Open Access Article
Open Access ArticleSupramolecular interaction of a molecular catalyst with a polymeric carbon nitride photoanode enhances photoelectrochemical activity and stability at neutral pH†
Sanjit
Mondal‡
a,
Martina
Salati‡
 bc,
Marco
Nicaso
bc,
Josep
Albero
bc,
Marco
Nicaso
bc,
Josep
Albero
 d,
Mireia
Segado-Centellas
d,
Mireia
Segado-Centellas
 b,
Michael
Volokh
b,
Michael
Volokh
 a,
Carles
Bo
a,
Carles
Bo
 *b,
Hermenegildo
García
*b,
Hermenegildo
García
 d,
Marcos
Gil-Sepulcre
*b,
Antoni
Llobet
d,
Marcos
Gil-Sepulcre
*b,
Antoni
Llobet
 *be and
Menny
Shalom
*be and
Menny
Shalom
 *a
*a
aDepartment of Chemistry and Ilse Katz Institute for Nanoscale Science and Technology, Ben-Gurion University of the Negev, Beer-Sheva 8410501, Israel. E-mail: mennysh@bgu.ac.il
bInstitute of Chemical Research of Catalonia (ICIQ), Barcelona Institute of Science and Technology (BIST), Av. Països Catalans 16, Tarragona 43007, Spain. E-mail: cbo@iciq.cat; mgil@iciq.cat; allobet@iciq.cat
cUniversitat Rovira i Virgili, Av. Països Catalans 35, Tarragona 43007, Spain
dInstituto Universitario de Tecnología Química CSIC-UPV, Universitat Politècnica de València, València 46022, Spain
eDepartament de Química, Universitat Autònoma de Barcelona Cerdanyola del Valles, Barcelona 08193, Spain
First published on 13th September 2024
Abstract
Polymeric carbon nitride (CN) emerged as an alternative, metal-free photoanode material for water-splitting photoelectrochemical cells (PECs). However, the performance of CN photoanodes is limited due to the slow charge separation and water oxidation kinetics due to poor interaction with water oxidation catalysts (WOCs). Moreover, operation under benign, neutral pH conditions is rarely reported. Here, we design a porous CN photoanode connected to a highly active molecular Ru-based WOC, which also acts as an additional photo-absorber. We show that the strong interaction between the π-system of the heptazine units within the CN with the CH groups of the WOC's equatorial ligand enables a strong connection between them and an efficient electronic communication path. The optimized photoanode exhibits a photocurrent density of 180 ± 10 μA cm−2 at 1.23 V vs. the reversible hydrogen electrode (RHE) with 89% faradaic efficiency for oxygen evolution with turnover numbers (TONs) in the range of 3300 and a turnover frequency (TOF) of 0.4 s−1, low onset potential, extended incident photon to current conversion, and good stability up to 5 h. This study may lead to the integration of molecular catalysts and polymeric organic absorbers using supramolecular interactions.
Introduction
Polymeric carbon nitride (CN) has gained significant interest as a low-cost and benign photoanodic material for water-splitting photoelectrochemical cells (PECs).1–7 However, low charge separation and transfer efficiency and slow water oxidation kinetics hinder the photoactivity and PEC performance of CN photoanodes.8–12 Moreover, a pristine CN photoanode is prone to degradation due to the moderate oxygen evolution reaction (OER), which leads to hole accumulation and self-oxidation of the CN layer.8,13,14 Therefore, low faradaic efficiencies (FEs) towards molecular oxygen and poor stability are usually observed. A common approach to overcoming the sluggish OER kinetics in PECs is introducing a co-catalyst, which acts as a hole sink and catalytic site for the OER.15 In recent years, only a few reports have shown that heterogeneous metal oxide-based co-catalysts can improve the CN photoanode activity towards the OER.15–18 Unlike metal oxide-based photoanodes (BiVO4 and Fe2O3), most known co-catalyst deposition methods did not lead to enhanced oxygen production on a CN-based photoanode.19–24 The poor hole transfer from the CN to the co-catalyst may stem from the insufficient interaction between the materials, although it is still not fully understood. Moreover, CN and many heterogeneous OER electrocatalysts based on oxides work efficiently only under alkaline conditions.8,15 Consequently, the performance of CN photoanodes in a neutral electrolyte medium is relatively poor.25,26 There is an allure in achieving a water-splitting PEC under neutral conditions as it offers gentler operating conditions than alkaline or acidic environments.High performance and robust molecular catalysts have been developed recently for the water oxidation reaction, mainly based on Ru complexes containing the so-called FAME (flexible, adaptable, multidentate, and equatorial) ligands that achieve high turnover numbers (TONs) and turnover frequencies (TOFs), with [RuII(tda-κ-N3O)(py)2] (Ru-tda, where tda2− is [2,2′:6′,2′′-terpyridine]-6,6′′-dicarboxylate and py is pyridine) being one of the best examples.27–29 The well-defined nature of these molecular catalysts, together with the capacity to spectroscopically characterize intermediates, has prompted a remarkable development of water oxidation catalysts (WOCs).30 In addition, the capacity to functionalize the ligands bonding to the metal center offers a wide variety of anchoring strategies.31–35 Recently, we have developed an oligomeric derivative of Ru-tda catalysts, using 4,4′-bpy as the bridging ligand to form [Ru(tda)(4,4′-bpy)]15(4,4′-bpy) (Ru15).34 The latter has the capacity to generate a large number of CH–π interactions with graphitic surfaces, generating robust hybrid materials for the efficient oxidation of water in a heterogeneous phase.
In the present work, we explore the capacity of the Ru15 catalyst to interact with the π system of heptazines, constituting the repeating unit of the CN material, via the CH groups of the tda ligand and generating a monolayer of the well-defined molecular catalyst on top of the CN surface. The CN synthetic procedure is chosen to form a porous layer with good adhesion to the conductive substrate (i.e., fluorine-doped tin oxide, FTO), allowing the exploration of the co-catalyst role in an FTO/CN/Ru15 photoanode. This should provide the needed electronic communication between the co-catalyst and the light absorbing material, leading to a superior performance of the hybrid material constituted by Ru15 and CN.
Results and discussion
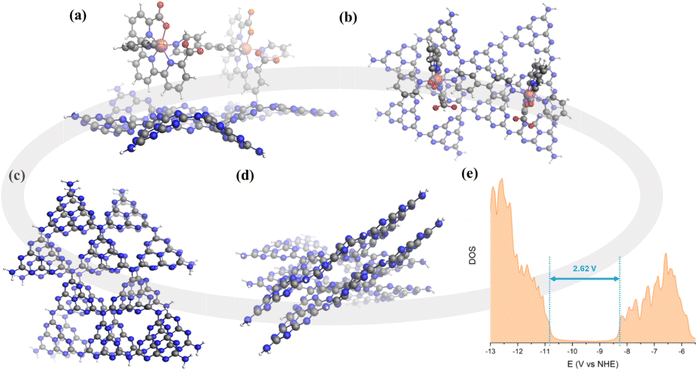
The fabrication process of a porous CN film over FTO-coated glass as the substrate is illustrated in Scheme 1. Thiourea and melamine were used to prepare CNTM photoanodes using a two-step method involving dipping and thermal treatment. The anchoring of the Ru15 oligomer into the CNTM film was performed by dipping the CNTM electrode in a solution containing 1 mg of Ru15 in 10 mL of 2,2,2-trifluoroethanol (TFE), for 20 min. Afterward, the electrode was removed from the solution and rinsed with TFE to generate the hybrid material, CNTM–Ru15. Further experimental details are given in the ESI.† The interaction of the Ru15 oligomeric water oxidation catalyst with the surface of the CN film occurs via CH–π interaction, as we have previously described in the case of graphitic surfaces.34–36 In order to further characterize this interaction and visualize it, we have carried out DFT calculations with a low molecular weight model system that involves 10 heptazine units (164 atoms: 60 C, 86 N, and 18 H) for the carbon nitride, labelled as CNRed, and a dinuclear Ru complex {[Ru(tda)(py)]2(μ-4,4′-bpy)} labeled as Ru2, to form CNRed–Ru2 (see Fig. 1, S14–S22 and Tables S4–S7†). Structural combinations of the two units were explored, including aqueous solvent effects, yielding, in the most favorable case, a stabilization energy of 12.7 kcal mol−1 per Ru center, which would imply 190.5 kcal mol−1 for the entire Ru15 oligomer with CNTM. This large stabilization energy is due to CH–π interactions between the CH aromatic groups of the tda ligands bonded to the Ru center and the π-system of the CNRed surface.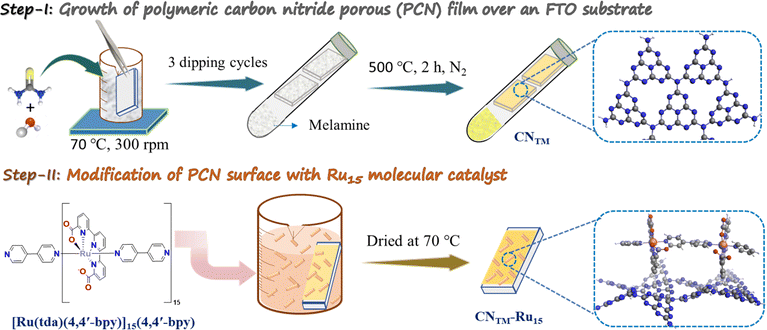 | ||
| Scheme 1 Procedure for the preparation of a CNTM film and the anchoring of the Ru15 oligomer to generate the hybrid material CNTM–Ru15. | ||
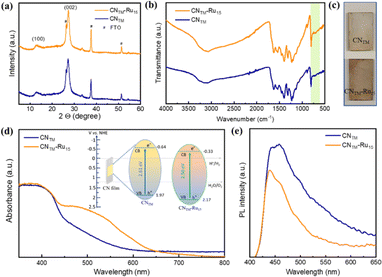
XRD (X-ray diffraction) and FTIR (Fourier transform infrared) spectroscopy were used to investigate the structural and functional properties of the films. The CNTM material exhibits two characteristic diffraction signals at 13.0° and 27.4°, which can be assigned to (100) and (002) planes, respectively, representative of the interplanar spacing and the conjugated aromatic system (Fig. 2a) in a heptazine-based CN.37,38 Upon the incorporation of the Ru15 oligomeric catalyst, the XRD pattern remains unchanged, highlighting the structural stability of CNTM films. The FTIR spectra of CNTM and CNTM–Ru15 films show a typical peak at 805 cm−1, which corresponds to the breathing mode of triazine units present in the sample (Fig. 2b). Additionally, the stretching modes of CN heterocycles were observed between 1200 and 1600 cm−1, with specific vibrations at 1400 and 1633 cm−1 in the CNTM film. Upon loading of the Ru15 catalyst onto the CNTM film, the stretching modes of the CN heterocycles are found at 1394 and 1625 cm−1. The broad band observed between 2980 and 3500 cm−1 in the spectra is attributed to either NH2 groups or surface-adsorbed water molecules.38 Additionally, 1H NMR, XRD, and FTIR data of the Ru15 oligomer are provided in Fig. S1.†
The modification of the CNTM film with the Ru15 oligomer has caused a visual change in the electrodes (Fig. 2c), which translates into the presence of a new broad band at approximately 480–550 nm, associated with the metal to ligand charge transfer (MLCT) band for the Ru complex, as can be observed in the diffuse reflectance spectrum (DRS) in Fig. 2d, and is comparable to the one visible in the UV-vis spectrum of Ru15 (Fig. S2†).
The direct optical bandgaps (Eg) of the CNTM and CNTM–Ru15 films are 2.61 and 2.50 eV, respectively (Fig. S3†). Valence band X-ray photoelectron spectroscopy (VB-XPS) discloses a more positive VB energy (EVB) position for the CNTM–Ru15 films of 2.17 V vs. NHE with regard to that of bare CNTM, which gives a value of 1.97 V vs. NHE and thus a better thermodynamic driving force for the former (Fig. S4†).10,38 Finally, the corresponding conduction band energy (ECB) of the CNTM and CNTM–Ru15 films is −0.64 and −0.33 V vs. NHE, respectively (see the inset of Fig. 2d for the energy diagram).
The electronic properties of the CNTM material were also analyzed based on TD-DFT calculations. A single sheet of CNRed made out of 10 heptazine units gave a band gap of 3.25 eV. Interestingly, a two-layer structure of heptazine units (CNRed)2 interacting via π–π stacking, as shown in Fig. 1, has a band gap of 2.62 eV closely matching the experimental value (2.61 eV), thus manifesting the importance of the delocalization of the electron density on the 2D network along with the π–π stacking interactions among the different layers to properly describe carbon nitride type materials.
Photoluminescence (PL) spectra are significantly different in the presence and absence of Ru15, as can be observed in Fig. 2e, where the intensity of the prominent emission peak at 450 nm in CNTM–Ru15 is partially quenched compared to pristine CNTM, suggesting the presence of an alternative non-radiative recombination path.39,40
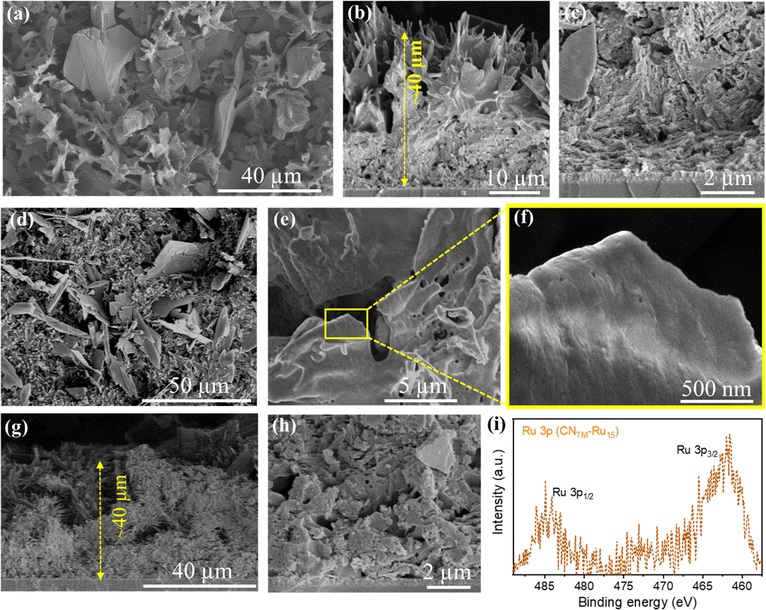
The SEM images of CNTM (Fig. 3a) indicate a porous sheet-like morphology with good adhesion to the FTO substrate with a thickness of 40–50 μm. The CNTM–Ru15 film images (Fig. 3d–f) suggest the preservation of the porous sheet-like morphology, with a rough surface. Cross-section analysis (Fig. 3g and h) indicates an intimate contact between the film and substrate with film thickness similar to that of the CNTM film. Energy dispersive X-ray spectroscopy (EDS) confirms the presence of Ru in the CNTM–Ru15 film (Fig. S5†). Moreover, the morphology of the CNTM–Ru15 sample was examined using a high-angle annular dark-field scanning transmission electron microscope (HAADF-STEM), revealing the layered structure of CN (see Fig. S6†). EDS mapping further confirms the presence and distribution of the Ru15 oligomer over the CN surface (Fig. S7†).
X-ray photoelectron spectroscopy (XPS) confirms the successful loading of Ru15 on the CNTM film, showing the presence of C, N, and Ru in the CNTM–Ru15 film (Fig. S8a†). The high-resolution XPS C 1s spectrum of the CNTM film (Fig. S8b†) exhibits three peaks centered around 284.7 and 288.3 eV, assigned to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups, sp2 C–C bonding, and N–C
O groups, sp2 C–C bonding, and N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bonding in the triazine units of carbon nitride, respectively. The high-resolution N 1s XPS curve shows three deconvoluted peaks attributed to C–N
N bonding in the triazine units of carbon nitride, respectively. The high-resolution N 1s XPS curve shows three deconvoluted peaks attributed to C–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, N–(C)3, and C–N–Hx bonds, respectively (Fig. S8c†).39,41,42 The deconvolution of the C 1s spectrum of the CNTM–Ru15 sample reveals six peaks. The additional three peaks are ascribed to C–C
C, N–(C)3, and C–N–Hx bonds, respectively (Fig. S8c†).39,41,42 The deconvolution of the C 1s spectrum of the CNTM–Ru15 sample reveals six peaks. The additional three peaks are ascribed to C–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C (sp2), N(sp2)–C, and Ru 3d3/2 (overlapping with C 1s), respectively, originating from the Ru oligomer (Fig. S8b†). The N 1s spectrum of CNTM–Ru15 shows five deconvoluted peaks, where two additional peaks are centered at 397.4 and 399.2 eV and originate from the Ru oligomer (Fig. S8c†). The high-resolution S 2p spectrum (Fig. S8d†) shows peaks centered at 167.9 and 169.6 eV, ascribed to an S–H bond (S 2p3/2 and S 2p1/2, respectively) in the CN film, which was prepared using a thiourea precursor as the S source.
C (sp2), N(sp2)–C, and Ru 3d3/2 (overlapping with C 1s), respectively, originating from the Ru oligomer (Fig. S8b†). The N 1s spectrum of CNTM–Ru15 shows five deconvoluted peaks, where two additional peaks are centered at 397.4 and 399.2 eV and originate from the Ru oligomer (Fig. S8c†). The high-resolution S 2p spectrum (Fig. S8d†) shows peaks centered at 167.9 and 169.6 eV, ascribed to an S–H bond (S 2p3/2 and S 2p1/2, respectively) in the CN film, which was prepared using a thiourea precursor as the S source.
It is noteworthy that the peaks related to C–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, N–(C)3, and C–N–Hx bonds stemming from the carbon nitride have shifted to higher binding energies after modification with Ru15 due to the Ru oligomer/CN interaction. The high-resolution XPS Ru 3p spectrum of the CNTM–Ru15 film displays peaks in the 485–460 eV range, attributed to the presence of Ru(II) species (Fig. 3i).43 Finally, inductively coupled plasma optical emission spectroscopy (ICP-OES) elemental analysis for the CNTM–Ru15 samples gives 6.5 μg of Ru per g of sample, which implies 64.3 nmol of Ru per g of CNTM–Ru15 (see Table S1†).
C, N–(C)3, and C–N–Hx bonds stemming from the carbon nitride have shifted to higher binding energies after modification with Ru15 due to the Ru oligomer/CN interaction. The high-resolution XPS Ru 3p spectrum of the CNTM–Ru15 film displays peaks in the 485–460 eV range, attributed to the presence of Ru(II) species (Fig. 3i).43 Finally, inductively coupled plasma optical emission spectroscopy (ICP-OES) elemental analysis for the CNTM–Ru15 samples gives 6.5 μg of Ru per g of sample, which implies 64.3 nmol of Ru per g of CNTM–Ru15 (see Table S1†).
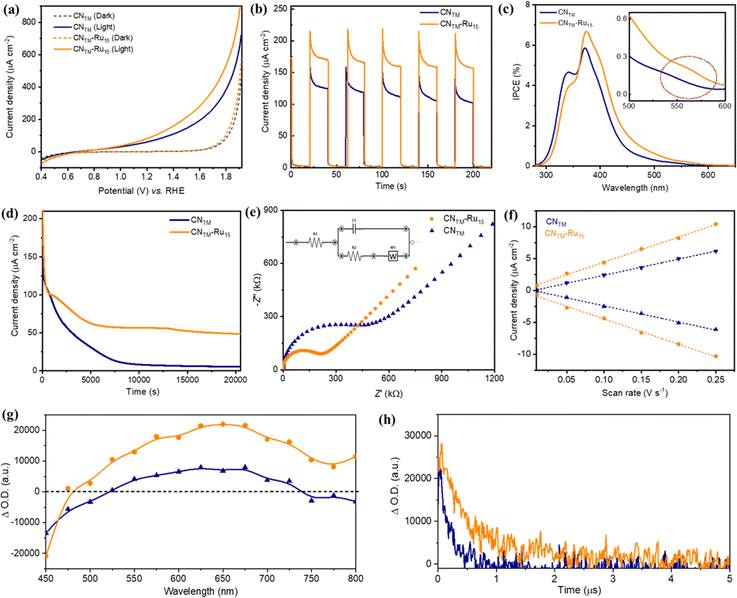
PEC measurements of CNTM and CNTM–Ru15 films were performed in a three-electrode system under simulated 1 sun illumination in a NaH2PO4/Na2HPO4 buffer solution (pH 7 and ionic strength 0.1 M) as a supporting electrolyte. The linear sweep voltammetry (LSV) curves (Fig. 4a) of CNTM and CNTM–Ru15 films demonstrate a typical PEC behavior, with an onset potential of 0.55 V vs. RHE. Chronoamperometry measurements at 1.23 V vs. RHE (Fig. 4b) reveal that the incorporation of Ru15 leads to an improvement in the photocurrent densities of about 40%, from 130 ± 8 μA cm−2 for the CNTM film to 180 ± 10 μA cm−2 for the CNTM–Ru15 film, both at an Eapp = 1.23 V vs. RHE. This improvement is attributed to the synergy between the CNTM and Ru15 anchored on the surface by CH–π interactions, facilitating charge transfer and separation and the additional capacity of Ru15 to efficiently catalyze the water oxidation reaction.
The measured incident photon-to-current conversion efficiency (IPCE) of CNTM and CNTM–Ru15 films at several illumination wavelengths ranging from 280 to 650 nm is displayed in Fig. 4c. The IPCE values are in good agreement with the absorption spectra of the films. The IPCE value of the CNTM–Ru15 film (6.7%) is higher than that of the CNTM film (5.8%) at 370 nm. In addition, the IPCE measurement reveals that the CNTM–Ru15 film is photoactive at longer wavelengths, up to ∼550 nm (inset of Fig. 4c), mainly due to the contribution of Ru15 (Fig. S2†).
Notably, the incorporation of Ru15 into the CNTM film significantly enhances the long-term stability. As shown in Fig. 4d, CNTM–Ru15 films in a neutral pH medium retain ∼35% photocurrent density even after 5.5 h. In sharp contrast, the CNTM film completely loses its photocurrent density (∼96%) within 2 hours. Importantly, O2 measurements indicate that most of the current is attributed to oxygen evolution and not to the self-oxidation of the CN layer. CNTM–Ru15 generates O2 at a rate of 0.014 μmol cm−2 min−1 (Fig. S9†) with a faradaic efficiency (FE) of up to 89% after 20 min. Overall, this implies a TON over 3000 after 5 h and a TOF of 0.4 s−1 (Table S2†).
A comparison table for the PEC performance using metal oxides as WOC co-catalysts with a CN-based film is given in Table S3.† It is worth mentioning that the molecular hybrid material is superior in terms of FE for O2 generation. It is also important to mention here that the amount of Ru used is in the range of micrograms of Ru per g of sample. This generally implies a loading of catalyst 4 to 6 orders of magnitude lower34,35 than related examples using Co, Ni, or Fe oxides.44,45
The CNTM–Ru15 film after the stability experiment was examined using PXRD, XPS and SEM (Fig. S10†), revealing minimal alterations in the film's structure and morphology. The improved durability of the CNTM–Ru15 film is associated with better charge separation and the high OER catalytic activity of Ru15. We analyzed the charge transfer kinetics behavior of CNTM and CNTM–Ru15 films using electrochemical impedance spectroscopy (EIS) and transient absorption spectroscopy (TAS) to elucidate the activity improvement. The EIS experiments (Fig. 4e and S11†) disclosed lower charge transfer resistance (Rct = 250 kΩ) for the CNTM–Ru15 film than for CNTM alone (470 kΩ), implying better hole transfer to the solution. An increased electrochemically active surface area (ECSA) is shown in Fig. 4f and S12,† indicating more active sites for water oxidation after modification with the Ru15 oligomer.
TAS measurements of CNTM and CNTM–Ru15 dispersions in MeCN upon 355 nm laser excitation further confirmed the improved photo-induced charge transfer kinetics in the presence of Ru15. The TA spectrum of CNTM (Fig. 4g) exhibits a negative feature up to ca. 525 nm, due to the bleaching of the ground state absorption of CNTM. The detected positive signal from 525 to 750 nm indicates the presence of excited states absorbing in the visible region, as previously reported for related CN materials. The TA spectrum of CNTM–Ru15 presents similar features with higher signal intensity. Still, it exhibits extended transient absorption in the NIR region (750–800 nm) due to the Ru15 incorporation. It is worth noticing that the increase in the positive signal intensity for CNTM–Ru15 is proportional to an enhancement in photo-induced charge carriers, in good agreement with the photocurrent and IPCE measurements, indicating a more efficient charge separation in CNTM–Ru15 compared to CNTM. These positive signals are attributed to photo-generated electrons in CNTM and CNTM–Ru15. Hole quenching experiments using MeOH as a sacrificial electron donor (Fig. S13†) confirm that the electrons are the main charge carriers detected under these experimental conditions.
The TA decays of CNTM and CNTM–Ru15 at 650 nm (Fig. 4h) reveal an almost one magnitude order longer electron half-lifetime of CNTM–Ru15 (1.60 μs) vs.CNTM (0.24 μs) thanks to a better charge separation and thus lower recombination rates. This agrees with the mechanism proposed in eqn (1)–(3):
| [CNTM–Ru15] + hν → [CNTM–Ru15]* (excited state) | (1) |
| [CNTM–Ru15]* → [−CNTM–Ru15+] (charge separated state) | (2) |
| 4[−CNTM–Ru15+] + 2H2O → 4[CNTM–Ru15] + O2 + 4H+ + 4e− (WOR) | (3) |
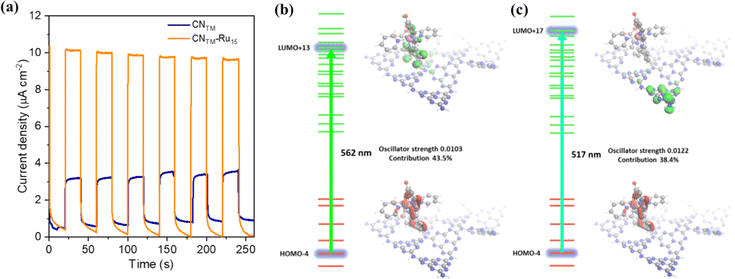
Furthermore, additional photocurrent measurements were performed using a 510 nm band-pass filter (FWHM 10 nm) for both electrodes, as presented in Fig. 5a, showing a photocurrent enhancement of approx. 3.2 times higher in the case of CNTM–Ru15 compared to CNTM due to the presence of the molecular catalyst. This points out the behavior of Ru15 as both a light absorber and a catalyst.46 To gain some insights into the processes occurring upon light excitation of the system, we computed the absorption spectra by means of TD-DFT of the model hybrid CNRed–Ru2, and the results are shown in Fig. 5 and S14–S22.† Two transitions are displayed in the figure, one at 562 nm (Fig. 5b) that is mainly intramolecular involving the Ru catalyst, which later on can further transfer an electron to the valence band of the CNRed moiety, resembling the typical Grätzel's dye-sensitized solar cells based on TiO2 and [Ru(bpy)3]2+.47,48 A second excitation at 517 nm, shown in Fig. 5c, would involve a direct charge transfer from the Ru center to the valence band of CNRed.
Conclusions
In this work, we introduced a new molecular hybrid material CNTM–Ru15 based on the anchoring of a highly active molecular water oxidation catalyst on a CNTM photoanode. The successful deposition of the Ru15 oligomer on polymeric carbon nitride photoanodes (CNTM) through CH–π interactions enables good photoelectrochemical water-splitting activity at neutral pH, enhanced long-term stability and high FE (>89%) for oxygen production. Detailed structural, photoelectrochemical, and mechanistic studies reveal that Ru15 markedly improves charge separation and hole extraction kinetics, enabling efficient water oxidation. Furthermore, the Ru15 oligomer leads to better light harvesting, a higher electrochemical surface area, and improved electronic conductivity. The optimized CNTM–Ru15 film demonstrates a photocurrent density of 180 ± 10 μA cm−2 with 89% FE for oxygen evolution, good stability up to 5 h, and IPCE values up to 6.7%. The amount of Ru-based catalyst loaded on the surface represents only 6.5 ppm of the electrode composition and leads to TONs in the range of 3300 and a TOF of 0.4 s−1. Furthermore, we have also shown that in the CNTM–Ru15 hybrid material, the Ru centers act both as a catalyst and as a photoabsorber.Finally, the present work is an example of positive synergy that can be obtained with the proper utilization of a molecular-based catalyst and a polymeric organic absorber.
Data availability
The data supporting this article have been included as part of the ESI.† The underlying raw data are available on request from the authors.Author contributions
S. M. performed most of the experiments, analyzed the data, and wrote the initial draft of the manuscript. M. S. synthesized and characterized the Ru15 oligomer and participated in manuscript writing. M. N. performed the DFT calculations and analysis. J. A. and H. G. performed the TAS measurements. M. S. C., C. B., and M. G. S. took part in the DFT study. M. V. took part in analysis, SEM imaging, and manuscript editing. M. S. and A. L. supervised the study, co-wrote and reviewed the paper, and acquired funding. All the authors discussed the results and reviewed the manuscript. All authors have given approval to the final version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This project has received funding from the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation program (Grant Agreement No. 849068). This work was also partially supported by the Israel Science Foundation (ISF), Grant No. 601/21. MS and MN acknowledge the Ministerio de Ciencia e Innovación (MICINN) for the grants PRE2020-093789 and PRE2020-093521, respectively. CB gratefully acknowledges MCINN/AEI/10.13039/501100011033 for projects PID2020-112806RB-I00 and CEX2019-000925-S, the ICIQ Foundation and the CERCA program of the Generalitat de Catalunya for funding. HG thanks MICINN (CEX-2021-001230-S and PDI2021-0126071-OB-CO21 funded by MCIN/AEI/10.13039/501100011033), Generalitat Valenciana (Prometeo 2021/038 and Advanced Materials programme Graphica MFA/2022/023 with funding from European Union Next Generation EU PRTR-C17.I1). JA thanks the MICINN for a Ramon y Cajal research associate contract (RYC2021–031006-I funded by MCIN/AEI/10.13039/501100011033 and by “European Union Next Generation EU/PRTR), and acknowledges the financial support (PID2022-141099OA-I00 funded by MCINN/AEI/10.13039/501100011033 and by “European Union Next Generation EU/PRTR). AL acknowledges MICINN through the project PID2022-140143OB-I00, Generalitat de Catalunya for the project 2017 SGR 1631 and Severo Ochoa (CEX2019-000925-S).References
- M. Volokh, G. Peng, J. Barrio and M. Shalom, Angew. Chem., Int. Ed., 2019, 58, 6138–6151 CrossRef CAS.
- N. P. Dharmarajan, D. Vidyasagar, J.-H. Yang, S. N. Talapaneni, J. Lee, K. Ramadass, G. Singh, M. Fawaz, P. Kumar and A. Vinu, Adv. Mater., 2023, 36, 2306895 CrossRef.
- X. Fan, Z. Wang, T. Lin, D. Du, M. Xiao, P. Chen, S. A. Monny, H. Huang, M. Lyu, M. Lu and L. Wang, Angew. Chem., Int. Ed., 2022, 61, e202204407 CrossRef CAS PubMed.
- G. Peng, J. Albero, H. Garcia and M. Shalom, Angew. Chem., Int. Ed., 2018, 57, 15807–15811 CrossRef CAS.
- J. Zhang, J. Zhang, C. Dong, Y. Xia, L. Jiang, G. Wang, R. Wang and J. Chen, Small, 2023, 19, 2208049 CrossRef CAS PubMed.
- T. H. Jeon, C. Park, U. Kang, G. Moon, W. Kim, H. Park and W. Choi, Appl. Catal., B, 2024, 340, 123167 CrossRef CAS.
- M. Fawaz, R. Bahadur, N. Panangattu Dharmarajan, J.-H. Yang, C. I. Sathish, A. M. Sadanandan, V. Perumalsamy, G. Singh, X. Guan, P. Kumar and A. Vinu, Carbon., 2023, 214, 118345 CrossRef CAS.
- J. Qin, J. Barrio, G. Peng, J. Tzadikov, L. Abisdris, M. Volokh and M. Shalom, Nat. Commun., 2020, 11, 4701 CrossRef PubMed.
- L. Jiang, X. Yuan, Y. Pan, J. Liang, G. Zeng, Z. Wu and H. Wang, Appl. Catal., B, 2017, 217, 388–406 CrossRef CAS.
- S. Mondal, G. Mark, L. Abisdris, J. Li, T. Shmila, J. Tzadikov, M. Volokh, L. Xing and M. Shalom, Mater. Horiz., 2023, 10, 1363–1372 RSC.
- Y. Yang, S. Wang, Y. Jiao, Z. Wang, M. Xiao, A. Du, Y. Li, J. Wang and L. Wang, Adv. Funct. Mater., 2018, 28, 1805698 CrossRef.
- S. Mondal, G. Mark, A. Tashakory, M. Volokh and M. Shalom, J. Mater. Chem. A, 2024, 12, 11502–11510 RSC.
- T. Shmila, S. Mondal, S. Barzilai, N. Karjule, M. Volokh and M. Shalom, Small, 2023, 19, 2303602 CrossRef CAS.
- F. Li, X. Yue, Y. Liao, L. Qiao, K. Lv and Q. Xiang, Nat. Commun., 2023, 14, 3901 CrossRef CAS.
- N. Karjule, C. Singh, J. Barrio, J. Tzadikov, I. Liberman, M. Volokh, E. Palomares, I. Hod and M. Shalom, Adv. Funct. Mater., 2021, 31, 2101724 CrossRef CAS.
- Y. Hou, F. Zuo, A. P. Dagg, J. Liu and P. Feng, Adv. Mater., 2014, 26, 5043–5049 CrossRef CAS PubMed.
- W. Zhang, J. Albero, L. Xi, K. M. Lange, H. Garcia, X. Wang and M. Shalom, ACS Appl. Mater. Interfaces, 2017, 9, 32667–32677 CrossRef CAS.
- R. Gong, D. Mitoraj, D. Gao, M. Mundszinger, D. Sorsche, U. Kaiser, C. Streb, R. Beranek and S. Rau, Adv. Sustainable Syst., 2022, 6, 2100473 CrossRef CAS.
- I. Cesar, A. Kay, J. A. Gonzalez Martinez and M. Grätzel, J. Am. Chem. Soc., 2006, 128, 4582–4583 CrossRef CAS.
- Z. Wang, Y. Gu, L. Zheng, J. Hou, H. Zheng, S. Sun and L. Wang, Adv. Mater., 2022, 34, 2106776 CrossRef CAS.
- J. Liu, Z. Luo, X. Mao, Y. Dong, L. Peng, D. Sun-Waterhouse, J. V Kennedy and G. I. N. Waterhouse, Small, 2022, 18, 2204553 CrossRef CAS PubMed.
- B. Liu, X. Wang, Y. Zhang, L. Xu, T. Wang, X. Xiao, S. Wang, L. Wang and W. Huang, Angew. Chem., Int. Ed., 2023, 62, e202217346 CrossRef CAS PubMed.
- Z. Zhang, X. Huang, B. Zhang and Y. Bi, Energy Environ. Sci., 2022, 15, 2867–2873 RSC.
- J. Lin, X. Han, S. Liu, Y. Lv, X. Li, Y. Zhao, Y. Li, L. Wang and S. Zhu, Appl. Catal., B, 2023, 320, 121947 CrossRef CAS.
- A. Tashakory, N. Karjule, L. Abisdris, M. Volokh and M. Shalom, Adv. Sustainable Syst., 2021, 5, 2100005 CrossRef CAS.
- J. Xia, N. Karjule, L. Abisdris, M. Volokh and M. Shalom, Chem. Mater., 2020, 32, 5845–5853 CrossRef CAS.
- P. Garrido-Barros, C. Gimbert-Suriñach, R. Matheu, X. Sala and A. Llobet, Chem. Soc. Rev., 2017, 46, 6088–6098 RSC.
- M. Gil-Sepulcre and A. Llobet, Nat. Catal., 2022, 5, 79–82 CrossRef CAS.
- N. Vereshchuk, M. Gil-Sepulcre, A. Ghaderian, J. Holub, C. Gimbert-Suriñach and A. Llobet, Chem. Soc. Rev., 2023, 52, 196–211 RSC.
- R. Matheu, M. Z. Ertem, J. Benet-Buchholz, E. Coronado, V. S. Batista, X. Sala and A. Llobet, J. Am. Chem. Soc., 2015, 137, 10786–10795 CrossRef CAS.
- R. Matheu, I. A. Moreno-Hernandez, X. Sala, H. B. Gray, B. S. Brunschwig, A. Llobet and N. S. Lewis, J. Am. Chem. Soc., 2017, 139, 11345–11348 CrossRef CAS PubMed.
- J. Creus, R. Matheu, I. Peñafiel, D. Moonshiram, P. Blondeau, J. Benet-Buchholz, J. García-Antón, X. Sala, C. Godard and A. Llobet, Angew. Chem., Int. Ed., 2016, 55, 15382–15386 CrossRef CAS PubMed.
- S. Grau, S. Berardi, A. Moya, R. Matheu, V. Cristino, J. J. Vilatela, C. A. Bignozzi, S. Caramori, C. Gimbert-Suriñach and A. Llobet, Sustainable Energy Fuels, 2018, 2, 1979–1985 RSC.
- M. A. Hoque, M. Gil-Sepulcre, A. de Aguirre, J. A. A. W. Elemans, D. Moonshiram, R. Matheu, Y. Shi, J. Benet-Buchholz, X. Sala, M. Malfois, E. Solano, J. Lim, A. Garzón-Manjón, C. Scheu, M. Lanza, F. Maseras, C. Gimbert-Suriñach and A. Llobet, Nat. Chem., 2020, 12, 1060–1066 CrossRef CAS PubMed.
- M. Gil-Sepulcre, J. O. Lindner, D. Schindler, L. Velasco, D. Moonshiram, O. Rüdiger, S. DeBeer, V. Stepanenko, E. Solano, F. Würthner and A. Llobet, J. Am. Chem. Soc., 2021, 143, 11651–11661 CrossRef CAS.
- D. Schindler, M. Gil-Sepulcre, J. O. Lindner, V. Stepanenko, D. Moonshiram, A. Llobet and F. Würthner, Adv. Energy Mater., 2020, 10, 2002329 CrossRef CAS.
- R. S. Roy, S. Mondal, S. Mishra, M. Banoo, L. Sahoo, A. Kumar, C. P. Vinod, A. K. De and U. K. Gautam, Appl. Catal., B, 2023, 322, 122069 CrossRef CAS.
- G. Mark, S. Mondal, M. Volokh, J. Xia and M. Shalom, Sol. RRL, 2022, 6, 2200834 CrossRef CAS.
- S. Mondal, L. Sahoo, Y. Vaishnav, S. Mishra, R. S. Roy, C. P. Vinod, A. K. De and U. K. Gautam, J. Mater. Chem. A, 2020, 8, 20581–20592 RSC.
- N. Karjule, J. Barrio, L. Xing, M. Volokh and M. Shalom, Nano Lett., 2020, 20, 4618–4624 CrossRef CAS.
- Y. Hou, Y. Zhu, Y. Xu and X. Wang, Appl. Catal., B, 2014, 156–157, 122–127 CrossRef CAS.
- Q. Zhang, X. Chen, Z. Yang, T. Yu, L. Liu and J. Ye, ACS Appl. Mater. Interfaces, 2022, 14, 3970–3979 CrossRef CAS.
- M. K. Awasthi, R. K. Rai, S. Behrens and S. K. Singh, Catal. Sci. Technol., 2021, 11, 136–142 RSC.
- J. Ma, X. Bai, W. He, S. Wang, L. Li, H. Chen, T. Wang, X. Zhang, Y. Li and L. Zhang, Chem. Commun., 2019, 55, 12567–12570 RSC.
- M. Volokh and M. Shalom, Ann. N. Y. Acad. Sci., 2023, 1521, 5–13 CrossRef CAS PubMed.
- I. N. Cloward, T. Liu, J. Rose, T. Jurado, A. G. Bonn, M. B. Chambers, C. L. Pitman, M. A. Ter Horst and A. J. M. Miller, Nat. Chem., 2024, 1–8 Search PubMed.
- G. Li, A. Yella, D. G. Brown, S. I. Gorelsky, M. K. Nazeeruddin, M. Grätzel, C. P. Berlinguette and M. Shatruk, Inorg. Chem., 2014, 53, 5417–5419 CrossRef CAS.
- M. Grätzel, Acc. Chem. Res., 2009, 42, 1788–1798 CrossRef.
Footnotes |
| † Electronic supplementary information (ESI) available: Materials, characterization, PEC and electrochemical measurements, detailed synthesis procedures of the Ru15 oligomer, photoelectrodes, computational details, figures, calculations, tables and detailed data of the theoretical part. See DOI: https://doi.org/10.1039/d4sc04678a |
| ‡ These authors have contributed equally. |
| This journal is © The Royal Society of Chemistry 2024 |