A reflection on ‘Side-chain fullerene polyesters: a new class of high refractive index polymers’
Sheng
Wang
,
Xiaohong
Li
and
Yingfeng
Tu
 *
*
Jiangsu Key Laboratory of Advanced Functional Polymer Design and Application, State and Local Joint Engineering Laboratory for Novel Functional Polymeric Materials, College of Chemistry, Chemical Engineering and Materials Science, Soochow University, Suzhou 215123, China. E-mail: tuyingfeng@suda.edu.cn
First published on 27th November 2024
Abstract
Polymers possessing a high refractive index of over 1.70 are commonly named as high refractive index polymers (HRIPs), and they have garnered enormous attention due to their vital application in organic light-emitting diodes, advanced display devices, antireflective coatings, optical lenses, etc. Our communication in Materials Horizons in 2014 (H. Yan, S. Chen, M. Lu, X. Zhu, Y. Li, D. Wu, Y. Tu and X. Zhu, Mater. Horiz., 2014, 1, 247–250, https://doi.org/10.1039/C3MH00105A) provided a series of side-chain fullerene polyesters with high fullerene contents displaying a quite high refractive index up to 1.79 at 589 nm of the sodium D line. This reflection briefly reviews the impacts and the significance of this work in the field of HRIPs.
1. Introduction
In the past two decades, optical materials with a high refractive index (n) have been extensively exploited due to their potential application in semiconductors, display devices, optical lenses, antireflective coatings, etc.1,2 In comparison with inorganic optical materials like silicon and glasses, polymeric optical materials possess prominent superiority in being lightweight and low cost, and are prized for their easy-processing and excellent fracture resistance. For traditional polymers, they commonly show a low refractive index, usually in the range of 1.20–1.70, while those possessing refractive indices over 1.70 are recognized as high refractive index polymers (HRIPs).3 In 2014, our communication published in Materials Horizons (https://doi.org/10.1039/C3MH00105A), reported for the first time a novel kind of HRIP by introducing fullerene C60 pendants in the side chains of polyesters (Fig. 1).4 Remarkably, these side-chain fullerene polyesters displayed high refractive indices above 1.70, which are much higher than that of corresponding polyesters bearing no fullerene pendants (n < 1.55).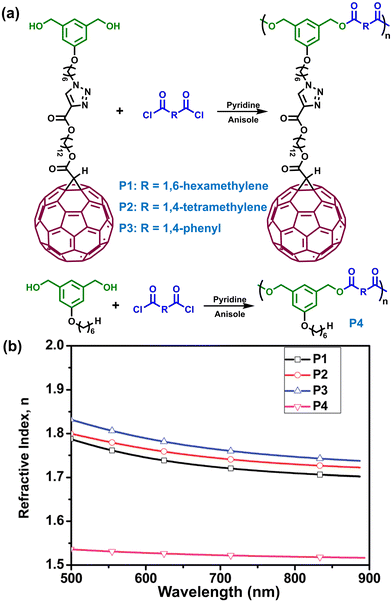 | ||
| Fig. 1 (a) Synthesis of fullerene polymers. (b) Refractive index of polymers P1–P4 changing with wavelength.4 | ||
Because of fullerene’s unique optical and electronic properties, fullerene-based polymers have drawn great attention. However, it remained rather difficult to synthesize fullerene polymers with well-defined structures through the conventional radical, anionic or metal-catalyzed polymerizations, owing to the existence of additional reactions from the free radical and nucleophile to fullerene moieties.5 On the other hand, the big spherical fullerene balls would increase the stiffness of polymer backbones, leading to their poor solubility and limiting their applications. Although the introduction of long alkyl chains can efficiently enhance the solubility, it may sacrifice the photoelectric properties of fullerene polymer materials owing to the low fullerene content.6 Developing a facile method for the synthesis of well-defined fullerene polymers possessing high fullerene contents and excellent solubility in common organic solvents is highly in demand but has rarely been achieved.
In our 2014 study (https://doi.org/10.1039/C3MH00105A), we successfully fabricated a series of high fullerene content (>50 wt%) polyesters via a polycondensation method between fullerene diol and various diacyl chlorides (Fig. 1a). By virtue of the Finkelmann’s decoupling effect of liquid crystalline polymers, long flexible alkyl spacers were introduced between the polymer backbone and fullerene pendants, which were able to minimize the influence of fullerene moieties on the chain stiffness, thereby enhancing their solubility. Interestingly, these fullerene polyesters exhibited very high refractive indices (Fig. 1b), the value of which at 589 nm of the sodium D line (nD) was up to 1.79, exceeding most of the common polymers (n = 1.30–1.70). Using the Lorentz–Lorenz equation, this high refractive index can be well explained and is attributable to the fullerene moieties’ high molar refraction. Moreover, the polymer density also contributed greatly to the refractive index. For P3 with the highest density among these fullerene polyesters, it displayed the highest nD value. Considering fullerene moieties possess unique semiconducting behavior, these fullerene polyesters may have potential applications as optoelectronics materials.
This communication not only reported a unique synthetic strategy for soluble fullerene polymers, but substantiated the feasibility of fullerene derivatives for HRIPs. After publication, it has attracted wide attention from researchers in different fields including in optical materials, organic fullerene materials, solar cells, and crystalline polyesters. In the following sections, we will track the influence of this communication not only on our own work but that of other research groups. Both its impact on the field of HRIPs and on the area of organic fullerene nanomaterials will be introduced. Finally, the future directions for HRIPs are discussed and outlined.
2. High refractive index polymers
Based on the understanding of the molecular design of HRIPs in the communication, our group further replaced aliphatic diacyl chlorides with a variety of aromatic diacyl chlorides to embed high molar refraction aromatic moieties in polyester backbones (Fig. 2a).7 Remarkably, the introduction of aromatic building blocks in the polyester mainchains was able to elevate the fullerene polyesters’ refractive index further (Fig. 2b). All polymers showed a quite high refractive index over 1.83 at 589 nm of the sodium D line, exceeding most existing HRIP examples in the literature. Among these polymers, the fullerene polyester (P5) bearing thiophene units displayed the best optical performance, not only possessing the highest refractive index (nD ∼ 1.86) but also the largest Abbe number (27.9). Its ultrahigh refractive index was ascribed to the synergistic effects among three high molar refraction units, including fullerene, aromatic rings and sulfur element.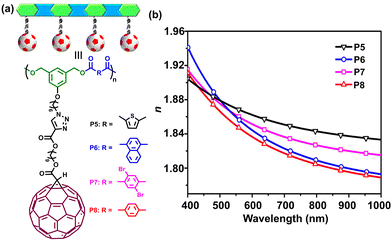 | ||
| Fig. 2 (a) Structure of fullerene polymers. (b) Refractive indices of polymers P5–P8 varying with wavelength.7 | ||
Since fullerenes are scavengers for free radicals and anions, the elegant controlled free radical polymerization developed in recent decades can not be applied for the synthesis of fullerene polymers. Due to the synthetic difficulties, in addition to the high price of fullerenes, researchers seldom utilized fullerene derivatives to construct HRIP materials in the subsequent works. Nevertheless, these two papers about fullerene-based HRIPs not only helped prove the feasibility of the Lorentz–Lorenz equation in guiding the molecular design of HRIPs, but further emphasized that an efficient method to elevate the polymers’ refractive indices was to embed some special groups possessing high molar refraction values, low molar volumes and high polarizability. During the last decade, researchers mainly followed this molecular design strategy and developed various HRIPs. An assortment of high molar refraction substituents, such like aromatic structures, sulfur, selenium, heavy halogen (I, Br, Cl), and other metal atoms, have been incorporated into polymer chains to enhance the refractive index.2,8–10
In our HRIP systems, the high molar refraction of fullerene is attributed to its π-electron density.4,7 In the subsequent decade, a variety of high molar refraction aromatic moieties including aromatic polyimides, carbazolyl, fluorenyl, pyrenyl, anthryl, naphthyl, phenanthrenyl, etc. also have been utilized to fabricate HRIPs by other research groups.11–17 Zhang and coworkers12 synthesized various transparent polyimides via an efficient thiol-Michael click reaction with an excellent tensile strength exceeding 106 MPa and a quite high refractive index over 1.70 at around 633 nm. Shibasaki et al. demonstrated that rigid polyguanamines containing pyrene, anthracene, or naphthalene pendants, were able to show a high refractive index reaching nD ∼ 1.80,13 which was apparently superior to that of traditional organic optical polymeric resins. Recently, Jian et al. demonstrated a powerful strategy to design high-refractive index all-hydrocarbon cyclic olefin copolymers using ethylene and cyclic olefins containing diverse aromatic groups as monomers through coordination–insertion copolymerizations.15 Because of the incorporation of planar conjugated aryl groups like pyrenyl substituent, these all-hydrocarbon polymers displayed a significantly high refractive index above 1.70 and a high optical transparency exceeding 90% in the whole visible region.
With the convenience of sulfur chemistry, diverse sulfur-containing polymers with high refractive indices have been exploited for optical applications.8,18–20 Pyun and coworkers21 developed sulfur-rich polymers with an ultrahigh refractive index of over 2.0 by inverse vulcanization between 1,3-diisopropenylbenzene, sulfur, and selenium. Afterwards, Lim and Im et al. exploited a novel sulfur-based HRIP thin film via a sulfur chemical vapor deposition method,22 which exhibited an excellent optical property with not only an outstanding refractive index exceeding 1.97 but a quite low birefringence Δn ∼ 0.0010. Recently, Fang et al. used biobased magnolol and commercial mercaptans and thiophenols as monomers, and prepared a series of crosslinked polymer networks via a fast photoinduced thiol–ene reaction.23 The resultant polymer films showcased both a splendid Abbe number of up to 35.2 and a very high refractive index reaching 1.72. Additionally, Costa and Oyaizu et al. demonstrated a “polarizable group synergy” concept design for aromatic poly(thiourea)s with an ultrahigh refractive index of 1.81.24 Oyaizu and coworkers reported a kind of visibly transparent dihydroxy-substituted poly(phenylene sulfide),25 which exhibited an ultrahigh refractive index of 1.85 due to the high-density amorphous networks generated by the synergistic effect of the multiple hydrogen bonds between hydroxy groups and the π–π interactions between rigid thiophenylene backbones. These results substantiate that introducing the sulfur element is an efficient strategy for HRIPs.
Compared with sulfur, selenium shows a higher molar refraction. Zhu and Pan et al. developed a variety of selenium-containing polymers including selenide-containing polystyrene, polyimides, and poly(methyl acrylate), whose refractive index is very high and in the range of 1.71–1.87.26–28 Tang and coworkers developed a novel metal-free multicomponent polymerization with dipropargyl alcohols, elemental selenium and diisocyanides as monomers.29 The resulting selenide-containing polymers displayed a notably high refractive index reaching 1.80 at about 633 nm, which even held at 1.78 at 1700 nm. Afterwards, this group further developed a novel kind of polyselenophenes or poly(1,4-diselenin)s which presented a higher refractive index reaching 1.85 at around 633 nm.30
Additionally, heavy halogen or metal atoms also have been incorporated into polymer chains for HRIPs. Kudo et al. fabricated iodine-containing polyacrylates by a conventional radical copolymerization of 2,4,6-triiodophenyl acrylate with 2-(2-ethoxyethoxy)ethyl/2-hydroxyethyl acrylate,31 showing a refractive index of up to 1.85. Ochiai and coworkers prepared types of transparent bismuth-containing polymer films through radical copolymerization of tristyrylbismuthine and diphenylstyrylbismuthine, which displayed quite high refractive indices exceeding 1.70 at 589 nm.32
The above findings have successfully built up the structure–property relationship of HRIP materials. Some HRIP systems have showcased significant applications in optical materials. In 2017, Pyun and coworkers developed chalcogenide hybrid inorganic/organic polymers with an ultrahigh refractive index greater than 2.0.21 After melt processing into windows, these optical devices were capable of highly resolved mid-infrared thermal imaging of human subjects. Recently, Oyaizu et al. integrated ultrahigh refractive index poly(thiourea)s into benchmark graphene-based lighting devices and obtained the remarkably enhanced external quantum efficiency owing to the raised light out-coupling of poly(thiourea) films.24 Additionally, Ochiai et al. demonstrated that bismuth-containing HRIPs also displayed excellent X-ray shielding performance.32 Nevertheless, when used as real high-performance optical devices, many significant parameters should be satisfied. Exploiting a polymer featuring both high refractive index and high Abbe number, and also low birefringence, as well as high visible transparency is greatly desirable but still remains challenging.
3. Supramolecular fullerene liquid crystals
Due to the difficulties of synthesizing fullerene polymers by traditional polymerization techniques, we believe that fullerene supramolecules can be developed much more easily by supramolecular polymerization via the interactions between fullerenes. Based on a similar synthetic approach to that reported in our communication, a series of organic fullerene derivatives possessing a unique soft-rod–soft-ball tetrablock structure were synthesized (Fig. 3).33–38 Driven by the π–π interactions between fullerene moieties, these fullerene dyads were able to undergo interesting self-organization to generate regular lamella assembly structures with triple-layer 2D fullerene crystals that were sandwiched between alkyl chain layers, subsequently further stacked into supramolecular liquid crystals (SLCs). The lamella thickness and phase diagrams of 2D crystals and SLCs could be modulated by altering the flexible alkyl spacer and tail length (Fig. 3b).35,36 Moreover, the mismatch of cross-sectional area between the fullerene spheres and the oversize hydrocarbon cones could lead to an ordered molecular flipping in the 2D crystal layers, with the formation of a fascinating lamellar superlattice (Fig. 3c), which was capable of remarkably improving the electron conductivity of the 2D nanosheets.39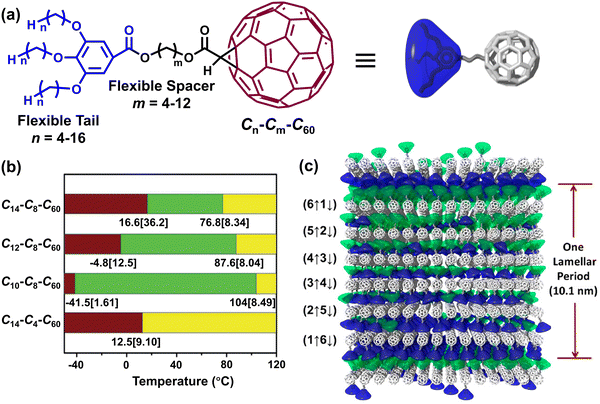 | ||
| Fig. 3 (a) Chemical structures of reported fullerene derivatives with a tetrablock structure. (b) Phase transition behaviors of fullerene derivatives. Reproduced with permission from ref. 33. Copyright (2015) John Wiley and Sons. (c) Representation of molecular packing of C7–C8–C60 in a superlattice. Reprinted with permission from ref. 39. Copyright (2020) American Chemical Society. | ||
Due to the SLCs’ strong self-assembly capacity and fullerene’s intrinsic high electron mobility rate, these fullerene dyads are able to provide a rapid channel for transporting charge-carriers and lower the trap densities and energetic disorder in bulk heterojunction (BHJ) films by increasing crystallinity. He and coworkers introduced our fullerene dyads into the BHJ’s active layers and showcased that the charge-carrier diffusion length could be efficiently increased, thereby enabling the apparent improvement of the power conversion efficiency (up to 15.23%) of organic solar cells.40
In addition, since PCBM, a famous fullerene derivative which is widely used as the electron transport layer material in inverted perovskite solar cells (PSCs), fullerene dyads should also have promising applications in this area. By linking a terpyridine chelating unit with C60, the fullerene dyad (FP-C8) showed much better power conversion efficiency, moisture and thermal stability than PCBM-based devices due to its enhanced molecule ordering and adhesion with the perovskite surface.41
4. Conclusion and future perspective
This short reflection article described the first example of high refractive index fullerene polyesters our group published in Materials Horizons in 2014, and introduced the subsequent research progress on HRIPs and fullerene-based nanomaterials in the last decade from our group and other research groups. Based on the Lorentz–Lorenz equation, an assortment of HRIPs have been successfully developed by incorporating high-density and highly polarizable atomic groups. Some polymers were even able to exhibit ultrahigh refractive indices exceeding 2.0. However, apart from the refractive index, other important parameters like Abbe number, birefringence, visible transparency, etc. are also required to be taken into account for designing a highly functional polymer in the near future. To realize its application in a real optical device, the polymeric material shall not only possess a high refractive index, but also low birefringence and a high Abbe number, as well as high transparency in the whole visible light wavelength range. On the other hand, machine learning or DFT calculations also can be applied to help optimize the structural parameters, thereby reducing the abovementioned challenge and promoting progress in exploiting diverse high-performance polymeric optical materials.Owing to the fullerenes’ intrinsic high refractive index, organic fullerene nanomaterials should also be high refractive index materials. However, due to the solubility and film-forming problems, it is difficult to get these materials. We are continuing trying our best to develop novel fullerene materials, gain deep insight into their structure–property relationships, and expand their application in organic solar cells and optoelectronics devices.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was funded by the National Natural Science Foundation of China (Grants 22071167 and 22231008). The financial support from the Natural Science Foundation of Jiangsu Higher Education Institutions of China (22KJA150005) and the Priority Academic Program Development of Jiangsu Higher Education Institutions is also acknowledged.References
- T. Higashihara and M. Ueda, Macromolecules, 2015, 48, 1915–1929 CrossRef CAS.
- S. Watanabe and K. Oyaizu, Bull. Chem. Soc. Jpn., 2023, 96, 1108–1128 CrossRef CAS.
- E. K. Macdonald and M. P. Shaver, Polym. Int., 2015, 64, 6–14 CrossRef CAS.
- H. Yan, S. Chen, M. Lu, X. Zhu, Y. Li, D. Wu, Y. Tu and X. Zhu, Mater. Horiz., 2014, 1, 247–250 RSC.
- Fullerene Polymers: Synthesis, Properties and Applications, ed. N. Martín and F. Giacalone, Wiley-VCH, Weinheim, 2009 Search PubMed.
- U. Hahn, E. Maisonhaute, C. Amatore and J. F. Nierengarten, Angew. Chem., Int. Ed., 2007, 46, 951–954 CrossRef CAS.
- S. Chen, D. Chen, M. Lu, X. Zhang, H. Li, X. Zhang, X. Yang, X. Li, Y. Tu and C. Y. Li, Macromolecules, 2015, 48, 8480–8488 CrossRef CAS.
- T. S. Kleine, R. S. Glass, D. L. Lichtenberger, M. E. Mackay, K. Char, R. A. Norwood and J. Pyun, ACS Macro Lett., 2020, 9, 245–259 CrossRef CAS PubMed.
- K. Mazumder, B. Voit and S. Banerjee, ACS Omega, 2024, 9, 6253–6279 CrossRef CAS.
- Y. Su, E. B. D. S. Filho, N. Peek, B. Chen and A. E. Stiegman, Macromolecules, 2019, 52, 9012–9022 CrossRef CAS.
- S. Xue, X. Lei, Y. Xiao, Y. Liu, Y. Zhang and Q. Zhang, ACS Appl. Polym. Mater., 2024, 6, 2315–2326 CrossRef CAS.
- S. Xue, X. Lei, Y. Xiao, G. Xiong, R. Lian, X. Xin, Y. Peng and Q. Zhang, Macromolecules, 2021, 54, 11256–11268 CrossRef CAS.
- T. Kotaki, N. Nishimura, M. Ozawa, A. Fujimori, H. Muraoka, S. Ogawa, T. Korenaga, E. Suzuki, Y. Oishi and Y. Shibasaki, Polym. Chem., 2016, 7, 1297–1308 RSC.
- M. Briesenick, M. Gallei and G. Kickelbick, Macromolecules, 2022, 55, 4675–4691 CrossRef CAS.
- Y. Zhao, L. Cui, Y. Zhang and Z. Jian, Macromolecules, 2024, 57, 8869–8876 CAS.
- H. Yuan, T. Kida, Y. Ishitobi, R. Tanaka, M. Yamaguchi, Y. Nakayama and T. Shiono, Macromolecules, 2021, 55, 125–132 CrossRef.
- T. Badur, C. Dams and N. Hampp, Macromolecules, 2018, 51, 4220–4228 CrossRef CAS.
- S. Silvano, C. F. Carrozza, A. R. de Angelis, I. Tritto, L. Boggioni and S. Losio, Macromolecules, 2020, 53, 8837–8846 CrossRef CAS.
- H. Zhou, F. Zhang, R. Wang, W.-M. Lai, S. Xie, W.-M. Ren and X.-B. Lu, Macromolecules, 2021, 54, 10395–10404 CrossRef CAS.
- K. Choi, W. Jang, W. Lee, J. S. Choi, M. Kang, J. Kim, K. Char, J. Lim and S. G. Im, Macromolecules, 2022, 55, 7222–7231 CrossRef CAS.
- L. E. Anderson, T. S. Kleine, Y. Zhang, D. D. Phan, S. Namnabat, E. A. LaVilla, K. M. Konopka, L. Ruiz Diaz, M. S. Manchester, J. Schwiegerling, R. S. Glass, M. E. Mackay, K. Char, R. A. Norwood and J. Pyun, ACS Macro Lett., 2017, 6, 500–504 CrossRef CAS.
- W. Jang, K. Choi, J. S. Choi, D. H. Kim, K. Char, J. Lim and S. G. Im, ACS Appl. Mater. Interfaces, 2021, 13, 61629–61637 CrossRef CAS.
- Z. Dou, J. Sun and Q. Fang, Biomacromolecules, 2024, 25, 6155–6163 CrossRef CAS PubMed.
- S. Watanabe, L. M. Cavinato, V. Calvi, R. van Rijn, R. D. Costa and K. Oyaizu, Adv. Funct. Mater., 2024, 34, 2404433 CrossRef CAS.
- S. Watanabe, H. Nishio, T. Takayama and K. Oyaizu, ACS Appl. Polym. Mater., 2023, 5, 2307–2311 CrossRef CAS.
- Q. Li, K. L. Ng, X. Pan and J. Zhu, Polym. Chem., 2019, 10, 4279–4286 RSC.
- Q. Li, S. Liu, M. Xu, X. Pan, N. Li, J. Zhu and X. Zhu, Eur. Polym. J., 2020, 122, 109358 CrossRef CAS.
- H. Jiang, X. Pan, N. Li, Z. Zhang, J. Zhu and X. Zhu, React. Funct. Polym., 2017, 111, 1–6 CrossRef CAS.
- X. Wu, J. He, R. Hu and B. Z. Tang, J. Am. Chem. Soc., 2021, 143, 15723–15731 CrossRef CAS PubMed.
- J. Peng, N. Zheng, P. Shen, Z. Zhao, R. Hu and B. Z. Tang, Chem, 2022, 8, 2301–2316 CAS.
- H. Maekawa, H. Amano, I. Nishina and H. Kudo, ChemistrySelect, 2022, 7, e202201543 CrossRef CAS.
- Y. Matsumura, H. Horikoshi, K. Furukawa, M. Miyamoto, Y. Nishimura and B. Ochiai, ACS Macro Lett., 2022, 11, 723–726 CrossRef CAS PubMed.
- X. Zhang, C. H. Hsu, X. Ren, Y. Gu, B. Song, H. J. Sun, S. Yang, E. Chen, Y. Tu, X. Li, X. Yang, Y. Li and X. Zhu, Angew. Chem., Int. Ed., 2015, 54, 114–117 CrossRef CAS PubMed.
- Y. Hu, K. Y. Wu, T. Zhu, P. Shen, Y. Zhou, X. Li, C. L. Wang, Y. Tu and C. Y. Li, Angew. Chem., Int. Ed., 2018, 57, 13454–13458 CrossRef CAS PubMed.
- T. Zhu, X. Zhang, Z. Li, C. H. Hsu, W. Chen, T. Miyoshi, X. Li, X. Yang, Y. Tu and C. Y. Li, Chem. Commun., 2017, 53, 8336–8339 RSC.
- P. Shen, X. Zhang, H. Lu, Z. Su, Y. Zhou, B. Song, X. Li, X. Yang, Y. Tu and C. Y. Li, Chem. – Asian J., 2019, 14, 125–129 CrossRef CAS PubMed.
- H. Lu, H. Zou, X. Chen, W. Zhang, B. Wang, Z. Cao and Y. Tu, J. Mater. Chem. C, 2023, 11, 2647–2652 RSC.
- H. Lu, X. Chen, X. Li, W. Sun, Y. Wang and Y. Tu, Chem. – Eur. J., 2023, 29, e202301015 CrossRef CAS.
- H. Lu, X. Zhang, T. Sakurai, X. Li, Y. Tu, J. Guo, S. Seki, C. Y. Li, G. Ungar and S. Z. D. Cheng, Nano Lett., 2020, 20, 8647–8653 CrossRef CAS.
- F. Zhao, D. He, C. Zou, Y. Li, K. Wang, J. Zhang, S. Yang, Y. Tu, C. Wang and Y. Lin, Adv. Mater., 2023, 35, e2210463 CrossRef.
- Y. Jiang, J. Wang, H. Zai, D. Ni, J. Wang, P. Xue, N. Li, B. Jia, H. Lu, Y. Zhang, F. Wang, Z. Guo, Z. Bi, H. Xie, Q. Wang, W. Ma, Y. Tu, H. Zhou and X. Zhan, J. Am. Chem. Soc., 2022, 142, 5400–5410 CrossRef.
| This journal is © The Royal Society of Chemistry 2025 |
