshmand and W. Zhang, Molecules, 2023, 28, 1642 CrossRef CAS PubMed.
- P. R. Varga and G. Keglevich, Molecules, 2021, 26, 2511 CrossRef CAS PubMed.
- H. Wu, Z. Wang and L. Tao, Polym. Chem., 2017, 8, 7290–7296 RSC.
- Â. de Fátima, T. C. Braga, L. D. S. Neto, B. S. Terra, B. G. F. Oliveira, D. L. da Silva and L. V. Modolo, J. Adv. Res., 2015, 6, 363–373 CrossRef PubMed.
- C. V. Robotham, C. Baker, B. Cuevas, K. Abboud and D. L. Wright, Mol. Divers., 2003, 6, 237–244 CrossRef CAS PubMed.
- A. Sehlinger and M. A. R. Meier, Multi-Component and Sequential Reactions in Polymer Synthesis, Springer Nature, 2015 Search PubMed.
- Y. Yang, L. Xu, J. Wang, Q. Meng, S. Zhong, Y. Gao and X. Cui, Carbohydr. Polym., 2022, 283, 119161 CrossRef CAS PubMed.
- A. E. J. de Nooy, G. Masci and V. Crescenzi, Macromolecules, 1999, 32, 1318–1320 CrossRef CAS.
- A. E. J. de Nooy, D. Capitani, G. Masci and V. Crescenzi, Biomacromolecules, 2000, 1, 259–267 CrossRef CAS PubMed.
- N. Hauck, N. Seixas, S. P. Centeno, R. Schlüßler, G. Cojoc, P. Müller, J. Guck, D. Wöll, L. A. Wessjohann and J. Thiele, Polymers, 2018, 10, 1055 CrossRef PubMed.
- I. D. Shulepov, K. V. Kozhikhova, Y. S. Panfilova, M. N. Ivantsova and M. A. Mironov, Cellulose, 2016, 23, 2549–2559 CrossRef CAS.
- H. Xuan, X. Tang, Y. Zhu, J. Ling and Y. Yang, ACS Appl. Bio Mater., 2020, 3, 1628–1635 CrossRef CAS PubMed.
- V. Crescenzi, A. Francescangeli, D. Capitani, L. Mannina, D. Renier and D. Bellini, Carbohydr. Polym., 2003, 53, 311–316 CrossRef CAS.
- V. Crescenzi, A. Francescangeli, A. L. Segre, D. Capitani, L. Mannina, D. Renier and D. Bellini, Macromol. Biosci., 2002, 2, 272–279 CrossRef CAS.
- A. Maleki, A.-L. Kjøniksen and B. Nyström, Carbohydr. Res., 2007, 342, 2776–2792 CrossRef CAS PubMed.
- H. Qiao, P. Qi, X. Zhang, L. Wang, Y. Tan, Z. Luan, Y. Xia, Y. Li and K. Sui, ACS Appl. Mater. Interfaces, 2019, 11, 7755–7763 CrossRef CAS PubMed.
- X. Chen, M. Fan, H. Tan, B. Ren, G. Yuan, Y. Jia, J. Li, D. Xiong, X. Xing, X. Niu and X. Hu, Mater. Sci. Eng., C, 2019, 101, 619–629 CrossRef CAS PubMed.
- H. Bu, A.-L. Kjøniksen, K. D. Knudsen and B. Nyström, Biomacromolecules, 2004, 5, 1470–1479 CrossRef CAS PubMed.
- H. Bu, A.-L. Kjøniksen and B. Nyström, Eur. Polym. J., 2005, 41, 1708–1717 CrossRef CAS.
- S. Javanbakht, M. Nabi and A. Shaabani, Materialia, 2021, 20, 101233 CrossRef CAS.
- Y. Li, L. Yang, Y. Zeng, Y. Wu, Y. Wei and L. Tao, Chem. Mater., 2019, 31, 5576–5583 CrossRef CAS.
- W. Li, Z. Wang, L. Jiang, M. Feng, X. Fan, H. Fan and J. Xiang, Polymers, 2023, 15, 3762 CrossRef CAS PubMed.
- V. X. Truong, F. Li and J. S. Forsythe, ACS Appl. Mater. Interfaces, 2017, 9, 32441–32445 CrossRef CAS PubMed.
- Y. Zeng, Y. Li, G. Liu, Y. Wei, Y. Wu and L. Tao, ACS Appl. Polym. Mater., 2020, 2, 404–410 CrossRef CAS.
- S. Zhang, F. He, X. Fang, X. Zhao, Y. Liu, G. Yu, Y. Zhou, Y. Feng and J. Li, J. Environ. Sci., 2022, 113, 55–63 CrossRef CAS PubMed.
- L. Wang, G. Yu, J. Li, Y. Feng, Y. Peng, X. Zhao, Y. Tang and Q. Zhang, J. Cleaner Prod., 2019, 226, 122–132 CrossRef CAS.
- X. He, L. Yang, G. Liu, Y. Wei, Y. Zeng and L. Tao, Chem. Mater., 2022, 34, 9558–9568 CrossRef CAS.
- X. He, Y. Zeng, G. Liu, Y. Tian, Y. Wei, L. Zhao, L. Yang and L. Tao, ACS Macro Lett., 2022, 11, 39–45 CrossRef CAS PubMed.
- Y. Tian, Y. Zeng, Y. Li, X. He, H. Wu, Y. Wei, Y. Wu, X. Wang and L. Tao, Eur. Polym. J., 2020, 133, 109773 CrossRef CAS.
- H. Nagarajaiah, A. Mukhopadhyay and J. N. Moorthy, Tetrahedron Lett., 2016, 57, 5135–5149 CrossRef CAS.
- R. Kaur, S. Chaudhary, K. Kumar, M. K. Gupta and R. K. Rawal, Eur. J. Med. Chem., 2017, 132, 108–134 CrossRef CAS PubMed.
- L. H. S. Matos, F. T. Masson, L. A. Simeoni and M. Homem-de-Mello, Eur. J. Med. Chem., 2018, 143, 1779–1789 CrossRef CAS PubMed.
- R. Yuan, Z. Fang, F. Liu, X. He, S. Du, N. Zhang, Q. Zeng, Y. Wei, Y. Wu and L. Tao, ACS Macro Lett., 2024, 13, 475–482 CrossRef CAS PubMed.
- M. Yuan, H. Liu, S. Hu, H. Xiao, Y. Li and H. Qi, Ind. Crops Prod., 2024, 216, 118824 CrossRef CAS.
- A. Hantzsch, Ber. Dtsch. Chem. Ges., 1881, 14, 1637–1638 CrossRef.
- E. Carosati, P. Ioan, M. Micucci, F. Broccatelli, G. Cruciani, B. S. Zhorov, A. Chiarini and R. Budriesi, Curr. Med. Chem., 2012, 19, 4306–4323 CrossRef CAS PubMed.
- A. Di Stilo, S. Visentin, C. Cena, A. M. Gasco and G. Ermondi, J. Med. Chem., 1998, 41, 5393–5401 CrossRef CAS PubMed.
- A. M. Vijesh, A. M. Isloor, S. K. Peethambar, K. N. Shivananda, T. Arulmoli and N. A. Isloor, Eur. J. Med. Chem., 2011, 46, 5591–5597 CrossRef CAS PubMed.
- S. Pan, N. Zhang, X. He, Z. Fang, Y. Wu, Y. Wei and L. Tao, ACS Macro Lett., 2023, 12, 1037–1044 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2024 |