Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
The photo-isomerization of the cyclononatetraenyl ligand and related rare earth complexes†
Lucie
Pedussaut‡
a,
Nolwenn
Mahieu‡
 a,
Camille
Chartier
a,
Camille
Chartier
 a,
Thayalan
Rajeshkumar
b,
Maxime
Tricoire
a,
Iskander
Douair
b,
Nicolas
Casaretto
a,
Laurent
Maron
b,
Grégory
Danoun
a,
Thayalan
Rajeshkumar
b,
Maxime
Tricoire
a,
Iskander
Douair
b,
Nicolas
Casaretto
a,
Laurent
Maron
b,
Grégory
Danoun
 *a and
Grégory
Nocton
*a and
Grégory
Nocton
 *a
*a
aLCM, CNRS, Ecole Polytechnique, Institut Polytechnique de Paris, Route de Saclay, 91120 Palaiseau, France. E-mail: gregory.nocton@polytechnique.edu; gregory.danoun@polytechnique.edu
bLPCNO, UMR 5215, Université de Toulouse-CNRS, INSA, UPS, Toulouse, France
First published on 18th October 2024
Abstract
The cyclononatetraenyl (Cnt) ligand is a large monoanionic ligand. It is easily synthesized by ring expansion after cyclopropanation of the cyclooctatetraenyl (Cot) ligand. The Cnt ligand can be reported as the cis–cis–cis–cis (cis) isomer, where the aromatic ring is flat, and all carbon atoms form a homogenous ring, and as the cis–cis–cis–trans (trans) isomer, where one carbon places itself inside the ring. The isomerization from the trans to the cis form has been reported numerous times in previous articles, but no quantitative analysis has been proposed due to contradictory data. This article proposes a detailed analysis involving light to rationalize this intrigue concerning isomerization. A careful synthesis at low temperatures and with light protection yields the ligand in its trans form (Cnt-trans). The controlled photo-isomerization of the Cnt-trans ligand is reported herein. A series of divalent or trivalent rare earth complexes, (Cnt)2Sm, and (Cot)(Cnt)Ln (Ln = Y, La, Ce, Pr, Nd, Sm, Gd, Tb, Er, Ho), which synthesis, solid-state X-ray diffraction and solution 1H NMR and UV-visible measurements, have been revised according to the synthesis using the Cnt-trans ligand. The photo-isomerization of the (Cnt-trans)2Sm evolves to the intermediate (Cnt-cis)(Cnt-trans)Sm and the (Cnt-cis)2Sm complex as the thermodynamical product. The photoisomerization of the trivalent (Cot)(Cnt)Ln complexes highlights the formation of a photostationary state (PSS) after several minutes of irradiation, in which both Cnt-trans and Cnt-cis ligands are present. The ratio of these two forms varies according to metal and irradiation wavelength: low-energy wavelengths favor the cis isomer, and high-energy wavelengths favor the trans isomer. DFT and TD-DFT were performed to provide a tentative orbital explanation.
Introduction
The cyclononatetraenyl ligand, C9H9− – or Cnt−, was synthesized in 1963 by Katz and coworkers.1,2 The ligand is anionic and aromatic, with 10 π electrons. Synthetic attempts to aim for sandwich complexes have been made, but – except for notable multimetallic complexes with Pd,3 they mostly yielded to the re-organization of this large ligand to the bicyclic 1,5-dihydroindenyl.4 Only recently, this ligand has been used with larger ions, first barium, then 4f-elements, the size of which suits the Cnt ligand well.5–7After the report of a series of complexes with Ba,5 the cyclononatetraenyl ligand has been used to develop bright luminescence dyes of Eu(II) in the (Cnt)2Eu complex.6 Soon after, the family of these sandwich complexes was continued with the classical divalent lanthanides Sm, Yb, and Tm, showing linear isostructures along the series.7 Heteroleptic complexes of formulae (Cot)(Cnt)Ln (Ln = La, Ce, Nd, Sm, Tb, Dy, Er, Ho, Tm, and Lu) complexes were made,8–10 accommodating the trivalent oxidation state, while the analogs to the historical (Cp)3Ln complexes with Cnt, the (Cnt)3Ln (Ln = Y, Gd, Tb, Dy, Ho, Er, Tm) complexes were also reported in recent years.11
Because of their potential for developing high-performance molecular magnets, lanthanide-based molecular compounds have developed strongly in recent years.12–19 Several sandwich complexes in the divalent and trivalent states containing the Cot and Cnt ligands have been employed.5–9,20–23 These have given rise to remarkable fundamental chemistry, describing several objects with fascinating structures.10
The Cnt ligand is a labile ligand easily displaced by competing coordinating solvents. Notably, it has been shown that the ligand bends and changes its coordination mode when complexed with lanthanide ions of different sizes.9 This characteristic has also been used to build complexes, modulating their physical properties by using solvents or controlling the ligand isomerization.7 For example, Vitova et al. recently demonstrated the photo-modulation of bond covalency in the (Cnt)2Sm complex.24
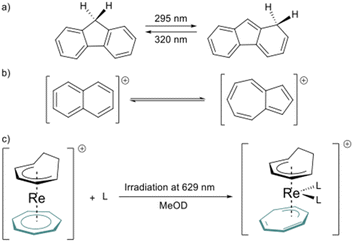
In addition to its coordination features, the Cnt ligand can be obtained as two different isomers (Fig. 1a). The first is the isomer in its usual aromatic form, with a planar symmetrical ligand and similar internal C–C distances. It is named the cis–cis–cis–cis (cis) isomer to recall the relative orientation of the alternating four double bonds originating from the 10 π-electrons. Its isomer is described by the reversal of a carbon atom in the ring, which induces the presence of a trans bond, hence the name cis–cis–cis–trans (trans). Aromaticity is limited to the six π-electrons in the ring, while the charge is localized on the reversed carbon. As a result, the NICS(0) value increases from the Cnt-cis ligand to the Cnt-trans from −15.8 to −20.3 in agreement with more localized aromaticity in the plane,25 while the NICS(1) has similar values of −13.7 and – 13.8 for Cnt-cis and Cnt-trans, respectively. The presence of both isomers has been described in previous work for the sole ligand and its metallic complexes without further explanation.7,9,26 For example, some of us used the difference in solubility of the Cnt-trans compared to the Cnt-cis to improve the yield of the (Cnt)2Ln complexes.7
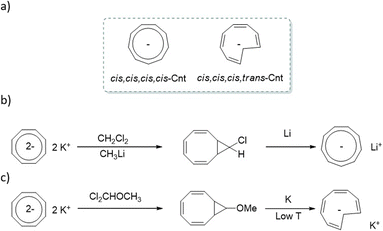 | ||
| Fig. 1 (a) Isomers of the cyclononatetraenyl ligand, (b) original synthetic paths by Lalancette and Benson2 and (c) synthetic path favoring the trans isomer.28 | ||
The question of the isomers returns to the original ligand synthesis. Initially, the synthesis was performed with dichloromethane or dichloromethyl methyl ether, leading to the cis isomer (Fig. 1b).27 A modification in the synthetic pathway favors the second isomer (trans) (Fig. 1c).28 At low temperatures, the methoxide group of the bicycle is favored in the anti-position. When electrons are injected into the system, it is reported that the anion radical is formed within the 8-member ring, leading to a conrotatory ring opening mechanism favoring the trans isomer.29 A comprehensive study by Boche in 1978 brought much information on the Cnt ligand and its isomerization.26 However, several questions remained: one particularly caught our attention. The isomerization was reported from the trans form to the cis form, implying that the cis form is the most thermodynamically stable. Additionally, the process was reported to be faster at 60 °C in THF than at 100 °C in the same solvent. This mention implies a counter-thermodynamic isomerization process, which differs from typical topoisomerization. Boche's comprehensive work is unquestionable, and we've been able to reproduce his results.
In addition to this intriguing result, some of us and Roesky have noted the presence of an isomerization phenomenon contrary to the thermodynamics of ligand isomerization. When the symmetrical (Cot)(Cnt)Tb complex (starting from the Cnt-cis ligand) is left at room temperature, the complex evolves towards the trans form.9 A fast mention in Boche's seminal work about a possible light-induced isomerization attracted our attention since it would lead to original photoswitch systems.26
Photochromism is a phenomenon in which molecules can undergo a photochemical reaction to switch between two stable isomers showing different structures or properties. Azobenzene is a prime example of a classical photoswitch.30,31 Many related examples exist, and photochromic properties have been used as a light trigger switch in polymers,32 surfaced materials, supramolecular chemistry, catalysis,7 and many biological applications.33 Complexes bearing azobenzene and related scaffolds are also reported to possess photo-switch behavior.34,35 One particularity of this motif is the possibility of switching from the E to the Z form and the reversal using a different light energy. Sometimes, the transfer from one to another isomer is incomplete, and several E![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Z ratios (PSS: photo stationary states) are reported as the stable form upon light irradiation.36
Z ratios (PSS: photo stationary states) are reported as the stable form upon light irradiation.36
However, this type of photo-induced isomerization is rare in aromatic hydrocarbon ligands. To our knowledge, the only instances reported of the photoinduced isomerization of C–H aromatics ligands are the isomerization of 9H-fluorene,37 naphthalene, and azulene cations.38 Another similar example is the light-induced hapticity switch of the C7H7 ligand in a Re sandwich complex (Fig. 2).39
Here, we report the synthesis and characterization of a series of rare earth complexes with the Cnt-trans ligand and study the photo conversion into the cis isomer. The finding particularly highlights the formation of a photo-stationary state (PSS), in which the cis![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) trans ratio evolves with the wavelength used and with the identity of the lanthanide cation. The study finalizes the rationalization of many observations made over the last 50 years on the cyclononatetraenyl ligand and opens various possibilities to control the cis
trans ratio evolves with the wavelength used and with the identity of the lanthanide cation. The study finalizes the rationalization of many observations made over the last 50 years on the cyclononatetraenyl ligand and opens various possibilities to control the cis![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) trans ratio.
trans ratio.
Results and discussion
Synthesis and solid-state studies
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 trans to cis ratio. This ratio can be brought to 95
20 trans to cis ratio. This ratio can be brought to 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 if the reaction and the work-up are protected from light.
5 if the reaction and the work-up are protected from light.
Thanks to the improved method to yield the KCnt ligand in a very high ratio of trans, which is reported herein, and also knowing that light causes problems in the reproducibility of the syntheses, the (Cnt-trans)2Ln were synthesized in the dark and in the absence of any coordinating solvent. The reaction vessel was protected by aluminum foil, and the light of the glovebox was switched off. The reaction between SmI2 and KCnt-trans was performed in toluene overnight. After filtration, the solvent was evaporated, and the resulting black powder was analyzed as pure (Cnt-trans)2Sm (1-trans) by 1H NMR (Fig. S2†). However, our attempts to obtain X-ray suitable single crystals of the pure trans compound failed. Despite our efforts to protect the crystallization from light, the few structurally characterized crystals always contained a considerable amount of the cis isomer in the solid state (82%). This is likely due to a slow isomerization process occurring even at 233 K over the time needed to grow the crystals and the solubility difference between 1-trans and 1-cis isomers. The latter being less soluble leads to an overestimation of the cis![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) trans ratio from the XRD perspective. This was confirmed by taking the NMR of the bulk material obtained after the crystallization step (See Fig. S3†) and revealed the presence of 49% of pure 1-trans and only 11% or conversion to pure 1-cis, the leftover 40% being the half-isomerized 1-cis–trans form.
trans ratio from the XRD perspective. This was confirmed by taking the NMR of the bulk material obtained after the crystallization step (See Fig. S3†) and revealed the presence of 49% of pure 1-trans and only 11% or conversion to pure 1-cis, the leftover 40% being the half-isomerized 1-cis–trans form.
The 1-cis isomer was previously reported in the literature in P21/n space group.71-trans crystallizes in the same space group. In our hands, because of the fast isomerization process while crystallizing, the crystals were found mostly in the cis form. Only one Cnt ring was modeled as the superposition of the two eclipsed Cnt isomers (18% trans). The carbon atom in the trans position crystalizes out of the plane, and is closer to the metal center. All C–C bonds are similar between the trans and cis isomers (Table 1).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
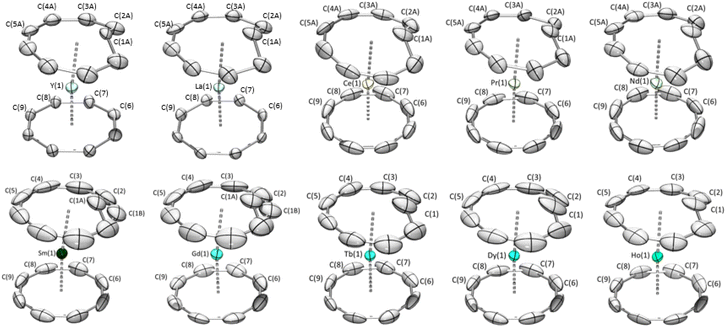
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cis ratios in the solid state. It was previously reported that the solid-state structure of the (Cot)(Cnt)Ln complexes (with the Cnt-cis ligand) could be modeled in two different space groups.8,9,40 For La, Ce, Nd, Sm, and Tb, the symmetry of the structure with the metallic ion localized on a given symmetry position led to the resolution in the Pnma space group. For the other lanthanide ions placed after Tb in the series, the P21/n space group was used. Thanks to the new method of synthesis of the KCnt ligand, the trans
cis ratios in the solid state. It was previously reported that the solid-state structure of the (Cot)(Cnt)Ln complexes (with the Cnt-cis ligand) could be modeled in two different space groups.8,9,40 For La, Ce, Nd, Sm, and Tb, the symmetry of the structure with the metallic ion localized on a given symmetry position led to the resolution in the Pnma space group. For the other lanthanide ions placed after Tb in the series, the P21/n space group was used. Thanks to the new method of synthesis of the KCnt ligand, the trans![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cis ratio is higher. However, despite all our precautions to avoid light during the syntheses, the crystals that were obtained almost all showed a trans
cis ratio is higher. However, despite all our precautions to avoid light during the syntheses, the crystals that were obtained almost all showed a trans![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cis ratio from 100% in the Tb, Dy, and Ho complexes to 71% and 69% in Gd and Sm, respectively and 58%, 55%, 50%, 25%, 73% in Nd, Pr, Ce, La and Y respectively (Fig. 3). In comparison to the previous work, all complexes crystallized in the Pnma space group and the hapticity switch of the Cnt ligand in 2-Dy-cis, 2-Ho-cis and 2-Y-cis complexes was not observed for their trans form. In this space group, only half of the complex needs to be modeled, and the other half will be constructed through the symmetry operation of this group.
cis ratio from 100% in the Tb, Dy, and Ho complexes to 71% and 69% in Gd and Sm, respectively and 58%, 55%, 50%, 25%, 73% in Nd, Pr, Ce, La and Y respectively (Fig. 3). In comparison to the previous work, all complexes crystallized in the Pnma space group and the hapticity switch of the Cnt ligand in 2-Dy-cis, 2-Ho-cis and 2-Y-cis complexes was not observed for their trans form. In this space group, only half of the complex needs to be modeled, and the other half will be constructed through the symmetry operation of this group.
Over the series, three types of behaviors were observed and modeled. First, for the early lanthanide ions (La, Ce, Pr, and Nd), the Cnt ring was modeled as the superposition of a Cnt-cis ligand and a Cnt-trans ligand in eclipsed positions, as represented in Fig. S35–39.† The occupation number in each ring was linked to the overall cis![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) trans ratio. For Sm and Gd, one carbon atom of the Cnt ligand presented a significant disorder with two favored positions: one following the ring and one inside it (Fig. S40 and 41†). The occupation number of each position can be used as a measure of the solid-state cis
trans ratio. For Sm and Gd, one carbon atom of the Cnt ligand presented a significant disorder with two favored positions: one following the ring and one inside it (Fig. S40 and 41†). The occupation number of each position can be used as a measure of the solid-state cis![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) trans ratio. Finally, for Tb, Dy, and Ho, the new synthesis led to the crystallization of purely trans complexes, and no disorder was modeled.
trans ratio. Finally, for Tb, Dy, and Ho, the new synthesis led to the crystallization of purely trans complexes, and no disorder was modeled.
The distances between the lanthanide centers and the carbon atoms of the Cot ligand follow the radius decrease along the series of the Ln with values of 2.695 (12) Å for the La and 2.55 (7) Å for Ho in the cis complexes and 2.694 (4) Å for the Ce and 2.478 (7) Å for Ho for the trans complexes (Table 2). A similar trend is observed with the Cnt-cis and Cnt-trans as well as the Ln–C(1) of the carbon atom inside the ring, with notable singularities observed for the Ho Cnt-cis due to the modification of the coordination mode described in earlier work.9
| Ln/Cnt-trans | 2-Y-trans | 2-La-trans | 2-Ce-trans | 2-Pr-trans | 2-Nd-trans | 2-Sm-trans | 2-Gd-trans | 2-Tb-trans | 2-Dy-trans | 2-Ho-trans |
|---|---|---|---|---|---|---|---|---|---|---|
| a Data taken from known literature.9 | ||||||||||
| Ln–C(Cot) average | 2.542 (8) | 2.694 (4) | 2.654 (4) | 2.636 (6) | 2.625 (9) | 2.595 (6) | 2.570 (7) | 2.552 (10) | 2.541 (5) | 2.478 (7) |
| Ln–C(Cnt–C8) average | 2.806 (9) | 2.905 (8) | 2.881 (15) | 2.86 (2) | 2.87 (2) | 2.833 (8) | 2.826 (9) | 2.818 (11) | 2.802 (8) | 2.792 (9) |
| Ln–C(Cnt) inside | 2.657 (10) | 2.851 (16) | 2.750 (12) | 2.749 (15) | 2.79 (2) | 2.687 (13) | 2.703 (17) | 2.664 (16) | 2.660 (9) | 2.673 (11) |
| Ln–C(Cnt) all average | 2.776 (9) | 2.894 (10) | 2.85 (1) | 2.84 (2) | 2.85 (2) | 2.804 (9) | 2.801 (11) | 2.787 (13) | 2.774 (8) | 2.768 (9) |
| Ln/Cnt-cis | 2-Y-cis | 2-La-cis40 | 2-Ce-cis40 | 2-Pr-cis | 2-Nd-cis8 | 2-Sm-cis8 | 2-Gd-cis | 2-Tb-cisa | 2-Dy-cisa | 2-Ho-cisa |
|---|---|---|---|---|---|---|---|---|---|---|
| Ln–C(Cot) average | 2.54 (2) | 2.695 (12) | 2.676 (8) | 2.65 (1) | 2.65 (1) | 2.624 (7) | 2.609 (16) | 2.58 (2) | 2.58 (4) | 2.55 (7) |
| Ln–C(Cnt) average | 2.75 (2) | 2.944 (15) | 2.91 (1) | 2.90 (2) | 2.88 (1) | 2.860 (7) | 2.82 (2) | 2.82 (3) | 2.81 (10) | 2.93 (33) |
| (Cot)^(Cnt) (plane) | 21.26 | 3.69 | 3.75 | 3.86 | 3.62 | 3.02 | 2.53 | 4.80 | 12.5 | 26.7 |
| Cot–Ln–Cnt (cent.) | 170.67 | 176.86 | 177.01 | 177.39 | 176.70 | 177.17 | 177.34 | 177.4 | 172.0 | 169.6 |
At this stage, it is essential to note that those solid-state studies and descriptions are only representative of the part of the mixture that did crystalize. Remarkably, the cis and the trans isomers possess different solubility, with the Cnt-trans being more soluble. As such, the observed ratio of isomers in the solid state is necessarily biased. Thus, a description of the speciation in the solution will be instrumental in accessing a complete characterization of the system.
Solutions studies
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
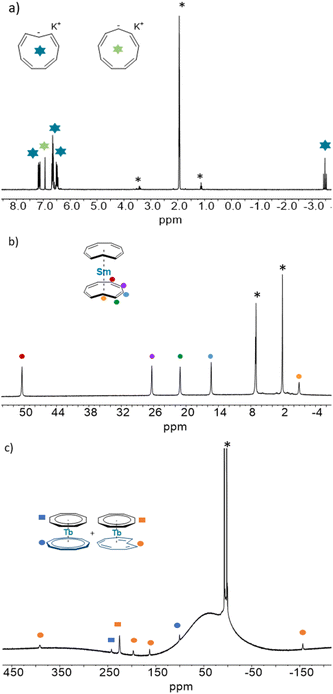
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio in agreement with equivalent Cnt ligands and a C2v symmetry in solution. The chemical shifts have been attributed by a COSY experiment and reflect the orientation of the principal axis of symmetry along the C2 axis with a negative shift for the proton attached to the C(1) carbon (−0.95 ppm) and a larger chemical shift for the proton in the farthest position in the aromatic backbone of the Cnt ligand (50.4 ppm, red dots in Fig. 4).42
2 ratio in agreement with equivalent Cnt ligands and a C2v symmetry in solution. The chemical shifts have been attributed by a COSY experiment and reflect the orientation of the principal axis of symmetry along the C2 axis with a negative shift for the proton attached to the C(1) carbon (−0.95 ppm) and a larger chemical shift for the proton in the farthest position in the aromatic backbone of the Cnt ligand (50.4 ppm, red dots in Fig. 4).42
The assignment of both isomers was reproduced for all of the compounds and is presented in ESI Fig. S11–S20.† Due to the paramagnetic nature of lanthanide ions, in our hands, only four signals coming from the Cnt-trans could be reliably identified in a broad spectral window. The proton attached to the C(1) carbon may be significantly influenced by the Ln ion, which disallowed its observation.
Photoisomerization of the cyclononatetraenyl ligand and 1
With a reliable method to correctly identify both isomers, the presumed photo-isomerization processes were studied for the Cnt ligand itself and all complexes reported in this article. Solution mixtures were irradiated directly into the NMR tubes at different wavelengths, and then the ratios were quantified by 1H NMR.With the syntheses of the cis compounds at hands,7–9,40 but also several of the trans compounds (see discussion above), UV-visible spectra of both isomers were recorded (see Fig. S47 to S59†) for all the compounds, except for the 1-trans–cis, which could not be isolated. In 2, Ln = Er, Tm, Lu, only the cis isomers were synthesized; thus, the spectrum of the PSS was generated under 370 nm irradiation before recording.
Under a 390 nm irradiation, the Cnt ligand isomerizes quickly from the Cnt-trans to the Cnt-cis. Thus, irradiation at a lower energy (427 nm) was used to monitor the process by 1H NMR (Fig. S62†). The complete conversion was noted after 5 min of irradiation. The apparent rate of decrease of the KCnt-trans in favor of the cis isomer displays a pseudo-first-order reaction rate. However, a complete photochemical analysis was not performed. The Cnt-trans shows two major absorption bands in the UV region at 345 nm and 275 nm with absorption coefficients of 5000 cm−1 M−1 and 45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cm−1 M−1, respectively. Meanwhile, the cis isomer displays two different absorption bands at 323 nm and 252 nm with absorption coefficients of 6000 cm−1 M−1 and 75
000 cm−1 M−1, respectively. Meanwhile, the cis isomer displays two different absorption bands at 323 nm and 252 nm with absorption coefficients of 6000 cm−1 M−1 and 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cm−1 M−1, respectively (Fig. 7). The strong absorption of the Cnt-cis isomer is in agreement with a larger HOMO–LUMO gap brought by the symmetry of the π system of the ligand, and thus the π to π* transfer requires more energy. Accordingly, the similar transition is less intense for the Cnt-trans ligand and is red-shifted. This energetic situation agrees with a trans-to-cis isomerization process, which occurs at energies lower than 300 nm, and the reverse process would need higher energy.26,43
000 cm−1 M−1, respectively (Fig. 7). The strong absorption of the Cnt-cis isomer is in agreement with a larger HOMO–LUMO gap brought by the symmetry of the π system of the ligand, and thus the π to π* transfer requires more energy. Accordingly, the similar transition is less intense for the Cnt-trans ligand and is red-shifted. This energetic situation agrees with a trans-to-cis isomerization process, which occurs at energies lower than 300 nm, and the reverse process would need higher energy.26,43
Under a 427 nm irradiation, the 1-trans isomerizes to the 1-cis using the 1-trans–cis as an intermediate within 30 min (Fig. S62†). The apparent rates of decrease of the 1-trans to the 1-trans–cis and the one from the 1-trans–cis to the 1-cis both display a pseudo-first-order reaction rate.
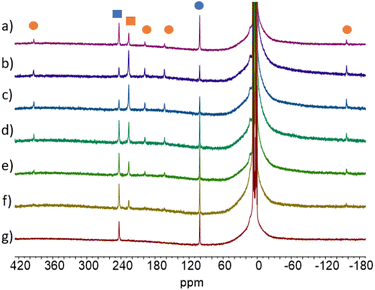
Photoisomerization of 2 and PSS
Because of the paramagnetic nature of the lanthanide ions and the superimposition of the two isomers' signals, the 1H NMR analyses were not possible for 2-Ln (Pr, Nd, Gd, Ho, Er, and Tm). However, for those where the integration is possible, irradiation at different wavelengths led to different ratios of Cnt-trans vs. Cnt-cis. Kinetic studies performed on the Sm complex (2-Sm) showed that the proper ratio is attained after 30 min and then does not change with time under irradiation. This phenomenon is in line with a PSS (photo-stationary state). In the experiments, the irradiation time was thus determined to ensure that the PSS was obtained in all cases. The PSS at different wavelengths can be reached either from the cis or from the trans complexes that can be obtained from different synthetic methods.8,40The PSS reached for the 2-Ln complexes are gathered in Table 3. Along the series, two main behaviors can be observed as illustrated in Fig. 5. First, for early La (2-La) and Ce (2-Ce) as well as Y (2-Y), the modification of the wavelength has only a modest impact on the PSS and high trans![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cis ratios are favored. For example, in La (2-La), the PSS ratio evolves from 93% to 99% at 370 nm and 440 nm, respectively, the Ce (2-Ce), from 97% to 64% at 370 nm and 467 nm, respectively, and the Y (2-Y) from 96% to 90% at 370 nm and 525 nm, respectively.
cis ratios are favored. For example, in La (2-La), the PSS ratio evolves from 93% to 99% at 370 nm and 440 nm, respectively, the Ce (2-Ce), from 97% to 64% at 370 nm and 467 nm, respectively, and the Y (2-Y) from 96% to 90% at 370 nm and 525 nm, respectively.
| 2-Y | 2-La | 2-Ce | 2-Sm | 2-Tb | 2-Dy | 2-Lu | |
|---|---|---|---|---|---|---|---|
| NMR | 61% | 55% | 79% | 89% | 87% | 75% | 0% |
| XRD | 73% | 25% | 50% | 69% | 100% | 100% | 0% |
| PSS 370 nm | 96% | 93% | 97% | 64% | 78% | 76% | 69% |
| PSS 390 nm | 99% | 98% | 100% | 52% | 79% | 75% | 71% |
| PSS 427 nm | 97% | 99% | 94% | 28% | 50% | 48% | 29% |
| PSS 440 nm | 95% | 99% | 86% | 23% | 39% | — | 20% |
| PSS 450 nm | — | — | — | — | — | 33% | — |
| PSS 467 nm | 92% | 99% | 64% | 13% | 28% | — | 11% |
| PSS 525 nm | 90% | 98% | 88% | 12% | 1% | 6% | 9% |
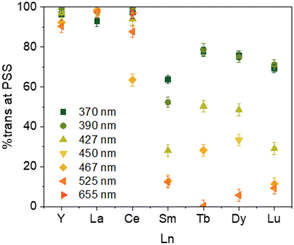 | ||
| Fig. 5 Ratio of 2-Ln at PSS at 370 nm irradiation up to 525 nm depending on the Ln (Y–Ce, Sm, Tb, Dy, Lu). | ||
The situation is different for Sm (2-Sm), Tb (2-Tb), Dy (2-Dy), and Lu (2-Lu) for which the ratios are significantly impacted by the irradiation wavelengths as shown in Fig. 5. For instance, for Tb (2-Tb), the trans![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cis ratio of the PSS at 370 nm is 78
cis ratio of the PSS at 370 nm is 78![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 22 while it drops to almost zero at 525 nm as shown in Fig. 6. A similar trend is observed for Sm (2-Sm) with values varying from 64% to 12% at 370 nm and 525 nm, respectively, for Dy (2-Dy) with values ranging from 76% to 6% at 370 nm and 525 nm, respectively, and for Lu (2-Lu) with values ranging from 69% to 9% at 370 nm and 525 nm, respectively.
22 while it drops to almost zero at 525 nm as shown in Fig. 6. A similar trend is observed for Sm (2-Sm) with values varying from 64% to 12% at 370 nm and 525 nm, respectively, for Dy (2-Dy) with values ranging from 76% to 6% at 370 nm and 525 nm, respectively, and for Lu (2-Lu) with values ranging from 69% to 9% at 370 nm and 525 nm, respectively.
To better understand this difference in speciation, UV-visible absorption spectra of 2 were recorded (Fig. S47–S59†). Similarly to what is known in the azobenzene PSS, it is notable that the overlapping of the absorption bands of both isomers does not favor an effective shift to one or the other isomers with a high PSS ratio upon light irradiation.43Fig. 8 reports the energy of the transitions observed before irradiation.
Similar to the KCnt ligand, 2 exhibits a strong absorption at low energy, but two maxima can be observed within the 300 to 370 nm region for the Y and late Ln (Sm–Lu). In contrast, only one can be observed for the early Ln (La–Nd) (Fig. 8). Additionally, 2-Ce-cis displays a third absorption band at 625 nm (800 cm−1 M−1) (Fig. 7b). As an additional note, it is important to stress that 2-Ce and 2-Tb were reported to be luminescent,40 but only when THF molecules were coordinated, so in the conditions reported here, no emitted light can interfere with the isomerization process.
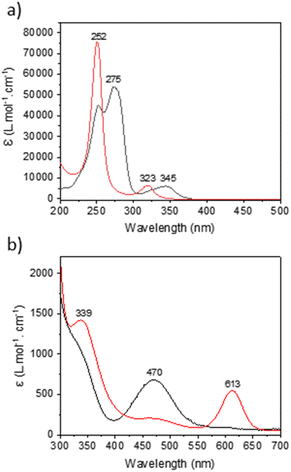 | ||
| Fig. 7 UV-visible of (a) KCnt-trans (black) and KCnt-cis (red) and (b) trans (black) and cis (red) complexes of 2-Ce. | ||
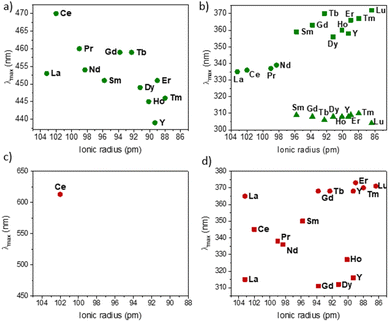 | ||
| Fig. 8 Evolution of the maximum absorption of 2-Ln (the cis/trans ratio is given in Table 3) complexes wavelength around (a) 450 nm and (b) 300–370 nm and of 2-Ln-cis (made from the methodology proposed in literature9,40 around) (c) 400–650 nm and (d) 300–380 nm. | ||
Based on the sole data we have in our hands, the rationalization of the data is not simple. First, we observe that the PSS of 2-Y and 2-La are unaffected by the different wavelengths. No matter what energy is used, the trans form is the most stable one under light irradiation. This is notable because the synthesis of the pure 2-Y-trans and 2-La-trans was not possible, and either in solution or in the solid-state, a significant amount of the cis material is made (See Table 3).
For Sm, Tb, Dy, and Lu, the PSS varies strongly depending on the wavelength used (See Fig. 5). The differences in the UV spectra compared to those of La, Y are minimal. One rationalization is that light brings enough energy to populate a transition state, which then relaxes on either the trans form or the cis one. Thus, when high energy is used, this allows for relaxing to the trans form, while without enough energy, the barrier to relax to the trans form is not crossed, and thus, the trans![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cis ratio remains small. This would mean that the barrier is lower in La and Y than in Sm, Tb, Dy, and Lu, which are similar.
cis ratio remains small. This would mean that the barrier is lower in La and Y than in Sm, Tb, Dy, and Lu, which are similar.
A second note is related to the absorption cross-section of the cis or the trans form at different wavelengths. While the cis compounds prepared with the published methodology9,40 have almost no absorption above 450 nm, the compounds with a higher trans![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cis ratio do (Fig. S60†). Thus, at lower energy wavelengths, only the trans form absorbs, which explains the high trans
cis ratio do (Fig. S60†). Thus, at lower energy wavelengths, only the trans form absorbs, which explains the high trans![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cis ratio for Sm, Tb and Lu. However, this does not explain the low trans
cis ratio for Sm, Tb and Lu. However, this does not explain the low trans![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cis ratio in Y and La (Fig. S45 and S46†). An interesting case is that of 2-Ce. 2-Ce-cis has one transition in the visible spectrum centered at 613 nm. At this value, the absorption of 2-Ce-trans is low. On the other hand, 2-Ce-trans has an absorption maximum at 470 nm and 2-Ce-cis has a weaker absorption at this wavelength (Fig. 7b). Following the ratio of trans
cis ratio in Y and La (Fig. S45 and S46†). An interesting case is that of 2-Ce. 2-Ce-cis has one transition in the visible spectrum centered at 613 nm. At this value, the absorption of 2-Ce-trans is low. On the other hand, 2-Ce-trans has an absorption maximum at 470 nm and 2-Ce-cis has a weaker absorption at this wavelength (Fig. 7b). Following the ratio of trans![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cis depending upon the energy of the wavelength is therefore informative. At high energy, the trans
cis depending upon the energy of the wavelength is therefore informative. At high energy, the trans![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cis ratio is high, similar to all compounds. At 467 nm, the trans
cis ratio is high, similar to all compounds. At 467 nm, the trans![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cis decreases to around 60%; following the case of Sm, Tb, Dy, and Lu, but then re-increases at lower energy (525, 88%, and 655 nm, 97%). This shows that the absorption cross-section of the cis form strongly influences the trans
cis decreases to around 60%; following the case of Sm, Tb, Dy, and Lu, but then re-increases at lower energy (525, 88%, and 655 nm, 97%). This shows that the absorption cross-section of the cis form strongly influences the trans![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cis ratio. Yet, compared to the KCnt ligand or with 1, where the photo-isomerization converts the trans to the cis, the reason for the stabilization at high light energies of the trans form compared to the cis one in 2-Ln was not straightforward. This is the reason why we have turned to theoretical computations.
cis ratio. Yet, compared to the KCnt ligand or with 1, where the photo-isomerization converts the trans to the cis, the reason for the stabilization at high light energies of the trans form compared to the cis one in 2-Ln was not straightforward. This is the reason why we have turned to theoretical computations.
DFT and TD-DFT calculations
To gain some insights into the electronic structure of 2, both in the cis and in the trans forms, DFT calculations were carried out (B3PW91, including dispersion corrections). The structures were optimized for Ce (2-Ce) and Sm (2-Sm), and in both cases, either the Cnt-cis or Cnt-trans ligands were considered. Interestingly, the two structures are found to be stable minima for both lanthanide metals. However, in the case of Ce (2-Ce), the 2-Ce-cis is found to be slightly more stable than the trans-one (3.7 kcal mol−1), while for Sm (2-Sm), the cis isomer is significantly more stable than the trans (6.9 kcal mol−1). These energy differences could explain the difference observed in the X-ray where Ce is a superposition of the two structures. In contrast, for Sm, only the disorder of one carbon is observed. In all cases, the optimized distances compare well with the experimental ones (see Tables S24 and S35 in ESI†). The fact that the 2-Ln-cis complexes are more stable than the 2-Ln-trans well agrees with the thermodynamical stability of 2-Ln-cis observed in previous studies.7–9,11Molecular and Natural Bonding Orbitals analysis (NBO) calculations were carried out on these complexes. The unpaired spin density appears to be mainly located at the lanthanide centers (Ce: 1.0, Sm: 5.5). However, these values imply the bonding mode in the two metals. Indeed, for Ce, the unpaired spin density is what is expected for a Ce(III) complex with a (Cot)2− ligand and, therefore, a (Cnt)1−. This is reflected in the Frontier Orbitals (see Fig. S88†), where the SOMO is a pure f-orbital, while the doubly occupied HOMO-1 and HOMO-2 describe the Ce–Cot interaction. The Canonical Molecular Orbital (CMO) analysis of NBO 6.0 shows that in these two orbitals, it is a donation from Cot to an empty mainly 5d (90%, 10% 4f) orbital of Ce. For the Sm, the expected unpaired spin density for a Sm(III) is 5, while a value of 5.5 is found, which would indicate the presence of a 2.5 oxidation at Sm. This would imply the formation of a (Cot)1.5−, which was proposed in the case of the cerocene complex.44–46 The latter situation is also proposed since 5 unpaired f electrons are found in the MOs as well as 4 MOs to describe the Sm–Ligand interactions. The CMO analysis clearly indicates that the two Cnt–Sm interactions are pure donation from ligand to metal, while for the Cot–Sm interaction, one is donation from ligand to metal, and the second one is donation from Sm to the Cot ligand (Table S41 and S45†).
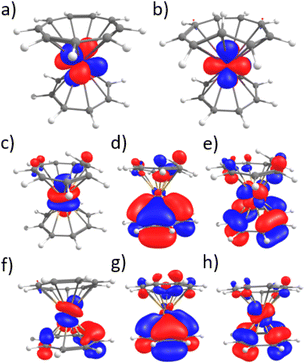
One important question that remained was the reason for the higher stabilization of the trans form with high-energy light. Although several populated orbitals have little ligand contribution, particularly with the carbon, which is out of the plane (see Fig. 9), the density of the ligand is minimal. However, it is notable that in virtual orbitals, the ligand contribution is significantly higher (see Fig. 9), which could play a role in what was observed in this work. Yet, this would imply a complete analysis of the excited-states and relaxation pathway, which is not in the scope of this article. However, TD-DFT was attempted to track the nature of the transitions, particularly those at lower wavelengths.
TD-DFT calculations were carried out on the different complexes of Ce (2-Ce) and Sm (2-Sm) (see ESI†). For 2-Sm, no remarkable difference could be observed. Most of the transitions are multiconfigurational, and the proximity and the similitude of the transitions between 2-Sm-trans and 2-Sm-cis do not allow a clear-cut analysis. This relies well on the difficulty of predicting the PSS and the isomerization patterns from the analysis of the optical spectra and of the molecular orbitals.
In the case of 2-Ce, a peak in the visible region is observed for the trans complex at 442 nm, in good agreement with the signal at 470 nm. The transition corresponds to an excitation from the unpaired f-electron to the LUMO, which is mainly located on the Ce–(Cnt) interaction. However, although the 2-Ce-cis complex shows a signal in lower energy at 613 nm, in our hand, the TD-DFT does not reproduce this data. This would then need a different theoretical framework of the excited states, which is outside the scope of this study.
Conclusions
This work describes a quantitative study of the isomerization of the Cnt ligand and its lanthanide sandwich complexes using light as the main trigger. A careful synthesis of the compounds was carried out in the absence of light to obtain the trans isomer for the Cnt ligand, the Sm(Cnt)2, and (Cot)(Cnt)Ln (Ln = Y, La, Ce, Pr, Nd, Sm, Gd, Tb, Er, Ho) complexes. These compounds were characterized through 1H NMR and X-ray crystallography.A fast isomerization rate was observed for the Cnt ligand potassium salt and for the (Cnt)2Sm complex, allowing trans to cis isomerization. While these systems were proven irreversible, a photostationary state (PSS) was observed for the (Cot)(Cnt)Sm complex, in which both isomers were present. A systematic study of the PSS was conducted at different wavelengths (from 370 nm to 525 or 655 nm) on all (Cot)(Cnt)Ln complexes, which were monitored by 1H NMR. Under high energy irradiation, with Ln = Sm, Tb, Dy, and Lu, the trans isomer was favored, while the cis isomer was favored under low energy irradiation. In the case of Ln = La, Ce, and Y, the trans isomer was strongly favored at any given wavelength.
Our measurements of the absorption spectra and the theoretical analyses show that a comprehensive analysis of the PSS and the photo-isomerization would require dedicated expertise, which was outside the scope of our synthetic and structural studies. However, we are confident that the findings gathered in this article will open a broad scope of future studies by us and the community.
Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
LP, NM, and CC performed all the experimental work, with equal contributions from LP and NM. TM, ID, and LM performed the theoretical work. NC, MT, and NM worked out the crystal structures with the help of LP. LM, GN, and GD analyzed and interpreted all the data together. GD and GN came up with the idea and oversaw all the work. GN wrote the structure of the article with contributions from all authors.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This project has received funding from the European Research Council (ERC) under the European Union's Horizon H2020 research program (grant agreement no. 101044892). CNRS and Ecole Polytechnique are thanked for funding. Ecole Normale Supérieure de Lyon is acknowledged for financial support for CC. LM is a senior member of the Institut Universitaire de France. CalMip is acknowledged for a generous grant of computing time. Dr Olivier Maury is thanked for insightful discussions.Notes and references
- T. J. Katz and P. J. Garratt, J. Am. Chem. Soc., 1963, 85, 2852–2853 CrossRef CAS.
- E. A. Lalancette and R. E. Benson, J. Am. Chem. Soc., 1963, 85, 2853 CrossRef CAS.
- T. Murahashi, R. Inoue, K. Usui and S. Ogoshi, J. Am. Chem. Soc., 2009, 131, 9888–9889 CrossRef CAS.
- S. Schäfer, H. Bauer, J. Becker, Y. Sun and H. Sitzmann, Eur. J. Inorg. Chem., 2013, 2013, 5694–5700 CrossRef.
- M. D. Walter, G. Wolmershäuser and H. Sitzmann, J. Am. Chem. Soc., 2005, 127, 17494–17503 CrossRef CAS PubMed.
- K. Kawasaki, R. Sugiyama, T. Tsuji, T. Iwasa, H. Tsunoyama, Y. Mizuhata, N. Tokitoh and A. Nakajima, Chem. Commun., 2017, 53, 6557–6560 RSC.
- M. Xémard, S. Zimmer, M. Cordier, V. Goudy, L. Ricard, C. Clavaguéra and G. Nocton, J. Am. Chem. Soc., 2018, 140, 14433–14439 CrossRef PubMed.
- L. Münzfeld, C. Schoo, S. Bestgen, E. Moreno-Pineda, R. Köppe, M. Ruben and P. W. Roesky, Nat. Commun., 2019, 10, 3135 CrossRef PubMed.
- M. Tricoire, L. Münzfeld, J. Moutet, N. Mahieu, L. La Droitte, E. Moreno-Pineda, F. Gendron, J. D. Hilgar, J. D. Rinehart, M. Ruben, B. Le Guennic, O. Cador, P. W. Roesky and G. Nocton, Chem.–Eur. J., 2021, 27, 13558–13567 CrossRef CAS PubMed.
- L. Münzfeld, S. Gillhuber, A. Hauser, S. Lebedkin, P. Hädinger, N. D. Knöfel, C. Zovko, M. T. Gamer, F. Weigend, M. M. Kappes and P. W. Roesky, Nature, 2023, 620, 92–96 CrossRef PubMed.
- O. Stetsiuk, L. La Droitte, V. Goudy, B. Le Guennic, O. Cador and G. Nocton, Organometallics, 2022, 41, 133–140 CrossRef CAS.
- C. A. P. Goodwin, F. Ortu, D. Reta, N. F. Chilton and D. P. Mills, Nature, 2017, 548, 439–442 CrossRef CAS PubMed.
- A. H. Vincent, Y. L. Whyatt, N. F. Chilton and J. R. Long, J. Am. Chem. Soc., 2023, 145, 1572–1579 CrossRef CAS PubMed.
- K. R. McClain, C. A. Gould, K. Chakarawet, S. J. Teat, T. J. Groshens, J. R. Long and B. G. Harvey, Chem. Sci., 2018, 9, 8492–8503 RSC.
- J. C. Vanjak, B. O. Wilkins, V. Vieru, N. S. Bhuvanesh, J. H. Reibenspies, C. D. Martin, L. F. Chibotaru and M. Nippe, J. Am. Chem. Soc., 2022, 144, 17743–17747 CrossRef CAS PubMed.
- C. A. Gould, K. R. McClain, D. Reta, J. G. C. Kragskow, D. A. Marchiori, E. Lachman, E.-S. Choi, J. G. Analytis, R. D. Britt, N. F. Chilton, B. G. Harvey and J. R. Long, Science, 2022, 375, 198–202 CrossRef CAS PubMed.
- S. Demir, M. Nippe, M. I. Gonzalez and J. R. Long, Chem. Sci., 2014, 5, 4701–4711 RSC.
- C. A. Gould, E. Mu, V. Vieru, L. E. Darago, K. Chakarawet, M. I. Gonzalez, S. Demir and J. R. Long, J. Am. Chem. Soc., 2020, 142, 21197–21209 CrossRef CAS PubMed.
- S. Demir, M. I. Gonzalez, L. E. Darago, W. J. Evans and J. R. Long, Nat. Commun., 2017, 8, 2144 CrossRef PubMed.
- F. Mares, K. Hodgson and A. Streitwieser, J. Organomet. Chem., 1970, 24, C68–C70 CrossRef CAS.
- K. O. Hodgson, F. Mares, D. F. Starks and A. Streitwieser, J. Am. Chem. Soc., 1973, 95, 8650–8658 CrossRef CAS.
- V. Lorenz, B. M. Schmiege, C. G. Hrib, J. W. Ziller, A. Edelmann, S. Blaurock, W. J. Evans and F. T. Edelmann, J. Am. Chem. Soc., 2011, 133, 1257–1259 CrossRef CAS PubMed.
- J. Moutet, J. Schleinitz, L. La Droitte, M. Tricoire, F. Pointillart, F. Gendron, T. Simler, C. Clavaguéra, B. Le Guennic, O. Cador and G. Nocton, Angew. Chem., Int. Ed., 2021, 60, 6042–6046 CrossRef CAS PubMed.
- T. Vitova, H. Ramanantoanina, B. Schacherl, L. Münzfeld, A. Hauser, R. S. K. Ekanayake, C. Y. Reitz, T. Prüßmann, T. S. Neill, J. Göttlicher, R. Steininger, V. A. Saveleva, M. W. Haverkort and P. W. Roesky, J. Am. Chem. Soc., 2024, 146, 20577–20583 CrossRef CAS PubMed.
- A. Stanger, ChemPhysChem, 2023, 24, e202300080 CrossRef CAS PubMed.
- G. Boche and A. Bieberbach, Chem. Ber., 1978, 111, 2850–2858 CrossRef CAS.
- T. J. Katz and P. J. Garratt, J. Am. Chem. Soc., 1964, 86, 5194–5202 CrossRef CAS.
- G. Boche, D. Martens and W. Danzer, Angew Chem. Int. Ed. Engl., 1969, 8, 984 CrossRef CAS.
- G. Boche, H. Weber, D. Martens and A. Bieberbach, Chem. Ber., 1978, 111, 2480–2496 CrossRef CAS.
- H. M. D. Bandara and S. C. Burdette, Chem. Soc. Rev., 2012, 41, 1809–1825 RSC.
- M.-M. Russew and S. Hecht, Adv. Mater., 2010, 22, 3348–3360 CrossRef CAS PubMed.
- Y.-F. Huang, W.-H. Chiang, P.-L. Tsai, C.-S. Chern and H.-C. Chiu, Chem. Commun., 2011, 47, 10978–10980 RSC.
- F. A. Jerca, V. V. Jerca and R. Hoogenboom, Nat. Rev. Chem, 2022, 6, 51–69 CrossRef PubMed.
- A. Bianchi, E. Delgado-Pinar, E. García-España, C. Giorgi and F. Pina, Coord. Chem. Rev., 2014, 260, 156–215 CrossRef CAS.
- Z. Freixa, Catal. Sci. Technol., 2020, 10, 3122–3139 RSC.
- P. Klan and J. Wirz, Photochemistry of Organic Compounds: From Concepts to Practice, John Wiley & Sons, Ltd, 2009, pp. 73–135 Search PubMed.
- T. Kumakura, N. Akai and M. Nakata, Chem. Phys. Lett., 2019, 714, 160–165 CrossRef CAS.
- U. Jacovella, C. Rossi, C. Romanzin, C. Alcaraz and R. Thissen, ChemPhysChem, 2023, 24, e202200474 CrossRef CAS PubMed.
- F. Battistin, R. Bolliger, M. L. Besmer, T. Fox, O. Blacque, H. Braband and R. Alberto, Inorg. Chem., 2024, 63, 2701–2708 CrossRef CAS PubMed.
- L. Münzfeld, M. Dahlen, A. Hauser, N. Mahieu, S. K. Kuppusamy, J. Moutet, M. Tricoire, R. Köppe, L. La Droitte, O. Cador, B. Le Guennic, G. Nocton, E. Moreno-Pineda, M. Ruben and P. W. Roesky, Angew. Chem., e202218107 Search PubMed.
- G. Boche, H. Weber and A. Bieberbach, Chem. Ber., 1978, 111, 2833–2849 CrossRef CAS.
- I. Bertini, C. Luchinat and G. Parigi, Solution NMR of Paramagnetic Molecules: Applications to Metallobiomolecules and Models, Elsevier, 2001 Search PubMed.
- M. Gao, D. Kwaria, Y. Norikane and Y. Yue, Nat. Sci., 2023, 3, e220020 CrossRef CAS.
- C. S. Neumann and P. Fulde, Z. Phys. B Condens. Matter, 1989, 74, 277–278 CrossRef.
- M. Dolg, P. Fulde, W. Küchle, C. Neumann and H. Stoll, J. Chem. Phys., 1991, 94, 3011–3017 CrossRef CAS.
- M. Dolg, P. Fulde, H. Stoll, H. Preuss, A. Chang and R. M. Pitzer, Chem. Phys., 1995, 195, 71–82 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Full experimental details, 1H NMR and IR spectra, X-ray crystallographic details and DFT calculations. CCDC 2370451–2370468. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4sc04767b |
| ‡ Both authors contributed equally to the work. |
| This journal is © The Royal Society of Chemistry 2024 |