A review on the active sites for titanium species in zeolites: coordination structure, synthetic strategies and activity
Yihao
Wang†
a,
Kaiwei
Wang†
a,
Fumin
Wang
 a,
Yi
Zhai
b,
Changhao
Bing
a,
Xiaolu
Fan
a,
Qi
Shen
a and
Xubin
Zhang
a,
Yi
Zhai
b,
Changhao
Bing
a,
Xiaolu
Fan
a,
Qi
Shen
a and
Xubin
Zhang
 *a
*a
aSchool of Chemical Engineering and Technology, Tianjin University, Tianjin 300350, P. R. China. E-mail: tjzxb@tju.edu.cn
bCollege of Chemical and Biological Engineering, Zhejiang University, Hangzhou 310027, P. R. China
First published on 14th November 2024
Abstract
Titanium species in titanosilicate zeolites exist in three forms: framework titanium species, framework-associated titanium species and anatase TiO2. They dominate the catalytic properties. Generally, the framework titanium species are considered as the active centers for catalytic reactions. However, the latest research has unveiled that additional titanium species within the framework, such as penta-coordinated and hexa-coordinate titanium species, can also exert their influence on catalytic processes. The catalytic activities of various titanium species, including penta- and hexa-coordinated titanium, exhibit superiority over traditional tetra-coordinated framework titanium species in some reactions. The urgent necessity lies in establishing a comprehensive understanding of the formation principles of various titanium species, characterization, and investigating their catalytic properties across diverse reactions. This review provides a comprehensive overview of contemporary advances in titanosilicate zeolites. The regulatory strategies, detection methods, and catalytic properties of titanium species are comprehensively summarized. Furthermore, a universal analysis is conducted on the mechanism of titanium species in the hydrogen peroxide catalytic system, offering valuable insights into both catalytic mechanism and precise regulation of microenvironmental conditions and spatial distribution of titanium species.
1. Introduction
The abundant acid sites and ordered pore structure of zeolites enable them to be extensively employed in the domains of catalysis, adsorption, and other related fields. Heteroatomic zeolites constructed by corner-sharing of TO4 tetrahedrons (T = Ti, Al, Sn etc.) introduce more active centers to show higher catalytic performance. The discovery of titanium silicalite-1 (TS-1) with the MFI topology in 1983 marked a significant milestone, garnering substantial research interest due to the exceptional catalytic capability exhibited by tetrahedrally coordinated Ti4+ ions towards peroxides.1 The Ti-MFI zeolite with high activity is currently the most extensively studied zeolite. TS-1 catalysts have gained significant importance in various selective oxidation reactions utilizing aqueous H2O2 as an oxidant, including the hydroxylation of phenol,2 ammoximation of cyclohexanone to cyclohexanone oxime,3–8 epoxidation of olefins,9–20 oxidation of pyridine derivatives and oxidative desulphurisation reactions.21–25 However, the catalytic activity of zeolites is directly influenced by factors such as the coordination structure, microenvironment, and stability of titanium species.26–29 T. Thomson et al. calculated by density functional theory that the new hexa-coordination titanium species showed higher catalytic performance for propylene epoxidation.30 The report indicates a pathway for enhancing catalytic performance through the adjustment of titanium active centers. Typically, the enhancement of catalytic behaviors can be achieved by both increasing the Lewis acid strength of titanium species and adjusting the coordination states of Ti.The catalytic activity in zeolites is attributed to the presence of Brønsted and Lewis acids, which have been extensively recognized as active centers. The construction of zeolite active centers can be classified into two categories: a bottom-up approach (where the metal source exists in the initial synthesis gels) or a top-down approach (where metal atoms are inserted into the already-formed framework). The different methods yield diverse targeted zeolites exhibiting significant variations in physicochemical properties, potentially leading to disparate catalytic active sites (open/closed, framework/framework-associated, and hydrated/dehydrated species).
The introduction of additional active centers in industry often involves impregnation loading or ion exchange methods.31,32 However, the incorporation of titanium species into the zeolite framework remains challenging using these approaches due to the lack of understanding of the coordination structure of titanium species within zeolites. Moreover, it is difficult to introduce titanium species into the zeolite skeleton by impregnation, and the maximum theoretical titanium content in the TS-1 framework is only 2.5 wt%. Furthermore, there still exist significant research gaps regarding the coordination structure of titanium species in zeolites. In recent decades, the advent of more sophisticated characterization techniques and computational methods has enabled researchers to unravel the mysteries of the coordination structures of different active species and catalytic mechanisms involved in reactions. The research on the methods of zeolite modification to obtain higher catalytic activity is now entering a completely new stage, and there is an urgent need to systematically summarise the current progress of research on the active centres with different coordination structures.
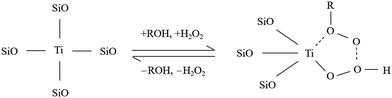
Titanium species have been extensively studied as active centers of the TS-1 zeolite.33–35 Borgida et al. have conducted an in-depth study of the working mechanism of framework titanium species in catalytic reactions. They discovered that in liquid-phase oxidation reactions utilizing aqueous H2O2 as the oxidizing agent, the tetra-coordinated framework of titanium species exhibited the ability to generate peroxidatively active intermediates (Ti–OOH) with hydrogen peroxide. Subsequently, these titanium peroxides effectively facilitated the oxidation of diverse organic reactants, which endowed the catalysts with enhanced oxidative properties.2,35–37Fig. 1 illustrates the mechanism of interaction between two distinct framework Ti species and H2O2 within TS-1.34 Moreover, Lamberti accurately characterised the stability of the perovskite complexes by resonance Raman spectra, demonstrating that in the absence of synergistic interaction between water molecules, H2O2 molecules form complexes with Ti(IV) with very low affinity. This finding highlights the pivotal role of water in the formation of titanium peroxide.37
 | ||
| Fig. 1 Equilibrium between framework Ti species and corresponding peroxide species in aqueous H2O2 solutions. Reproduced with permission.34 Copyright 2007, Royal Society of Chemistry. | ||
The advancements in precision instruments and characterization methods have led to the discovery of a significant number of new titanium species in recent years, such as Ti-OH defects, octahedrally coordinated Ti species (TiO6), and binuclear titanium species (Ti–O–Ti), which have demonstrated exceptional catalytic properties in oxidation reactions.38–42 Yu et al. detected mononuclear octahedrally coordinated Ti species using the 266 and 325 nm-excited UV-Raman spectra and DFT calculations, which showed good catalytic activity in the epoxidation of cyclohexene.43 Li et al. detected Ti(OH)4(OSi)2 by the 244 nm-excited UV-Raman spectra, demonstrating excellent catalytic performance in propylene epoxidation.44 Therefore, summarising the catalytic activities of different titanium species in various types of reactions is of great research value for the in-depth investigation of the reaction mechanism and the enhancement of the catalytic activity of zeolites or the targeted design of zeolites with high selectivity and catalytic activity through the adjustment of titanium species distribution and microenvironment.
In this review, we provide a comprehensive overview of the recent progress in the TS-1 zeolite, encompassing an overview of detection methods and synthesis strategies for various titanium coordination species, as well as a detailed comparison of the catalytic performance and reaction mechanism of TS-1 under different reactions. Notably, an extensive array of tables is employed to elucidate the coordination structure of Ti active species. In Sections 2 to 4, the structure and catalytic properties of tetra- coordination, hexa-coordination titanium species and anatase are introduced respectively, and the synthesis strategies and formation mechanisms of various titanium species are compared. Section 5 presents a study of additional novel titanium species based on the current works. Section 6 summarizes the strategies of spatial distribution of titanium species. In Section 7, the reaction mechanisms of 1-hexene epoxidation, cyclohexanone aminoximation and propylene epoxidation are summarized. Finally, we provide our outlook on the research prospect of Ti-zeolite catalysts.
2. Tetrahedrally coordinated framework Ti species
2.1. Catalytic performance
Tetra-coordinated titanium species (TiO4) represent a class of titanium species in which Ti atoms replace Si atoms at the TO4 site and are incorporated into the zeolite framework in the form of tetrahedral coordination. This framework titanium species was identified as being associated with catalytic activity as early as in the original patent by Taramasso et al.1 Subsequently, a similar conclusion was reached by Mantegazza et al., who observed that the activity of TS-1 in ammonia oxidation, cyclohexanone aminoximation and propylene epoxidation was proportional to the molar fraction of Ti in the framework at low Ti concentrations. It is widely acknowledged that TiO4 can be categorised into two distinct groups: perfect “closed” sites (Ti(OSi)4) and defective “open” sites (Ti(OSi)3OH). The Ti(OSi)4 species are found within the zeolite framework, while the Ti(OSi)3OH species are primarily located within the zeolite framework, with a small part on the outer surface. Both Ti(OSi)4 and Ti(OSi)3OH species have the capacity to activate H2O2, thereby enhancing the catalytic performance. The structures are depicted in Fig. 2(a and b).34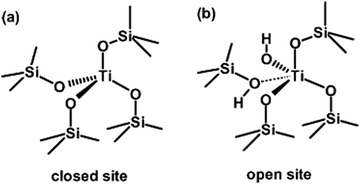 | ||
| Fig. 2 Schematic representation of (a) perfect “closed” titanium sites and (b) defective “open” titanium sites. Reproduced with permission.34 Copyright 2007, Royal Society of Chemistry. | ||
He et al. designed four Ti active sites in a Ti-MWW type catalyst through post treatment and investigated their impacts on oxidation pathways. They proposed that the activity follows the order that, Ti(OSi)4(OTiO5)2 ≪ Ti(OSi)4 < Ti(OSi)3OH < Ti(OSi)3OH(HO–Si)n. The activation of H2O2 is hindered by the presence of “OTiO5” (TiO6) species surrounding the tetrahedral Ti framework in titanosilicate, thereby rendering Ti(OSi)4(OTiO5)2 inactive during oxidation reactions.45 After acid treatment, the foreign framework TiO6 in the catalyst gradually decreases and the active sites of Ti(OSi)4 increase. The Ti(OSi)4 active site gradually transformed into Ti(OSi)3OH with enhanced catalytic activity as the acid treatment time increased.45 Liu et al.41 successfully synthesized a highly active catalyst enriched with controllable defects (Ti(OSi)3OH species) for the first time through hydrothermal treatment of TS-1 with ethylamine. The synthetic procedure is illustrated in Fig. 3. The Ti(OSi)3OH species, generated by the selective dissolution of Si species near the Ti(OSi)4 active site, exhibits higher catalytic activity compared to the Ti(OSi)4 active site due to its stronger Lewis acidity, which contributes to the formation of Ti–OOH intermediates. The potential impact of Si–OH groups in the vicinity of the Ti active site has garnered increasing attention from researchers.41,46–49 Wells et al. disclosed that various Ti active-site microenvironments in titanosilicates performed different activity on propylene epoxidation in the order of Ti–OH(HO–Si)3 > Ti–OH > Ti–OH(HO–Si).50,51 The reaction is due to the fact that the Ti(OSi)3OH has a much smaller spatial site-barrier effect, resulting in a lower reactive energy barrier for the reaction of Ti with H2O2. Other researchers have proposed that the enhanced Lewis acidity of the Ti(OSi)3OH relative to the Ti(OSi)4 would be more conducive to the formation of reactive intermediates through binding to H2O2.52–55 Thomson et al. employed DFT to demonstrate that defective “open” sites are more active than perfect “closed” sites.48 For the Ti sites within the framework, they concluded that Ti(OSi)3OH has a lower energy barrier than Ti(OSi)4 in the propylene epoxidation reaction, based on Gibbs free energy analysis at 298 K.48,51,56–58 This is attributed to the less restrictive environment of the transition state due to neighboring vacancies, and the favorable interaction between the silanol group associated with the Si vacancy and the olefin molecule for trapping oxygen from the Ti–OOH intermediate.
 | ||
| Fig. 3 Construction strategy for defective Ti(OSi)3OH reactive species in TS-1.41 Copyright 2018, Royal Society of Chemistry. | ||
The precise manipulation of the microenvironment surrounding the active site of titanium in titanosilicate has garnered significant attention owing to its exceptional catalytic properties. Generally, the incorporation of fluorine as a potent electron-accepting agent aids in enhancing the electrophilicity of Ti sites and inducing Oα–Oβ polarization in Ti–OOH species (Ti–Oα–Oβ–Hend) through induction effects. Similarly, other heteroatoms such as Zn, La, and Cd have been observed to exert analogous influences on modified catalysts leading to their remarkable catalytic performance.45,59 Fang et al. successfully introduced fluorine into the framework by post-treatment of Ti-MWW with NH4F. The condensation of fluorine with silanol to produce SiO3/2F− structural units increased the positive electronegativity of the framework titanium in the neighbouring position, which improved the Lewis acid strength. However, fluoride ions in titanosilicate zeolites synthesised in fluorine-containing media mainly existed in the form of SiO4/2F− structural units. The extreme electronegativity of these units caused a substantial decrease in the electron density near the Si at adjacent positions, which led to a decrease in the catalytic activity of the zeolites. In order to enhance the catalytic efficacy of Ti-MWW, it is essential to selectively mitigate the detrimental effects of SiO4/2F− structural units while striving to minimise the impairment of SiO3/2F− structural units. They selectively removed the SiO4/2F− structural unit by mild treatment with KCl, which further improved the catalytic activity of fluorine-treated Ti-MWW in a 1-hexene epoxidation reaction.60 In order to investigate clearly the impact factors on the microenvironment of titanium species and the catalytic activity of zeolites during this process of NH4F post-treatment of Ti-MWW, Wu et al.61 continued their research into the post-treatment of Ti-MWW with fluoride. They successfully replaced Si–OH with Si–F by acid treatment of Ti-MWW in the presence of NH4F, generating three fluorine species in Ti-MWW: SiF62−, SiO4/2F− and SiO3/2F. The fluorine species, the titanium species and the zeolite structure were all affected by the acid treatment temperature and the amount of ammonium fluoride added. A series of characterisation studies revealed that the acid treatment was unable to fully remove the framework-associated titanium species when the temperature was low, and that the zeolite framework structure was damaged when the temperature was high. The optimal temperature for the incorporation of F into the framework of Ti-MWW was found to be 377 K. The doping of F was also related to the Ti content, with the higher the Ti content, the more difficult it was to dope F. The doping of F also improved the hydrophobicity of Ti-MWW and served to stabilise the structure of the framework and the structure of the active titanium species. The presence of hydrogen bonding between SiO4/2F− species impeded the electrophilicity of active titanium sites, thereby hindering epoxidation. This phenomenon can be counteracted by employing a treatment with KCl, as demonstrated in the preceding study. The above study started from the introduction of other elements into the zeolite framework to change the microenvironment of the TiO4 to improve the catalytic activity of titanosilicate zeolites, which provided a new idea for the subsequent studies on the modulation of the microenvironment of titanium species.
Both theoretical and experimental findings suggest that defective Ti(OSi)3 has been shown to be more active than Ti(OSi)4 due to the former's stronger Lewis acidity and the influence of adjacent Si–OH groups.33,41,48,62,63 Bregantė et al. proposed that the catalytic performance of olefin epoxidation improves as the density of (Si–OH)4 near the Ti active site increases. This is attributed to the interactions46 between the transition state, H2O clusters and the catalyst surface.64 Typically, TiO4 exhibits excellent catalytic activity in reactions such as phenol hydroxylation,2 olefin epoxidation,9–11,20 cyclohexanone aminoxidation3 and oxidative desulfurisation.21 It is worth noting that in the olefin epoxidation reaction, P. Ratnasamy et al.65 found that Ti(OSi)4 had higher selectivity for epoxidation products compared to Ti(OSi)3OH, but this experimental result did not contradict the previous conclusion. The stronger acidity of the Ti(OSi)3OH is more likely to catalyse the ring-opening reaction of the epoxidation product, resulting in a decrease of selectivity. Therefore, when targeting the microenvironment of TiO4, the relative proportions of defective and perfect titanium species should be rationally distributed to achieve optimal catalytic performance. Table 1 summarizes the catalytic performance of TS-1, mainly TiO4, in common reactions. These findings provide a deeper understanding of the influence of the microenvironment on the catalytic activity of framework titanium species, as well as examples of reference for targeted adjustment of the generation of defective framework titanium species to enhance the catalytic activity of catalysts.
| Sample | Probe reaction | Si/Ti | Quantity of catalyst (mg) | Reaction time (h) | Temperature (K) | Conversion (%) | Selectivity (%) | Ref. |
|---|---|---|---|---|---|---|---|---|
| TS-1 | 1-Hexene epoxidation | 75 | 50 | 2 | 333 | 44.9 | 94.0 | 41 |
| TS-1 | Phenol hydroxylation | 40 | 20 | 6 | 333 | 27.4 | 94.7 | 64 |
| TS-1 | Cyclohexanone amoximisation | 35 | 25 | 1 | 353 | 99.1 | 99.8 | 66 |
| TS-1 | Oxidative desulphurisation | 16 | 10 | 0.5 | 333 | 76.4 | — | 21 |
| Ti-MWW | Oxidative desulphurisation | 42 | 40 | 3 | 323 | 78.0 | — | 67 |
| Ti-MCM-41 | Styrene oxidation | 50 | 41.6 | 12 | 313 | 18.0 | 24.0 | 19 |
| Ti-Beta | Styrene oxidation | 50 | 41.6 | 12 | 313 | 56.0 | 62.0 | 19 |
| TS-1 | Styrene oxidation | 30 | 41.6 | 12 | 313 | 71.0 | 87.0 | 65 |
| TS-1-PPA | n-Hexene epoxidation | — | 100 | 4 | 333 | 41.0 | 65.85 | 6 |
| Ti-MWW(P)-AT-24 | 1-Hexene epoxidation | — | 50 | 2 | 333 | 51.2 | 100.0 | 63 |
2.2. Characterization
The catalytic activity of catalysts is contingent upon the existence form of titanium species and the complex and variable microenvironment. Consequently, the detection and study of the coordination state of titanium species are fundamental steps in the analysis of the mechanism of catalytic reactions.The application of ultraviolet-visible (UV-Vis) spectra represents a reliable technical method for the detection of coordination states of titanium ions. For TiO4, researchers agree that TS-1 and TS-2 correspond to the band at 210 nm in the UV-Vis region, and Ti-MCM-41 corresponds to the band at 220 nm.65,68 The Ti-beta synthesized in fluorine medium corresponds to the band at 205–220 nm,69 and the spectral differences of these same coordination Ti in different environments may be related to different Ti–O–Si bond angles.70 The formation of these bands is attributed to the 2p electron of oxygen transitioning to the 3d orbital of the tetravalent titanium ion.71–73 For instance, Ratnasamy et al. synthesised several common classes of titanosilicate zeolites using different synthetic methods or treatments. The UV-Vis spectral data of their five classes of samples are listed in Table 2. Samples 1 and 2 were prepared through conventional hydrothermal synthesis, while sample 3 was synthesised by a fluorine mediated method.33
| Titanosilicate | Deconvoluted bands and assignments: λmax, nm (relative intensity, %) | ||||
|---|---|---|---|---|---|
| Band 1 (Ti(OSi)4) | Band 2 (Ti(OH)(OSi)3) | Band 3 (Ti(OH)(H2O)(OSi)3) | Band 4 (Ti(OH)2 (H2O)2(OSi)2) | Band 5 (anatase-like) | |
| TS-1 (sample 1) | 206 (85) | 228 (8) | 258 (6) | 293 (1) | Nil |
| TS-1 (sample 2) | 203 (72) | 228 (10) | 255 (8) | 288 (5) | 328 (5) |
| TS-1 (sample 3) | 206 (78) | 229 (11) | 260 (7) | 293 (4) | Nil |
| TS-2 | 201 (58) | 229 (13) | 255 (24) | 288 (5) | Nil |
| Ti-MCM-41 | 207 (27) | 227 (49) | 263 (8) | 290 (16) | Nil |
Fourier transform infrared (FT-IR) spectra can also be employed for the detection of titanium species. The band at 960 cm−1 corresponds to the telescopic vibration of the Ti–O–Si bond or the perturbation of the Si–O bond by the framework Ti atoms. The band at 800 cm−1 corresponds to the MFI topology.35,74–76 Consequently, for MFI titanosilicate zeolites, the relative values of the 960 and 800 cm−1 band intensities (I960/I800) can be employed to quantitatively analyse the framework titanium content.77–79 The higher the framework titanium content, the larger the value of I960/I800. However, it has been postulated that this band may be attributed to surface hydroxyl groups (e.g. Si–OH)73,80 or defect sites.81 Consequently, the precise identification of framework titanium atoms remains a pressing issue. UV-Raman resonance spectroscopy82,83 is an technique capable of more sensitive identification of transition metals in the zeolite framework at lower levels and thus overcomes the problem of conventional spectroscopic techniques in detecting titanium atoms at lower concentrations.84 Li et al. presented UV-Raman resonance spectroscopic data which, for the first time, clearly distinguished between framework titanium, framework silicon and framework-associated titania. UV-Raman resonance spectra significantly improved the quality of zeolite Raman spectra by avoiding fluorescence interference in zeolite samples.83,85 They found that the resonance Raman bands induced by framework titanium atoms were selectively enhanced when UV laser excitation of charge transfer transitions associated with framework titanium. As a result, a clear distinction can be made among framework titanium atoms, framework-associated titanium species and other defect sites. The Raman bands at 960 and 1125 cm−1 together correspond to asymmetric stretching and symmetric stretching vibrations of the framework [TiO4] unit. The Raman bands at 490, 530, and 1125 cm−1 correspond to the bending vibrations of the framework Ti–O–Si species, symmetric stretching, and asymmetric stretching vibrations. Oxygen-17 has an NMR-active quadrupole nucleus whose spectral properties can be easily measured by solid-state NMR and calculated by DFT. The NMR feature (chemical shifts and quadrupole coupling) is highly sensitive to the symmetry and electronic structure around the oxygen atoms. Gordon et al. therefore reasoned that 17O NMR spectroscopy could be used to detect the signature of the active sites in TS-1.38
X-ray photoelectron spectra (XPS) is a technique employed to ascertain the microenvironment of Ti atoms. In XPS spectra, it is generally accepted that the peaks of titanosilicate zeolites at 459 and 465 eV correspond to Ti 2p3/2 and Ti 2p1/2, respectively. Deconvolution of these two peaks yields four components. The two low-binding-energy components, 460.6 eV for Ti 2p3/2 and 466.4 eV for Ti 2p1/2, correspond to the TiO4.86–88 The X-ray absorption spectra of TiO4 exhibit a pronounced XANES peak at 4969 eV,89,90 which is a notable feature. However, as the majority of zeolite samples, the XANES peak at 4969 eV cannot be attributed solely to TiO4. Instead, it also encompasses small quantities of penta- and hexa-coordinated titanium species.91 Furthermore, this technique lacks the requisite sensitivity to differentiate between various TiO4.92,93 Photoluminescence spectra can be employed to distinguish between various types of tetrahedrally coordinated or nearly tetrahedrally coordinated titanium species, such as Ti(OSi)4 and Ti(OSi)3OH, when the sample is excited at 250 nm. Upon excitation at 250 nm, the emission band at 500 nm is indicative of Ti(OSi)4,33 while the emission band at 430 nm is Ti(OSi)3OH.94 The complex and variable microenvironments of titanium species usually do not yield reliable results with only one assay technique, and a reasonable combination of multiple assays should be used to verify each other. Table 3 summarizes some characteristic peaks corresponding to TiO4 in characterization.
| Structure associated with TiO4 | Characterization | Peak position | Ref. |
|---|---|---|---|
| Ti(OSi)4 | UV-Vis | 200–210 nm | 33, 65, 68 and 69 |
| Ti(OSi)4 | Photoluminescence spectra | Excitation at 250 nm: 500 nm | 33 |
| Ti(OSi)3OH | UV-Vis | 230 nm | 33, 63, 65, 68 and 69 |
| Ti(OSi)3OH | Photoluminescence spectra | Excitation at 250 nm: 430 nm | 94 |
| Ti-O-Si | FT-IR spectra | 960 cm−1 | 77–79 |
| Ti–O–Si | UV-Raman | 490, 530 and 1125 cm−1 | 83 and 85 |
| [TiO4] | UV-Raman | 960 and 1125 cm−1 | 83 and 85 |
| [TiO4] | XPS spectra | 460.6 and 466.4 eV | 86–88 and 95 |
| [TiO4] | XANES spectra | 4969 eV, a single peak is produced at the intense pre-edge peaks | 89, 90 and 96 |
2.3. Synthetic strategy
Different titanium species have different catalytic activities in different oxidation reactions. In order to prepare titanosilicate zeolites aiming at a certain reaction, targeted modulation of the generation of titanium species with different coordination states and the microenvironment of titanium species to improve the reactivity and lifetime of the catalysts are of great industrial significance. Table 4 summarizes the current strategies for the synthesis of tetracordinate titanosilzeolite. Wang et al. synthesised hollow TS-1@S-1 (HCS-TS) with a core–shell structure for the first time. The higher catalytic activity of this catalyst in olefin epoxidation was attributed to the generation of defective tetra-coordinated titanium species (Ti(OSi)3OH). In this approach, post-processing represented a pivotal stage in the synthesis of HCS-TS, as it not only introduces secondary pores within the zeolite framework, but also modified the coordination environment of the tetra-coordinated Ti sites.97 With regard to the Ti(OSi)3OH, researchers have conducted a comprehensive investigation into various aspects, resulting in a unified conclusion.| Sample | Strategy | Method | Advantage | Ref. |
|---|---|---|---|---|
| TS-1 | Post-treatment | Construct the hollow TS-1@S-1 zeolite of the core–shell structure | Production of Ti(OSi)3(OH) with higher activity | 97 |
| TS-1 | Post-treatment | TS-1 modified by acid phosphate solution | TiO6 is passivated while the TiO4 microenvironment is regulated | 98 |
| Ti-MWW | Post-treatment | Add the right amount of phosphoric acid | Formation of highly active TiO4 species with a Ti-O-P structure | 99 |
| Ti-MWW | Post-treatment | Acid treatment of Ti-MWW containing boron | The acid treatment not only removes about half of the TiO6, but also converts the other part to TiO4 | 100 |
| Ti-MWW | Post-treatment | Fluorine was implanted into Ti-MWW | Increase in Lewis acid strength | 101 |
| Ti-MWW | Post-treatment | Fluoride was introduced into the Ti-MWW framework in the presence of ammonium fluoride | The hydrophobicity of the zeolite surface is improved, and the electropositivity near tetragordinate Ti species is changed | 102 |
| TS-1-PPA | Additive | Poly(acrylic acid) as the gelating agent | The catalyst has a high titanium content and is free of extra-framework Ti species | 6 |
| TS-1-PC | Additive | Dissolve TBOT in H2O2 | Ti enters the framework mainly within 5–8 hours of crystallization | 103 |
| Ti-MWW | Post-treatment | Ti-MWW(P) (Si/Ti = 25) is treated with 2.0 M HNO3 for 24 h at a solid-to-liquid weight ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 under refluxing conditions (105 °C) 50 under refluxing conditions (105 °C) |
Accelerate the conversion of Ti(OSi)4 material to Ti(OSi)3OH material in zeolite | 63 |
| TS-1 | Additive | Add less urea | Increasing surface defects and unsaturated TiO4 | 104 |
Thomas et al. used a grafting method to attach titanocene-derived catalyst precursors to the pore walls of MCM-41 to generate epoxidation catalysts for cyclohexenes and larger volumes of cycloolefins. The use of titanocene dichloride as a grafting reagent avoided the generation of anatase TiO2 and favoured the generation of TiO4, as the relatively stable cyclopentadienyl ligand protected the titanium centre, thus preventing dimerisation and/or oligomerisation.105 The microenvironment of Ti species can be effectively improved by introducing phosphorus. Previous studies have demonstrated that Ti-MWW undergoes changes in the coordination state and content of titanium species in the following treatment with acid.63,106,107 Guo et al. employed this principle to investigate the efficacy of acid treatment of TS-1 with acid phosphates (ammonium dihydrogen phosphate, potassium dihydrogen phosphate, and sodium dihydrogen phosphate solutions). The catalyst samples exhibited enhanced catalytic activity in the propylene liquid-phase epoxidation reaction. This phenomenon could be attributed to the passivation of TiO6 by phosphate species and the subsequent impact on the microenvironment of TiO4.98 The mechanism of acid treatment of Ti-MWW was further investigated by Yang et al. They prepared phosphorus-modified Ti-MWW by adding different amounts of phosphoric acid during the synthesis of Ti-MWW. The 1-hexene epoxidation reaction was employed as a probe reaction, and various characterisation techniques were employed to demonstrate that the addition of an appropriate amount of phosphoric acid can enhance the catalytic performance of Ti-MWW in the 1-hexene epoxidation reaction. Conversely, the occurrence of epoxidation of the reactants is inhibited by the addition of excessive phosphoric acid. This is due to the fact that an optimal quantity of phosphoric acid can combine with the central titanium atom to form TiO4 with a Ti–O–P structure, which is more active than conventional TiO4 with a Ti–O–Si structure. Otherwise, an excess of phosphoric acid combines with the central titanium atom forming titanium phosphate, which clogs the pores and degrades the catalytic performance.108 Their study offers novel insights into the rational control of phosphoric acid addition for the construction of highly active TiO4. Yang et al. conducted a subsequent study to elucidate the mechanism of the transformation of the coordination state of titanium species during acid treatment. For the first time, they characterised the evolution of titanium species after acid treatment of Ti-MWW using UV-Raman resonance spectra with 244 nm excitation. They discovered that following acid treatment of Ti-MWW before calcination, nearly half of the TiO6 were removed, while the remaining half underwent transformation into TiO4. Conversely, when the Ti-MWW after calcination was subjected to further acid treatment, the TiO6 were converted to anatase TiO2.109 TiO6 is usually the only titanium species present in conventionally synthesized MWW zeolites, as titanium is inherently challenging to incorporate into the MWW zeolite framework. The acid treatment of the parent zeolite results in the removal of a significant number of framework boron atoms, which then form silanol nests. This allows the incorporation of the TiO6 into the zeolite framework transforming into TiO4 as a restorative agent. The newly transformed Ti(OSi)4 exhibits minimal catalytic activity in the 1-hexene epoxidation reaction. Whereas, the catalytic activity of the TiO4 gradually increases with the gradual extension of the acid treatment time, which is attributed to the gradual transformation of the Ti(OSi)4 into the Ti(OSi)3OH.63,110 The rational design and regulation of the content of TiO4 and the distribution of different types of TiO4 in titanosilica zeolites are of great significance in improving the catalytic activity of titanosilica zeolites.
3. Hexa-coordination framework-associated titanium species
3.1. Catalytic performance
In addition to framework titanium species, framework-associated titanium species were formed during the synthesis, which are amorphous TiO2 and crystalline TiO2. Researchers have postulated that the former are partially aggregated octa-coordinated Ti or isolated TiOx attached to the zeolite lattice, and their activity in catalytic reactions is still controversial.111 Bianchi et al. prepared the modified TS-1(TS-1B), which exhibited a clear absorption peak at 270 nm in the UV-vis spectrum and demonstrated high activity and selectivity in the oxidation of benzene to phenol.112 The discovery of this new type of titanium species has aroused wide attention. Li et al.52 discovered a new active titanium species, the “TiO6” octahedral species, in TS-1, as shown in Fig. 4. This species exhibits a characteristic Raman spectral band at 695 cm−1 and an absorption peak in the UV/visible spectrum at 270 nm. This newly discovered titanium species is active in the reactions of propylene epoxidation and benzene oxidation to phenol, but its acidity would lead to the ring-opening reaction of epoxy products. The selectivity of the epoxide product is lower compared to the tetrahedrally coordinated titanium species. Subsequently, Wang et al. reported that this titanium species was also catalytically active in the epoxidation reaction of 1-hexene. With increasing research, the understanding of TiO6 has been gradually deepened. TiO6 is a common framework-associated titanium species, which is typically obtained by first isolating the TiO4, followed by selective solubilisation of the silica species and finally recrystallisation.95 This review presents a table of catalytic reactions in which hexa-coordinated Ti species exhibit extraordinary catalytic activity (Table 5).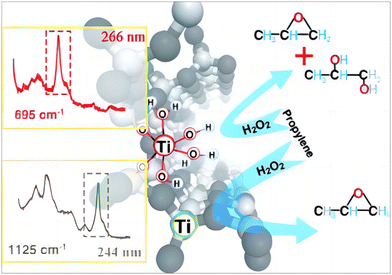 | ||
| Fig. 4 New reactive Ti species (TiO6) and their characteristic Raman bands. Reproduced with permission.52 Copyright 2012, John Wiley and Sons. | ||
| Sample | Probe reaction | Si/Ti ratio | Quantity of catalyst (mg) | Reaction time (h) | Temperature (K) | Conversion (%) | Selectivity (%) | Ref. |
|---|---|---|---|---|---|---|---|---|
| a The difference between TS-1A and TS-1B is that the latter sample has TiO6. b The difference between TS-2 and TS-2+ is that the latter sample has TiO6. | ||||||||
| TS-1 | Propylene epoxidation | 33 | 40 | 1.5 | 333 | 97.4 | 92.1 | 113 |
| TS-1Aa | Propylene epoxidation | 50 | 20 | 1 | 333 | 98.2 | 99.0 | 114 |
| TS-1Ba | Propylene epoxidation | 50 | 20 | 1 | 333 | 99.3 | 86.6 | 114 |
| TS-1 | Hydroxylation of benzene | — | 5000 | 2 | 373 | 8.6 | 91.0 | 112 |
| TS-1 | 1-Hexene epoxidation | 36.9 | 50 | 2 | 333 | 33.0 | 95.0 | 95 |
| TS-2b | 1-Hexene epoxidation | 54 | 50 | 2 | 333 | 6.7 | 100.0 | 40 |
TS-2+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b b |
1-Hexene epoxidation | 54 | 50 | 2 | 333 | 17.7 | 90.2 | 40 |
| TS-1-AM | 1-Hexene epoxidation | 80 | 50 | 2 | 333 | 28.0 | 90.0 | 96 |
| N2-Al-TS-1 | Ethylene epoxidation | — | 200 | 1 | 333 | 87.2 | 91.3 | 115 |
Wells et al. and Grosso-Giordano et al. utilized DFT calculations and found that the defective TiO6 adjacent to Si vacancies were more catalytically active than the TiO4 in propylene epoxidation reactions. This is attributed to the fact that the missing Si atoms reduce the spatial site resistance and provide a wider space for Ti atoms to move, which makes it easier for the defective TiO6 combining with hydrogen peroxide to form a peroxygen-active intermediate through propylene epoxidation reaction.51,116 Liu et al. prepared catalysts with Ti(OSi)2(OH)2(H2O)2 species and a good distribution of TiO4 and TiO6 by hydrothermal treatment of conventional TS-1 with ethylamine and tetrapropylammonium bromide.40 The TiO6 was found to exist in the mononuclear state and exhibited superior catalytic activity to the TiO4 in the epoxidation reaction of 1-hexene. Guo et al. investigated the kinetics of propylene epoxidation before and after hydrothermal treatment of TS-1 with TPAOH. Their findings demonstrated that the formed TiO6 were more favourable for the conversion of H2O2, had lower energy barriers to epoxidation, and had better catalytic activity in propylene epoxidation.117 Furthermore, Li et al. demonstrated by UV-Raman spectra that the TiO6 in TS-1 existed in a mononuclear state (Fig. 3).52 They also provided a new strategy to solve the problem of low selectivity of the TiO6 for the epoxide products. This involved the addition of a weak base reagent, which could inhibit the occurrence of the side reaction. Consequently, they concluded that the introduction of TiO6 significantly augmented the number of active sites of titanium. However, under specific conditions, the TiO6 would obstruct the TiO4 and deactivate them in the oxidation reaction.45 Consequently, the optimal incorporation of TiO6 and the regulation of the distribution and microenvironment of TiO6 and TiO4 can markedly enhance the number of active titanium sites, which can facilitate superior catalytic performance in olefinic epoxidation reactions.42,118,119
3.2. Characterization
The identity of the titanium species indicated by the band at 240–280 nm in the UV-Vis spectra is currently a matter of contention. Some researchers propose that the band represents the presence of TiO6 formed by isolated [TiO4] or [HOTiO3] with two water molecules.91,120 It has been proposed that the observed band is indicative of the condensation of Ti–O–Ti bonds of TiO6.121,122 In the UV-Raman resonance spectra, the Raman bands at 270 and 695 cm−1 collectively correspond to TiO6.52 Li et al. named a new Ti species (Ti(OH)4(OSi)2) for the first time through UV-Raman spectroscopy detection and verification by DFT calculation. For the new Ti species detected by the 266 nm UV-Raman spectroscopy, the Raman peaks at 441, 705, 1125 and 1342 cm−1 are associated with Ti(OH)4(OSi)2.39 In the XPS spectra, as previously stated, the peaks of titanosilicate zeolite at 459 and 465 eV are generally accepted to correspond to Ti 2p3/2 and Ti 2p1/2, respectively. Back-convolution of these two peaks yields four components. The peaks yield four components, of which the two high-binding-energy components, 459.2 eV for Ti 2p3/2 and 464.9 eV for Ti 2p1/2, correspond to the TiO6. It is noteworthy that the peak positions of the TiO4 and the TiO6 are closely aligned. The latter contain amorphous TiO2 and amorphous TiO2–SiO2 binary oxides, which could not be reliably analysed by XPS spectra alone. Table 6 summarizes some characteristic peaks corresponding to TiO6 in characterization.| Structure associated with TiO6 | Characterization | Peak position | Reference |
|---|---|---|---|
| (TiO4)(H2O)2 | UV-Vis | 240–280 nm | 91 and 120 |
| HOTiO3(H2O)2 | UV-Vis | 240–280 nm | 91 and 120 |
| Ti-O-Ti | UV-Vis | 240–280 nm | 121 and 122 |
| Mononuclear TiO6 | UV-Raman 325 nm | 270 and 695 cm−1 | 96 and 114 |
| TiO6 | XPS spectra | 459.2 and 464.9 eV | 86–88 and 95 |
| TiO6 | EXAFS fitting | Coordination number is more than 4 | 96 |
| Ti(OH)4(OSi)2 | UV-Raman 266 nm | 441, 705 and 1342 cm−1 | 123 |
3.3. Synthetic strategy
It has been demonstrated that TiO6 exhibits superior catalytic activities to TiO4 in olefin epoxidation and oxidative desulfurisation reactions. Consequently, the regulation of the vast quantity of TiO6 in titanosilicate zeolites has become a significant research topic. Table 7 summarizes the current strategies for the synthesis of hexacoordination titanosilicate zeolite.| Sample | Strategy | Method | Advantage | Ref. |
|---|---|---|---|---|
| TS-1 | Hydrothermal treatment | Tetrabutyl-n-titanate tetramer was used as the titanium source | More TiO6 and less TiO2 | 124 |
| TS-1 | The seed-assisted and the microwave synthesis | 2 wt% calcined seeds + a microwave reactor at 170 °C for 1 h | Both seed and microwave radiation can effectively promote the formation of TiO6 and greatly reduce the necessary crystallization time | 96 |
| TS-1 | Two-step crystallisation + L-lysine | The crystallization process was conducted at 80 °C for 48 h and then at 170 °C for 24 h + L-lysine | The two-step crystallization process prevented the formation of anatase TiO2, and L-lysine stabilized TiO6 | 95 |
| TS-1 | Two-step crystallisation | Add polyacrylamide (PAM) | Following the non-classical mechanism, abundant TiO6/TiO4 species were generated | 125 |
| Ti-MWW | Post-treatment | Add piperidine (PI) | Induce the conversion of TiO4 to TiO6 | 126 |
| TS-1 | Post-treatment | Add ethylamine (EA) and tetrapropyl ammonium bromide (TPABr) | Construct “TiO6” species without leaching any framework Si and Ti species | 40 and 127 |
| Al-TS-1 | Post-treatment | (NH4)2CO3 hydrothermal reprocessing of parent zeolite | Generates Ti(OH)4(OSi)2 | 115 |
Starting from the generation and induction of titanium species, the directional regulation of titanium species can be effectively realized. Bai et al. selected tetrabutyl orthotitanate tetramer as the titanium source to prepare titanium-rich TS-1 at the nanoscale, rather than tetrabutyl titanate. Following UV-Vis and UV-Raman spectroscopic studies, it was demonstrated that the samples prepared utilising tetrabutyl orthotitanate tetramer as the titanium source exhibited an abundance of TiO6 and a lower concentration of anatase TiO2. Furthermore, the content of the TiO6 increased with the incorporation of tetrabutyl orthotitanate tetramer, reflecting a higher catalytic activity in the oxidative desulphurisation of dibenzothiophene.124 Xu et al. achieved a rapid one-step synthesis of titanium-rich TS-1 by introducing an active crystal species during the synthesis of titanosilicate zeolite and employing microwave amplitude irradiation. The microwave amplitude irradiation method has been demonstrated to achieve more uniform heating compared with the traditional hydrothermal synthesis method. Furthermore, the addition of active crystal species has been shown to effectively promote the formation of highly coordinated titanium species precursors.42 X-ray absorption spectra (XAS) has enabled us to ascertain that the titanium-rich TS-1 synthesised with microwave amplitude irradiation assisted by crystalline species contains a considerable number of TiO6. The X-ray diffraction (XRD) patterns demonstrate that the titanium-rich TS-1 exhibits greater crystallinity than the conventional TS-1. This indicates that the crystal species and microwave irradiation can accelerate the crystallisation of the zeolite. Furthermore, it was observed that both the active crystal seed and microwave irradiation can induce the generation of TiO6.
Changing the crystallization mode of zeolite and adding additives are also good ways to adjust the formation of titanium species. Wang et al. prepared TS-1 containing both TiO4 and TiO6 by adding L-lysine during the synthesis of anatase TiO2-free TS-1 by two-step crystallisation. The amino acid-assisted zeolite synthesis strategy developed is based on the existence of a synergistic interaction between amino acids and titanium species. L-Lysine acts as a stabilising agent for TiO6, thereby enabling regulation of the coordination state and number of titanium species by changing the kinetics of zeolite growth.95 Zhang et al. also employed a two-step crystallisation method for the synthesis of titanosilicate zeolites. Their findings indicated that the addition of PAM facilitated the structural evolution of the TS-1 precursor, resulting in a shift from a classical to a non-classical crystallisation mechanism. The alteration in the crystallisation process makes TS-1 a coarser surface and a more porous multilevel structure, which is conducive to the diffusion of reactant and product molecules, as well as the enhancement of the accessibility of the active titanium sites. Ultimately, this leads to the regulation of titanium species coordination states and microenvironments.125
In addition to regulating the type of titanium during zeolite synthesis, post-treatment is also a common, direct and effective way to introduce TiO6 into titanosilicate zeolite. Xu et al. prepared a nanoscale flaky Ti-MWW with PI as a ligand, which exhibited high catalytic activity and product selectivity in olefin epoxidation reactions. The results of UV-Vis spectroscopic studies indicated that the majority of titanium species in Ti-MWW existed in a tetra-coordinated state. However, a notable quantity of TiO6 was observed in the synthesised Ti-MWW, following PI post-treatment, and exhibited a reduction in the content of TiO4, with a partial restoration observed when PI was removed. This indicates that the post-treatment of Ti-MWW with PI resulted in the conversion of TiO4 to TiO6.118 Wu et al. subjected TS-1 to post-treatment with EA and TPABr, subsequently comparing the resulting samples with those of the classical TS-1. They observed the generation of TiO6 in the TS-1 treated with EA and TPABr. Additionally, a decrease in the number of active centres of the TiO4 was observed in the pyridine infrared spectral pattern, suggesting that some of the TiO4 had undergone conversion to TiO6. They conducted an in-depth study on the synthesis process of TS-1 by post-treatment with EA and TPABr. The post-treatment of titanium with only EA or TPABr was unable to generate TiO6. However, the simultaneous use of EA and TPABr was found to produce TiO6, with the content of TiO6 being positively correlated with the amount of them. This was due to the selective dissolution of silicon species, which controlled the dissolution rate of silicon species to match the recrystallisation rate, thus promoting the generation of TiO6.40 A comparable study was conducted by Yu et al., who synthesised multistage porous TS-1 with more TiO6 through a rationally designed sequential post-treatment. This process not only altered the coordination state of the titanium species but also introduced mesopores into the microporous zeolites. Their in-depth study concluded that only the sequential post-treatment of TPAOH-EA was able to synthesise multistage porous TS-1 with abundant surface TiO6. Furthermore, the initial step of the sequential post-treatment, involving TPAOH, serves to introduce mesopores into the microporous framework. The second step, which involves EA, is the key to removing the amorphous TiO2 on the outer surface of the zeolite and generating the TiO6. The multistage porous TS-1 synthesised by sequential post-treatment exhibited extremely high catalytic activity in the 1-octene epoxidation reaction.127 This provides a new design idea for the construction of titanosilicate zeolites more suitable for macrocyclic olefin oxidation reactions.
4. TiO2 titanium species
4.1. Catalytic performance
Anatase TiO2 is an octahedrally coordinated titanium species. It is commonly believed that in catalytic systems where hydrogen peroxide is used as the oxidant, the presence of anatase TiO2 will decompose most of the hydrogen peroxide, as well as covering the active site, which is detrimental to the reaction.128 Therefore, the generation of anatase TiO2 should be avoided as much as possible in the synthesis strategy of titanosilicate zeolites. The generation of anatase TiO2 is due to the mismatch between the hydrolysis rates of titanium and silicon sources, which results in the formation of Ti–OH from the hydrolysis of the titanium source further converting into anatase TiO2. Consequently, a large number of studies have been devoted to the elimination of anatase TiO2 as an alternative to the pickling step, such as the introduction of different crystallisation modifiers (inorganic ammonium salts, X-100 tralatone, Tween-20, hydrogen peroxide, isopropanol, etc.) during the zeolite's synthesis process, or the preparation of suitable titanium sources to match the hydrolysis rate of titanium and silicon sources, and the use of crystalline species and UV assistance.2,129–134Su et al. succeeded in synthesising TS-1 containing amorphous Ti, as well as an amorphous Ti oligomer containing an octahedral-coordinated Ti–O–Ti structure in the presence of Na+ or after treatment with ammonia solution.111 The presence of amorphous Ti in the propylene gas-phase epoxidation reaction resulted in the generation of by-products and the decomposition of H2O2, which significantly impaired the catalytic performance of TS-1. Zhang et al. proposed that amorphous TiO2 species in TS-1 are also responsible for propylene epoxidation.113 In recent years, taking a deep dive into anatase TiO2 has revealed that it not only has an inhibitory effect on the oxidation reaction, but promotes the reaction in specific instances. Li et al. found that in styrene epoxidation reaction, TS-1 containing anatase TiO2 exhibited the best styrene conversion and product selectivity. The reason was that the synergistic interaction between framework Ti species and anatase TiO2 in the styrene epoxidation reaction resulted in higher catalytic activity of TS-1.135 Subsequently, Xiao et al. demonstrated that anatase TiO2 has a positive promotion effect in the TS-1 system during the cyclohexanone aminoximation reaction. The cyclohexanone ammoximisation reaction is an alkaline process, and titanosilicate zeolites are susceptible to desilication in an alkaline environment, which reduces the catalyst's operational lifespan. They discovered that anatase TiO2 has the capacity to strongly interact with ammonia molecules and can adsorb ammonia molecules effectively, which facilitates ammonia uptake and simultaneously enhances the corrosion resistance of the zeolite framework. This results in a significant improvement in the catalyst's operational lifetime and catalytic activity.66 The promotion of certain oxidation reactions by anatase TiO2 provides a new idea to study the catalysis of titanium species.
4.2. Characterization
In the UV-vis range, there is a more uniform view of the 310–330 nm band, which points to anatase TiO2.33 In the UV-Raman resonance spectra, the Raman bands at 144, 390, 516 and 637 cm−1 collectively correspond to anatase TiO2.77 In XPS, the binding energy band of titanosilicate zeolite around 458 eV is indicative of anatase TiO2.86,87 In XANES, the pre-edge features give rise to three weak peaks (in the range of 4960 eV to 4975 eV) referring to anatase TiO2.136 A summary of the current assays for structures related to anatase TiO2 is presented in Table 8.| Structure associated with TiO2 | Characterization | Peak position | Ref. |
|---|---|---|---|
| TiO2 | UV-Vis | 310–330 nm | 33 |
| UV-Raman | 144, 390, 516 and 637 cm−1 | 77 | |
| XPS | 458 eV | 86, 87 and 95 | |
| XANES | The pre-edge features give rise to three weak peaks (in the 4960 eV to 4975 eV range) | 96 |
4.3. Synthesis strategy
As previously stated, anatase TiO2 is an unfavourable factor in the majority of reactions. Consequently, researchers have placed a significant focus on the avoidance of anatase TiO2 generation in the adjustment strategy of titanium species. The current research primarily centers on the mechanisms underlying TiO2 formation and strategies for reducing its generation. Relevant studies have been summarized in Table 9.| Sample | Strategy | Method | Advantage | Ref. |
|---|---|---|---|---|
| TS-1 | Hydrothermal treatment | (NH4)2CO3 is added to the synthetic gel | The formation of anatase is avoided, and the distribution of Ti in the framework is more uniform | 2 |
| TS-1 | Hydrothermal treatment | Different ammonium salts are added to the synthetic gel | Avoid anatase formation | 137 |
| TS-1 | Hydrothermal treatment | 1,3,5-Phenyl tricarboxylic acid was used as a crystallization modifier | The traditional liquid phase mechanism is transformed into a solid–liquid binding mechanism, and the formation of anatase is inhibited | 138 |
| TS-1 | Hydrothermal treatment | Starch as an additive | The production of anatase TiO2 and TiO6 was inhibited | 77 |
| TS-1 | Spin crystallisation | Triton X-100 is a mesoporous template agent | Avoid anatase formation | 129 |
| TS-1 | Hydrothermal treatment | Isopropyl alcohol and hydrogen peroxide were added to tetrabutyl titanate | Reduce the hydrolysis rate of the titanium source | 139 and 140 |
| TS-1 | Hydrothermal treatment | Tetrabutyl-n-titanate tetramer was used as the titanium source | The crystallization process of zeolite is slowed down, thus balancing the incorporation rate of titanium and the crystal growth rate | 124 |
| TS-1 | Hydrothermal treatment | The TEOS was pre-hydrolyzed | Avoid anatase formation | 141 |
| TS-1 | Solid-phase conversion method | The low amount of TPAOH (TPA/SiO2 = 0.13) and the suitable crystallization temperature | Avoid anatase formation | 142 |
The formation of TiO2 is mainly caused by the different hydrolysis rates of titanium source and silicon source, and the best combination can be obtained by combining different silicon and titanium sources. Initially commonly reported as a study of the hydrolysis behaviour of titanium sources, the hydrolysis behaviour of different silicon sources has now given us new inspiration that the modulation of titanium species can also be accomplished by controlling the hydrolysis behaviour of silicon sources. Bai et al. selected tetrabutyl orthotitanate tetramer as the titanium source, rather than the more conventional tetrabutyl titanate, as it has been demonstrated that this can effectively slow down the crystallisation rate of zeolites.124 This allows for a more precise matching of the titanium doping rate with the crystallisation rate of zeolite, and the generation of a reduced amount of anatase TiO2. It displays an augmented external surface area and enhanced accessibility of active titanium species. It has been reported that the zeolites prepared when the silica source is ethyl orthosilicate have small particles and a high framework titanium content, while silica sol would cause large particles and a low framework titanium content. Silica sol is formed by dispersing 10–20 nm SiO2 particles uniformly in an aqueous phase to create a sol system. In contrast, ethyl orthosilicate undergoes hydrolysis to generate orthosilicate molecules, which are subsequently dehydrated and polymerised into specific silicon species. The formation of small-particle silica species with a reticulation structure allows for enhanced compatibility with titanium species, preventing the aggregation of titanium species and increasing the framework titanium content.143–146 Geng et al. studied the hydrolysis behaviour of tetraethyl orthosilicate in depth using Fourier infrared spectra,141 and analyzed the effect of hydrolysis time on the coordination state of titanium species in titanosilicate zeolite using X-ray fluorescence spectra and UV-vis. The following pattern was found: when 1-hexene epoxidation was employed as the probe reaction, the catalyst activity exhibited an initial increase followed by a subsequent decrease with a prolonged hydrolysis time of tetraethyl orthosilicate, reaching its peak at 2 hours. In other words, the titanosilicate zeolite synthesized through the hydrolysis of tetraethyl orthosilicate possessed a higher titanium content within its framework and effectively suppressed the formation of anatase TiO2 to the maximum extent.
The pH level in the solution also significantly impacts the rate of hydrolysis for silicon and titanium sources. Fan et al. added (NH4)2CO3, NH4F, NH4Cl, NH4Br, NH4I, CH3COONH4, NH4NO3, (NH4)2CO3, (NH4)2SO4, and (NH4)3PO4 to the synthesised gel. All ammonium salts promote the incorporation of titanium species into the framework, with the degree of promotion varying according to the type of ammonium salt. Among them, (NH4)2SO4, (NH4)3PO4 and (NH4)2CO3 can significantly reduce the pH value, thus slowing down the crystallisation rate and inhibiting the formation of anatase TiO2. This approach better balanced the rate of titanium doping into the zeolite framework with the rate of crystallisation and altered the crystallisation mechanism, thereby improving the hydrophobicity of the zeolite and enhancing the titanium doping rate. This ultimately led to a notable reduction in the generation of anatase TiO2.2,137
The incorporation of additives in the precursor can also significantly influence the hydrolysis rate of the titanium source and prevent the formation of titanium ore. Li et al. successfully synthesised anatase TiO2-free nano-TS-1 within a single day by incorporating 1,3,5-benzenetricarboxylic acid (H3BTC) as a crystallisation modifier. Upon the addition of H3BTC, two routes of doping the framework with Ti atoms were observed: firstly, titanium species in the amorphous solid can be directly inserted into the framework lattice; secondly, titanium species in the liquid phase insert into the framework lattice rapidly as the crystallisation process occurs. Consequently, the titanium species are inclined to enter the zeolite framework, thus preventing aggregation and the formation of anatase TiO2.138 Zhang et al. found that the addition of the additive starch to the hydrothermal system of TPAOH reduced the crystallisation rate, balanced the doping rate of titanium and silicon atoms, and reduced the production of anatase TiO2.77 Wang et al. reported the use of an anionic polyelectrolyte, polyacrylic acid (PAA), as a gelling agent. This approach could achieve partial conversion of liquid-phase precursors to solid-phase precursors, and the generation of anatase TiO2 could be avoided by utilising the synergistic effect of the liquid-phase and solid-phase conversion mechanisms in combination with rotational crystallisation. Nevertheless, this approach would result in the aggregation of nanoscale TS-1, which would reduce the external surface area to a degree that was insufficient to enhance the catalytic activity.6 Zhang et al. selected the inexpensive Triton X-100 as a template agent and employed rotational crystallisation to synthesise nano-titanosilicate zeolites with uniform mesopores and large external surface areas. Additionally, they achieved complete titanium doping into the zeolite framework in a tetrahedrally coordinated state, effectively circumventing the formation of anatase TiO2. Triton X-100 serves to reduce the crystallisation rate, while the conditions of rotational crystallisation accelerate the doping of titanium and make the doping rates of titanium and silicon more compatible.129 Thangaraj et al.139 and Sasidharan et al.140 added isopropanol and hydrogen peroxide to tetrabutyl titanate and stirred them to facilitate the formation of complexes. Both studies employed the complexation between isopropanol and tetrabutyl titanate to reduce the rate of hydrolysis of the titanium source. The two-step crystallisation synthesis strategy has also been widely employed by researchers in the synthesis of titanosilicate zeolites devoid of anatase TiO2. Additionally, the low-temperature pretreatment in the initial step gives rise to the formation of a substantial number of 5-membered and 4-membered rings, which exhibit the distinctive structure of MFI. This facilitates the capture of titanium and, consequently, the doping of the zeolite framework during the subsequent high-temperature crystallisation process.64
Furthermore, post-treatment represents an efficacious approach to the elimination of anatase TiO2. Alba-Rubio et al. investigated the framework-associated titanium species in TS-1 and identified the presence of two types of framework-associated titanium species: anatase TiO2 located on the outer surface of the zeolite, and titanium oxide nanodomains present within the zeolite pores.147 The effective removal of anatase TiO2 on the outer surface of zeolite and nano titanium oxide blocked in the pores can be achieved by utilizing an appropriate concentration of HCl treatment. Zhai et al. discovered that nutrients capable of influencing the microenvironment of titanium species were present in the mother liquor of titanosilicate zeolite synthesis.88 They economically synthesised hollow TS-1 free of anatase TiO2 with a controlled microenvironment and spatial distribution of titanium species through functional mother liquor-induced dissolution recrystallisation, and for the first time proposed a thermodynamic-kinetic dual-regulation pathway describing the reversible changes of the titanium species’ microenvironment during dissolution recrystallisation. The microenvironment and spatial distribution of titanium species in the dissolution and recrystallisation process induced by the functional masterbatch are summarised by combining the results of different characterisations: The functional masterbatch can inhibit the formation of anatase TiO2 at low temperatures, but the low crystallisation temperature is not conducive to the incorporation of Ti, which is conducive to the formation of extra-framework titanium species; unsaturated silica species in the functional masterbatch are the functional species that facilitate the regulation of the microenvironment of titanium species; the liquid–solid ratio is an important indicator for regulating the microenvironment of titanium species, and a high liquid–solid ratio can promote the dissolution and re-insertion of anatase TiO2, which is conducive to improving the crystallinity and titanium content of zeolite, and a low liquid–solid ratio generates less anatase TiO2, thus, the titanium content in the framework is higher, but at the same time, fewer unsaturated silica species cannot adequately capture the anatase TiO2. It is therefore clear that the microenvironment of titanium species undergoes reversible changes at different liquid–solid ratios, and this evolution was corroborated by the results of UV-vis combined with ICP-OES characterisation. The elimination of anatase TiO2 and the modulation of the titanium species microenvironment are achieved using unsaturated silica species in the mother liquor through this synthetic method. Liu et al. presented a one-step solid phase conversion strategy for the synthesis of anatase-free hollow TS-1 without the use of a mesoporous agent.142 By employing an appropriately low amount of TPAOH (TPA/SiO2 = 0.13) and selecting a suitable crystallization temperature, the hollow structures can be induced and more titanium can be incorporated into the framework. The phase transition mechanism was elucidated by investigating both the crystallization rate and dissolution rate during the phase transition process.
5. Novel titanium species
5.1. Catalytic performance
Gordon et al. proposed that dinuclear titanium sites, rather than isolated titanium atoms in the framework, explain the high efficiency of TS-1 in propylene epoxidation with H2O2. Density functional theory calculations indicate that cooperativity between two titanium atoms enables propylene epoxidation via a low-energy reaction pathway with a key oxygen-transfer transition state similar to that of olefin epoxidation by peracids.40 The pentahedrally coordinated Ti species (TiO5) is a relatively uncommon occurrence in the synthesis of titanosilicate zeolites, and it is a framework-associated titanium species. Shakeri et al. prepared TS-1 in the presence of polyvinyl alcohol and crystal seed using TPABr as a structure-directing agent and detected the presence of defective titanium species, TiO5, TiO6, and TiO4 at the embryonic stage of TS-1 during the synthesis.148 Furthermore, they concluded that TiO5 exhibited a higher catalytic performance in olefin epoxidation reactions compared to TiO4.40,41,149 Tang et al. constructed TS-1 with highly active hydrogen-bonded titanium species through a subcrystalline aggregation crystallisation and bottom-up strategy. This hydrogen-bonded titanium species has a pentahedrally coordinated structure and Brønsted acidity, and the catalytic activity of the new catalyst in the chloropropylene epoxidation reaction is approximately 70% higher than that of conventional TiO6 and TiO4.150 Other analyses indicate that this TS-1 has extended lattices and narrower cross-pore channels. Wang et al. subjected pre-prepared TS-1, containing only “TiO4”, to post-treatment with aqueous TPAOH, aqueous TPAOH after hydrothermal treatment, and mother liquor, respectively.39 Each stage of the post-treatment resulted in the rupture of some Ti–O–Si bonds in “TiO4” and the formation of new bonds through a desilication and recrystallisation process. These processes resulted in the formation of new TiO5 and TiO6, which were identified for the first time by detailed and systematic UV-Raman spectroscopic studies combined with DFT calculations. The coordination structures of the “new” titanium sites in the three catalysts were established. The calculated DFT results and experimentally observed Raman peaks are listed in Table 10, and the new TiO5 were designated Ti-V (Ti(OH)2(OSi)3), with their structures illustrated in Fig. 5. In the propylene epoxidation reaction, the catalytic activity of the sample containing Ti-V and Ti-IV was superior to that of the sample comprising solely of “TiO4”.| Raman Peak (cm−1) | Raman peak assignment | Coordination structure | Ti-site | |||
|---|---|---|---|---|---|---|
| DFT calculated | Experimentally measured | Coordination number | Ti–O–Si bonds | Ti–O–H bond (s) | ||
| 493 | 490 | Ti–O–Si bending | 4 | 3 | 1 | Ti-IV |
| 1130 | 1125 | Ti–O–Si stretching | ||||
| 776 | — | Ti–O–H stretching | ||||
| 511 | 510 | Ti–O–H wagging | 5 | 3 | 2 | Ti-V |
| 680 | 685 | Ti–O–H rocking | ||||
| 1119 | 1125 | Ti–O–Si stretching | ||||
| 440 | 441 | Ti–O–H wagging | 6 | 2 | 4 | Ti-VI |
| 709 | 705 | Ti–O–H rocking | ||||
| 1119 | 1125 | Ti–O–Si stretching | ||||
| 1339 | 1342 | Si–O–H rocking | ||||
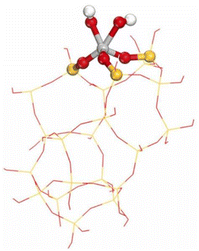 | ||
| Fig. 5 Structure of Ti-V (Ti(OH)2(OSi)3) proposed by discrete Fourier transform, with grey balls representing Ti atoms, yellow balls representing Si atoms, red balls representing O atoms, and white balls representing H atoms. Reproduced with permission.39 Copyright 2023, Elsevier. | ||
The existence of titanium species is dependent on their form and microenvironment, which are complex and variable. These variables are affected by a number of factors. In addition to the titanium species that often exist in the synthesis process of the titanosilicate zeolite, other new titanium species will also be formed in the post-processing process of the parent zeolite. The study of novel titanium species is still in its infancy. Apart from the fact that the synthesis methods and generation mechanisms have not been clearly proposed, the lack of appropriate characterizations represents an important limiting factor. Therefore, only an individual example of titanosilicon zeolite synthesis is presented in this review. Xiao et al. observed the generation of a distinctive new peak at 5.1 ppm in the 29Si MAS NMR spectrum during the silylation of Ti-MCM-41 with trimethylchlorosilane.151 This peak was attributed to the interaction of titanol with trimethylchlorosilane, resulting in the formation of a titanium species with a structure of (CH3)3SiOTi(OSi)3. The application of the silylated Ti-MCM-41 to benzene hydroxylation and 1-octene epoxidation revealed that it exhibited superior catalytic activity compared to the conventional Ti-MCM-41. This suggests that the (CH3)3SiOTi(OSi)3 titanium species displays enhanced catalytic activity compared to the conventional TiO4 and TiO6 in benzene hydroxylation and 1-octene epoxidation.
5.2. Characterization
In UV-Vis spectra, it has been suggested that at least two titanium species exist in the band at 240–280 nm, TiO6 and TiO5.77 In UV Raman resonance spectra, when excited at 266 nm, the Raman bands at 510 and 685 cm−1 correspond to the Ti–O–H wagging and rocking of TiO5.150 When excited at 257 nm, the Raman bands at 510, 685 and 1125 cm−1 together correspond to the Ti(OH)2(OSi)3.39 In XPS, the additional peak at 459.0 eV could be attributed to TiO5.150As previously stated, the peak at 5.1 ppm in the 29Si NMR spectrum was attributed to the novel titanium species (CH3)3SiOTi(OSi)3 in a study by Xiao et al.151 This paper presents a summary of the current methods for detecting titanium species with TiO5 and such novel titanium species, as presented in Table 11.
6. Adjustment strategies for the spatial distribution of titanium species
In addition to the coordination, the spatial distribution of heteroatoms also has a great influence on the catalytic activity. The important role of the distribution of Al acid sites in the zeolite framework (ZSM-5, MCM-22, Y and ITQ-13) in improving the catalytic activity and regulating the product distribution has been widely reported.152 However, how to regulate the distribution of titanium atoms in the framework has been less studied.153 Therefore, the preparation of titanosilicate zeolites with a controllable spatial distribution of titanium species is an important guide to selectively improve the catalytic activity of zeolites (Table 12). The mass transfer process and the accessibility of active sites greatly affect the catalytic activity of zeolite, and regulating the spatial distribution of titanium sites can promote the distribution of more titanium sites on the more accessible crystal surface rather than inside the crystal, which can improve the accessibility of active titanium species. At the same time, with the improvement of pore structure, the mass transfer process of substrate molecules can be promoted. Takaishi et al. synthesised TS-1 using S-1 as a silica source. When the synthetic gel was highly alkaline, the initial stage of the synthesis of S-1 underwent violent dissolution, with Ti being uniformly distributed into the TS-1 crystals. Conversely, when the alkalinity of the gel was low, the initial stage of dissolution was suppressed and recrystallisation occurs on the surface of the parent S-1, resulting in the distribution of Ti predominantly on the outer surface of the incompletely dissolved S-1 core. The synthesised samples demonstrated enhanced H2O2 efficiency in the 1-hexene oxidation reaction.154 Wang et al. prepared a core–shell zeolite (S-1@TS-1) through the recrystallisation of silica and titanium species on the surface of hollow S-1 enriched with titanium atoms in the shell. This process facilitated the diffusion of components to the active site during the reaction process, thereby increasing the reactivity.155 In a study by Feng et al., the synthesis of TS-1 was conducted using UV irradiation, which resulted in the creation of additional Ti sites within the zeolite. This process led to the construction of a more inert Si shell layer, which inhibited the decomposition of H2O2 and the ring opening of epoxide during the diffusion of the product from the inner surface to the outer in the propylene epoxidation reaction.132 Chen et al. achieved the successful synthesis of TS-1 with uniform distributions of Ti atoms in the framework through the precise adjustment of the alkalinity of the organic precursor by a crystal dissolution crystallisation method. Their findings revealed the pivotal role of the states of the silica species in this process. As illustrated in Fig. 6, the pincer-like silica species in the Q2 and Q3 states contain a substantial number of Si–OH groups, which can effectively bind titanium atoms and form stable isolated Si–O–Ti structural units within the synthetic sol. These structural units remain unaltered throughout the liquid-phase conversion crystallisation process, protected by the silicon species. Consequently, the generation of framework-associated titanium species is suppressed, and the titanium atoms exhibit a uniform spatial distribution in the framework.153,156 Jiang et al. fabricated TS-1 devoid of an anatase phase, featuring abundant titanium species within its framework and a surface rich in titanium by employing a two-step crystallization strategy.64 The influence of the first step's crystallization temperature and duration on the properties of zeolite was investigated.| Sample | Strategy | Method | Advantage | Ref. |
|---|---|---|---|---|
| TS-1 | Hydrothermal treatment | S-1 zeolite is used as the silicon source | When the gel pH is higher, Ti is uniformly distributed in MFI. When the gel pH is low, Ti is mainly distributed on the outer surface of zeolite | 154 |
| TS-1 | Hydrothermal treatment | Recrystallization of silicon and titanium species on the surface of hollow S-1 zeolite | The leaching of framework Ti in the conventional solution-recrystallization process is avoided | 155 |
| TS-1 | Dissolution and recrystallization | The dissolution and recrystallization of zeolite was induced by functional mother liquor | The microenvironment of titanium species will change reversibly under different liquid–solid ratios | 88 |
| TS-1 | Ultraviolet light | Ultraviolet irradiation | UV irradiation can match the hydrolysis rate of Ti and Si precursors | 157 |
| TS-1 | Bottom-up approach | Precisely regulate the alkalinity of organic precursors | The Ti atoms in the skeleton are evenly distributed in TS-1 | 153 and 156 |
| Ti-MWW | Hydrothermal treatment | Heteroatom Al is incorporated | The introduction of Al exposes more active titanium species to the outer surface of zeolite and improves the accessibility of active Ti sites | 158 |
| TS-1 | Hydrothermal treatment | Two-step crystallization | Anatase free TS-1 with abundant skeleton titanium species and a titanium-rich surface was synthesized | 64 |
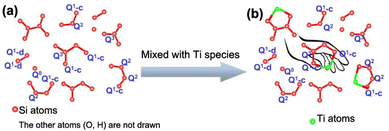 | ||
| Fig. 6 Possible structures of (a) silicon species and (b) interaction patterns between silicon and titanium species. Reproduced with permission.153 Copyright 2021, Elsevier. | ||
In addition to the zeolite framework atoms, heteroatoms can also modulate the spatial distribution of Ti species. In Ti-MWW, there are eight potential crystal sites within the framework. DFT calculations have demonstrated that Ti4+ in the form of Ti(OSi)4 within the framework exhibits a tendency to occupy the T1 and T3 sites. Conversely, the heteroatom B tends to occupy the T2 and T4 sites.159 It can be concluded that the mechanism of site competition between heteroatoms plays a regulatory role in the spatial distribution of Ti species.
Tang et al. employed a straightforward doping method to introduce Al into conventional Ti-MWW, resulting in the formation of Al-Ti-MWW with enhanced Ti species distributed on the zeolite surface.158 These zeolites exhibited superior catalytic activity compared to conventional Ti-MWW in cyclohexene epoxidation and cyclohexanone aminoximetation reactions. It has been reported that other researchers have identified T7 and T1 as the most suitable sites for Al in the MCM-22 framework, followed by T5, T3 and T4.160 In light of these findings, Wang et al.161 sought to regulate the spatial distribution of Ti species with precision. They observed that the influence of heteroatom B on the distribution of heteroatoms Ti and Al could be harnessed to achieve this. The Al-Ti-MWW demonstrated enhanced accessibility of active Ti sites due to the introduction of Al. This resulted in a greater relative amount of active Ti species exposed to the outer surface of the zeolite.
7. The reaction mechanism
The mechanism of the epoxidation of 1-hexene has been proposed by many groups. Linda J et al. proposed an Eley–Rideal-type reaction mechanism when capturing reaction kinetics data, as shown in Fig. 7. Firstly, the titanium species reacts with a hydrogen peroxide molecule to form the Ti–OOH species. Secondly, the Ti–OOH reacts with a physisorbed 1-hexene molecule to form chemisorbed 1,2-epoxyhexane and water. Lastly, the 1,2-epoxyhexane is desorbed, and the titanium species is regenerated. Shen et al. confirmed this mechanism through the specifically designed TS-1 and used it to catalyze the epoxidation of 1-hexene. They found that the presence of hierarchical pores in TS-1 was very important because the formation of the Ti–OOH species was a rate control step and suitable pore size reduced the barrier of mass transfer.162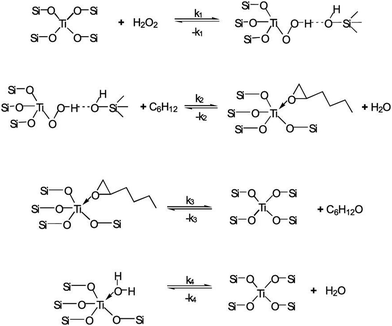 | ||
| Fig. 7 Proposed reaction mechanism for the liquid-phase epoxidation of 1-hexene in TS-1. Reproduced with permission.162 Copyright 2008, Elsevier. | ||
There are two views on the mechanism of cyclohexanone aminoximation: the hydroxylamine mechanism, and imine mechanism. The hydroxylamine mechanism is followed in reactions using water as a solvent while the imine mechanism is followed in reactions using acetic acid. For the former, the process begins with the interaction of ammonia and hydrogen peroxide with TS-1 to produce adsorbed ammonia and titanium peroxide, and then the intermediates react to form ammonium salts, which decompose to form hydroxylamine.
For the latter, the reaction pathway is shown in Fig. 8. TS-1 and hydrogen peroxide form Ti-OOH, and cyclohexanone reacts with ammonia to form imines. Then imines and Ti-OOH are produced directly to cyclohexanone oxime.163
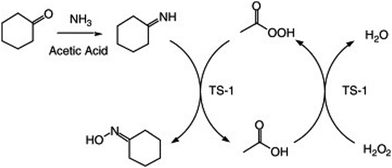 | ||
| Fig. 8 Proposed reaction pathway for cyclohexanone ammoximation using acetic acid as a solvent. Reproduced with permission.163 Copyright 2005, Elsevier. | ||
For propylene epoxidation, the reaction mechanism proposed by Clerici was generally accepted. This hypothesis also suggests that titanium species first react with hydrogen peroxide to form Ti–OOH. Methanol, 2-propanol and 2-butanol are commonly used as solvents in this reaction. In the case of a proton alcohol, ROH can coordinate with Ti–OOH to form a five-membered ring. Then, the electrophilic attack of the five-membered ring intermediate on the P-electron cloud of the double bond in propylene leads to the epoxidation of propylene. The pathway of formation of five-membered ring intermediates is shown in Fig. 9.
Using density functional theory, Wells made the first attempt to uniformly use the BPW91/LANL2DZ level of theory and a larger cluster model than in the original study to calculate the energy barriers for the rate control step for each of the five propylene epoxidation reaction mechanisms (Sinclair and Catlow56 mechanism, Vayssilov and van Santen57 mechanism, Munakata et al.58 mechanism, Ti/defective mechanism: partial silane alcohol nesting model51 and Ti/defect mechanism: full silanol nesting model48) and the results are summarised in Table 13.
| Mechanism and step | ΔEa (kcal mol−1) | ΔEZPEb (kcal mol−1) | ΔUc (kcal mol−1) | ΔGd (kcal mol−1) | Pre-exponential factors (s−1) |
|---|---|---|---|---|---|
| a The difference in the electronic energies of the transition state and the reactants at 0 K. b The difference in the electronic energies of the transition state and the reactants at 0 K with proper accounting of the zero point energy. c The difference in the internal energies of the transition state and the reactants at 298 K and 1 atm pressure. d The difference in the Gibbs free energies of the transition state and the reactants at 298 K and 1 atm pressure. e Note that all the energy differences correspond to the activation energies. Whenever the activation barrier is less than kBT, it is reported as negligible. | |||||
| Sinclair and Catlow for external site | |||||
| Hydroperoxy formation | 7.40 | 6.37 | 5.57 | 7.90 | 2.69 × 1012 |
| Epoxidation | 8.50 | 7.51 | 7.15 | 7.91 | 1.69 × 1013 |
| Vayssilov and van Santen | |||||
| Epoxidation | 19.03 | 17.96 | 18.37 | 19.16 | 4.87 × 1012 |
| Munakata et al. | |||||
| First step in peroxo formation | 21.22 | 20.21 | 20.44 | 19.80 | 6.85 × 1013 |
| Second step in peroxo formation | 7.28 | 6.22 | 6.90 | 4.31 | 9.21 × 1014 |
| Epoxidation | 1.20 | Negligible | 1.00 | Negligible | 4.52 × 1014 |
| Defect (partial silanol nest) | |||||
| Hydroperoxy formation | 15.39 | 13.71 | 13.92 | 14.49 | 2.89 × 1013 |
| Epoxidation | 12.98 | 12.32 | 11.89 | 14.26 | 7.01 × 1011 |
| Defect (full silanol nest) | |||||
| Hydroperoxy formation | 10.09 | 8.83 | 8.26 | 8.92 | 4.45 × 1013 |
| Epoxidation | 6.12 | 5.50 | 6.53 | 4.62 | 7.74 × 1013 |
| Gas-phase (noncatalytic) | |||||
| Epoxidation | 21.09 | 19.77 | 18.98 | 22.40 | 6.87 × 1011 |
When TS-1 is used as the catalyst, the reactants of the reaction have no obvious diffusion limitation. Steric hindrance mainly comes from five-membered rings. The larger the molecular size of alcohol, the larger the steric hindrance of the intermediate. Therefore, from the point of view of spatial factors, the reactivity increases with the decrease in solvent molecular size.48
In summary, in the Ti/H2O2 catalytic system, Ti–OOH is often used as the reactive intermediate, and the properties of Ti–OOH determine the catalytic activity of titanosilicate zeolite in most reactions. The intermediates produced by different titanium species would have different activity. However, the current research on the mechanism mostly takes Ti(OSi)4 as an example. In order to have a clearer understanding of the reaction mechanism and a more accurate regulation of the catalytic activity of titanosilicate zeolite, it is necessary to have advanced characterization techniques and precise regulation strategies of titanium species.
8. Conclusion and perspective
Both titanium species in the framework and framework-associated titanium silicalite are active centers for catalytic reactions using H2O as an oxidant. The active sites in titanium silicalite are highly abundant, facilitating the generation of Ti–OOH oxygen intermediates through multiple titanium species, thereby promoting chemical reaction processes. The following conclusions, challenges, and future directions accompany their synthesis and catalytic applications.(1) The titanium content in titanium-silicalite zeolites is subject to an upper limit, making it crucial to selectively synthesize specific titanium species for enhancing its content. To achieve precise regulation of Ti species, a range of strategies have been developed, including the design of template agents, introduction of crystallization regulators, implementation of post-treatment methods, utilization of seed-assisted crystallization with specialized silicon or titanium sources, adoption of a two-step crystallization method and application of microwave radiation. The aforementioned strategies have resulted in the generation of titanium species with diverse requirements, which can be verified through characterization techniques. The adjustable titanium species exhibit distinct coordination structures and microenvironments, enabling reversible transformation between these species and enhancing the relative content of the dominant titanium species to achieve optimal catalytic activity. Consequently, titanium species play a crucial role as materials in the field of chemistry and catalysis.
(2) Titanium species have the potential to modulate zeolite acidity owing to their variable electron cloud structure. However, the precise mechanism by which titanium species participate in catalytic reactions remains unclear. The majority of current hypotheses and evidence suggest that Ti–OOH is formed through the interaction between titanium species and hydrogen peroxide. Nevertheless, further research is necessary to validate this hypothesis. The lack of perfect characterization technology has posed significant challenges in detecting TiO5 and novel titanium species. Consequently, there is an urgent need to update characterization technology to gain a deeper understanding of the structure and microenvironment of these titanium species. In the future, we should continue improving the precise regulation system for titanium species, exploring more strategies for regulating TiO5 and new titanium species, as well as considering advancements in spectroscopic and microscopic techniques to reveal the fine structure of titanium species.
Each regulatory strategy for titanium species has its own set of advantages and disadvantages, catering exclusively to specific types or a limited number of titanium structures. Enhancing existing synthetic methodologies, devising novel synthetic approaches, and unearthing a synthesis strategy tailored for individual titanium active sites represent an inspiring avenue of research that effectively bridges the gap between catalyst structure and function.
Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Conflicts of interest
The authors declare no competing financial interests.Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No: 22211540711, 21978198 and 22002052). This work was supported by the China Postdoctoral Science Foundation (Certificate Number: 2023M742998).References
- M. Taramasso, G. Perego and B. Notari, Preparation of porous crystalline synthetic material comprised of silicon and titanium oxides, 1983.
- W. Fan, R.-G. Duan, T. Yokoi, P. Wu, Y. Kubota and T. Tatsumi, Synthesis, crystallization mechanism, and catalytic properties of titanium-rich TS-1 free of extraframework titanium species, J. Am. Chem. Soc., 2008, 130, 10150–10164 CrossRef CAS
.
- M. Capel-Sanchez, J. Campos-Martin and J. Fierro, Impregnation treatments of TS-1 catalysts and their relevance in alkene epoxidation with hydrogen peroxide, Appl. Catal., A, 2003, 246, 69–77 CrossRef CAS
.
- M. Mantegazza, G. Petrini, G. Spano, R. Bagatin and F. Rivetti, Selective oxidations with hydrogen peroxide and titanium silicalite catalyst, J. Mol. Catal. A: Chem., 1999, 146, 223–228 CrossRef CAS
.
- D. Huybrechts, L. D. Bruycker and P. Jacobs, Oxyfunctionalization of alkanes with hydrogen peroxide on titanium silicalite, Nature, 1990, 345, 240–242 CrossRef CAS
.
- J. Wang, Y. Zhao, T. Yokoi, J. N. Kondo and T. Tatsumi, High-Performance Titanosilicate Catalyst Obtained through Combination of Liquid-Phase and Solid-Phase Transformation Mechanisms, ChemCatChem, 2014, 6, 2719–2726 CrossRef CAS
.
- P. Roffia, M. Padovan, E. Moretti and G. De Alberti, Catalytic process for preparing cyclohexanone-oxime, 1988.
- P. Roffia, G. Leofanti, A. Cesana, M. Mantegazza, M. Padovan, G. Petrini, S. Tonti and P. Gervasutti, Cyclohexanone ammoximation: a break through in the 6-caprolactam production process, Stud. Surf. Sci. Catal., 1990, 55, 43–52 CrossRef CAS
.
- D. Bianchi, R. D’Aloisio, R. Bortolo and M. Ricci, Oxidation of mono-and bicyclic aromatic compounds with hydrogen peroxide catalyzed by titanium silicalites TS-1 and TS-1B, Appl. Catal., A, 2007, 327, 295–299 CrossRef CAS
.
- Y. Li, Q. Fan, Y. Li, X. Feng, Y. Chai and C. Liu, Seed-assisted synthesis of hierarchical nanosized TS-1 in a low-cost system for propylene epoxidation with H2O2, Appl. Surf. Sci., 2019, 483, 652–660 CrossRef CAS
.
- A. Wróblewska and A. Fajdek, Epoxidation of allyl alcohol to glycidol over the microporous TS-1 catalyst, J. Hazard. Mater., 2010, 179, 258–265 CrossRef
.
- M. G. Clerici and P. Ingallina, Epoxidation of lower olefins with hydrogen peroxide and titanium silicalite, J. Catal., 1993, 140, 71–83 CrossRef CAS
.
- X. Wang, X. Zhang, Y. Wang, H. Liu, J. Qiu, J. Wang, W. Han and K. L. Yeung, Performance of TS-1-coated structured packing materials for styrene oxidation reaction, ACS Catal., 2011, 1, 437–445 CrossRef CAS
.
- Y. Kuwahara, K. Nishizawa, T. Nakajima, T. Kamegawa, K. Mori and H. Yamashita, Enhanced catalytic activity on titanosilicate molecular sieves controlled by cation−π interactions, J. Am. Chem. Soc., 2011, 133, 12462–12465 CrossRef CAS PubMed
.
- P. J. Cordeiro and T. D. Tilley, Enhancement of the catalytic activity of titanium-based terminal olefin epoxidation catalysts via surface modification with functionalized protic molecules, ACS Catal., 2011, 1, 455–467 CrossRef CAS
.
- M. Signorile, V. Crocella, A. Damin, B. Rossi, C. Lamberti, F. Bonino and S. Bordiga, Effect of Ti speciation on catalytic performance of TS-1 in the hydrogen peroxide to propylene oxide reaction, J. Phys. Chem. C, 2018, 122, 9021–9034 CrossRef CAS
.
- F. Schmidt, M. Bernhard, H. Morell and M. Pascaly, HPPO process technology a novel route to propylene oxide without coproducts, Chim. Oggi, 2014, 32, 31–35 CAS
.
- V. Russo, R. Tesser, E. Santacesaria and M. Di Serio, Kinetics of propene
oxide production via hydrogen peroxide with TS-1, Ind. Eng. Chem. Res., 2014, 53, 6274–6287 CrossRef CAS
.
- V. Russo, R. Tesser, E. Santacesaria and M. Di Serio, Chemical and technical aspects of propene oxide production via hydrogen peroxide (HPPO process), Ind. Eng. Chem. Res., 2013, 52, 1168–1178 CrossRef CAS
.
- L. Bonoldi, C. Busetto, A. Congiu, G. Marra, G. Ranghino, M. Salvalaggio, G. Spano and E. Giamello, An ESR study of titanium-silicalite in presence of H2O2, Spectrochim. Acta, Part A, 2002, 58, 1143–1154 CrossRef CAS
.
- G. Lv, S. Deng, Z. Yi, X. Zhang, F. Wang, H. Li and Y. Zhu, One-pot synthesis of framework W-doped TS-1 zeolite with robust Lewis acidity for effective oxidative desulfurization, Chem. Commun., 2019, 55, 4885–4888 RSC
.
- L. Kong, G. Li and X. Wang, Mild oxidation of thiophene over TS-1/H2O2, Catal. Today, 2004, 93–95, 341–345 CrossRef CAS
.
- G. Gao, S. Cheng, Y. An, X. Si, X. Fu, Y. Liu, H. Zhang, P. Wu and M. Y. He, Oxidative desulfurization of aromatic sulfur compounds over titanosilicates, ChemCatChem, 2010, 2, 459–466 CrossRef CAS
.
- Q. Lv, G. Li and H. Sun, Synthesis of hierarchical TS-1 with convenient separation and the application for the oxidative desulfurization of bulky and small reactants, Fuel, 2014, 130, 70–75 CrossRef CAS
.
- C. Shen, Y. Wang, J. Xu and G. Luo, Synthesis of TS-1 on porous glass beads for catalytic oxidative desulfurization, Chem. Eng. J., 2015, 259, 552–561 CrossRef CAS
.
- Y. Li, L. Li and J. Yu, Applications of Zeolites in Sustainable Chemistry, Chem, 2017, 3, 928–949 CAS
.
- M. Shamzhy, M. Opanasenko, P. Concepción and A. Martínez, New trends in tailoring active sites in zeolite-based catalysts, Chem. Soc. Rev., 2019, 48, 1095–1149 RSC
.
- J. Liang, Z. Liang, R. Zou and Y. Zhao, Heterogeneous catalysis in zeolites, mesoporous silica, and metal–organic frameworks, Adv. Mater., 2017, 29, 1701139 CrossRef PubMed
.
- A. Corma, State of the art and future challenges of zeolites as catalysts, J. Catal., 2003, 216, 298–312 CrossRef CAS
.
- D. H. Wells Jr, W. N. Delgass Jr and K. T. Thomson, Evidence of Defect-Promoted Reactivity for Epoxidation of Propylene in Titanosilicate (TS-1) Catalysts
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A DFT Study, J. Am. Chem. Soc., 2004, 126, 2956–2962 CrossRef
A DFT Study, J. Am. Chem. Soc., 2004, 126, 2956–2962 CrossRef .
- C. Martínez and A. Corma, Inorganic molecular sieves: Preparation, modification and industrial application in catalytic processes, Coord. Chem. Rev., 2011, 255, 1558–1580 CrossRef
.
- M. Campanati, G. Fornasari and A. Vaccari, Fundamentals in the preparation of heterogeneous catalysts, Catal. Today, 2003, 77, 299–314 CrossRef CAS
.
- P. Ratnasamy, D. Srinivas and H. Knözinger, Active sites and reactive intermediates in titanium silicate molecular sieves, Adv. Catal., 2004, 1–169 CAS
.
- S. Bordiga, F. Bonino, A. Damin and C. Lamberti, Reactivity of Ti(IV) species hosted in TS-1 towards H2O2–H2O solutions investigated by ab initio cluster and periodic approaches combined with experimental XANES and EXAFS data: a review and new highlights, Phys. Chem. Chem. Phys., 2007, 9, 4854–4878 RSC
.
- G. Tozzola, M. Mantegazza, G. Ranghino, G. Petrini, S. Bordiga, G. Ricchiardi, C. Lamberti, R. Zulian and A. Zecchina, On the structure of the active site of Ti-silicalite in reactions with hydrogen peroxide: A vibrational and computational study, J. Catal., 1998, 179, 64–71 CrossRef CAS
.
- S. Bordiga, A. Damin, F. Bonino, G. Ricchiardi, C. Lamberti and A. Zecchina, The Structure of the Peroxo Species in the TS-1 Catalyst as Investigated by Resonant Raman Spectroscopy, Angew. Chem., Int. Ed., 2002, 41, 4734–4737 CrossRef CAS PubMed
.
- F. Bonino, A. Damin, G. Ricchiardi, M. Ricci, G. Spanò, R. D'Aloisio, A. Zecchina, C. Lamberti, C. Prestipino and S. Bordiga, Ti-peroxo species in the TS-1/H2O2/H2O system, J. Phys. Chem. B, 2004, 108, 3573–3583 CrossRef CAS
.
- C. P. Gordon, H. Engler, A. S. Tragl, M. Plodinec, T. Lunkenbein, A. Berkessel, J. H. Teles, A.-N. Parvulescu and C. Copéret, Efficient epoxidation over dinuclear sites in titanium silicalite-1, Nature, 2020, 586, 708–713 CrossRef CAS PubMed
.
- Y. Wang, H. Yang, Y. Zuo, D. Tian, G. Hou, Y. Su, Z. Feng, X. Guo and C. Li, New penta-and hexa-coordinated titanium sites in titanium silicalite-1 catalyst for propylene epoxidation, Appl. Catal., B, 2023, 325, 122396 CrossRef CAS
.
- L. Wu, X. Deng, S. Zhao, H. Yin, Z. Zhuo, X. Fang, Y. Liu and M. He, Synthesis of a highly active oxidation catalyst with improved distribution of titanium coordination states, Chem. Commun., 2016, 52, 8679–8682 RSC
.
- L. Wu, Z. Tang, Y. Yu, X. Yao, W. Liu, L. Li, B. Yan, Y. Liu and M. He, Facile synthesis of a high-performance titanosilicate catalyst with controllable defective Ti(OSi)3OH sites, Chem. Commun., 2018, 54, 6384–6387 RSC
.
- W. Xu, T. Zhang, R. Bai, P. Zhang and J. Yu, A one-step rapid synthesis of TS-1 zeolites with highly catalytically active mononuclear TiO6 species, J. Mater. Chem. A, 2020, 8, 9677–9683 RSC
.
- R. Bai, M. T. Navarro, Y. Song, T. Zhang, Y. Zou, Z. Feng, P. Zhang, A. Corma and J. Yu, Titanosilicate zeolite precursors for highly efficient oxidation reactions, Chem. Sci., 2020, 11, 12341–12349 RSC
.
- Y. Wang, H. Yang, Y. Zuo, Z. Feng, X. Guo and C. Li, Macro-meso-microporous titanium silicalite-1 catalyst featuring self-assembled nanocrystals and multiple active species for propylene epoxidation, Chem. Eng. Sci., 2023, 280, 118981 CrossRef CAS
.
- Y. Yu, R. Wang, W. Liu, Z. Chen, H. Liu, X. Huang, Z. Tang, Y. Liu and M. He, Control of Ti active-site microenvironment in titanosilicate catalysts and its effect on oxidation pathways, Appl. Catal., A, 2021, 610 Search PubMed
.
- D. T. Bregante, A. M. Johnson, A. Y. Patel, E. Z. Ayla, M. J. Cordon, B. C. Bukowski, J. Greeley, R. Gounder and D. W. Flaherty, Cooperative effects between hydrophilic pores and solvents: catalytic consequences of hydrogen bonding on alkene epoxidation in zeolites, J. Am. Chem. Soc., 2019, 141, 7302–7319 CrossRef CAS
.
- L. Wang, J. Sun, X. Meng, W. Zhang, J. Zhang, S. Pan, Z. Shen and F.-S. Xiao, A significant enhancement of catalytic activities in oxidation with H2O2 over the TS-1 zeolite by adjusting the catalyst wettability, Chem. Commun., 2014, 50, 2012–2014 RSC
.
- D. H. Wells, A. M. Joshi, W. N. Delgass and K. T. Thomson, A quantum chemical study of comparison of various propylene epoxidation mechanisms using H2O2 and TS-1 catalyst, J. Phys. Chem. B, 2006, 110, 14627–14639 CrossRef CAS PubMed
.
- T. Ge, Z. Hua, J. Lv, J. Zhou, H. Guo, J. Zhou and J. Shi, Hydrophilicity/hydrophobicity modulated synthesis of nano-crystalline and hierarchically structured TS-1 zeolites, CrystEngComm, 2017, 19, 1370–1376 RSC
.
- D. H. Wells, A. M. Joshi, W. N. Delgass and K. T. Thomson, Thomson, A Quantum Chemical Study of Comparison of Various Propylene Epoxidation Mechanisms Using H2O2 and TS-1 Catalyst, J. Phys. Chem. B, 2006, 110, 14627–14639 CrossRef CAS PubMed
.
- D. H. Wells, A. M. Joshi, W. N. Delgass and K. T. Thomson, Evidence of defect-promoted reactivity for epoxidation of propylene in titanosilicate (TS-1) catalysts: a DFT study, J. Am. Chem. Soc., 2004, 126, 2956–2962 CrossRef CAS PubMed
.
- Q. Guo, K. Sun, Z. Feng, G. Li, M. Guo, F. Fan and C. Li, A thorough investigation of the active titanium species in TS-1 zeolite by in situ UV resonance Raman spectroscopy, Chem. – Eur. J., 2012, 18, 13854–13860 CrossRef CAS PubMed
.
- J. Zhuang, G. Yang, D. Ma, X. Lan, X. Liu, X. Han, X. Bao and U. Mueller, In situ magnetic resonance investigation of styrene oxidation over TS-1 zeolites, Angew. Chem., Int. Ed., 2004, 43, 6377–6381 CrossRef CAS
.
- L. Wang, G. Xiong, J. Su, P. Li and H. Guo, In Situ UV Raman Spectroscopic Study on the Reaction Intermediates for Propylene Epoxidation on TS-1, J. Phys. Chem. C, 2012, 116, 9122–9131 CrossRef CAS
.
- W. Lin and H. Frei, Photochemical and FT-IR probing of the active site of hydrogen peroxide in Ti silicalite sieve, J. Am. Chem. Soc., 2002, 124, 9292–9298 CrossRef CAS
.
- P. E. Sinclair and C. R. A. Catlow, Quantum chemical study of the mechanism of partial oxidation reactivity in titanosilicate catalysts: active site formation, oxygen transfer, and catalyst deactivation, J. Phys. Chem. B, 1999, 103, 1084–1095 CrossRef CAS
.
- G. N. Vayssilov and R. A. Van Santen, Catalytic activity of titanium silicalites—A DFT study, J. Catal., 1998, 175, 170–174 CrossRef CAS
.
- H. Munakata, Y. Oumi and A. Miyamoto, A DFT study on peroxo-complex in titanosilicate catalyst: hydrogen peroxide activation on titanosilicalite-1 catalyst and reaction mechanisms for catalytic olefin epoxidation and for hydroxylamine formation from ammonia, J. Phys. Chem. B, 2001, 105, 3493–3501 CrossRef CAS
.
- X. Fang, L. Wu, Y. Yu, L. Sun and Y. Liu, Improving the catalytic performance of TS-1 through Zn(AC)2 modification, Catal. Commun., 2018, 114, 1–5 CrossRef CAS
.
- X. Fang, Q. Wang, A. Zheng, Y. Liu, Y. Wang, X. Deng, H. Wu, F. Deng, M. He and P. Wu, Fluorine-planted titanosilicate with enhanced catalytic activity in alkene epoxidation with hydrogen peroxide, Catal. Sci. Technol., 2012, 2, 2433–2435 RSC
.
- X. Fang, Q. Wang, A. Zheng, Y. Liu, L. Lin, H. Wu, F. Deng, M. He and P. Wu, Post-synthesis, characterization and catalytic properties of fluorine-planted MWW-type titanosilicate, Phys. Chem. Chem. Phys., 2013, 15, 4930–4938 RSC
.
- H. Xu, Y. Guan, X. Lu, J. Yin, X. Li, D. Zhou and P. Wu, Relation of Selective Oxidation Catalytic Performance to Microenvironment of TiIV Active Site Based on Isotopic Labeling, ACS Catal., 2020, 10, 4813–4819 CrossRef CAS
.
- Z. Tang, Y. Yu, W. Liu, Z. Chen, R. Wang, H. Liu, H. Wu, Y. Liu and M. He, Deboronation-assisted construction of defective Ti(OSi)3OH species in MWW-type titanosilicate and their enhanced catalytic performance, Catal. Sci. Technol., 2020, 10, 2905–2915 RSC
.
- L. Jiang, F. Wang, Y. Zhai, K. Wang, Z. Xu, X. Zhang, M. Li, M. Li and X. Zhang, Two-step synthesis of Ti-rich surface TS-1 with controllable microenvironment of titanium species, Appl. Catal., A, 2023, 651, 119023 CrossRef CAS
.
- D. Srinivas, P. Manikandan, S. Laha, R. Kumar and P. Ratnasamy, Reactive oxo-titanium species in titanosilicate molecular sieves: EPR investigations and structure–activity correlations, J. Catal., 2003, 217, 160–171 CAS
.
- C. Niu, X. Yang, Q. Zhang, Y. Zhang, X. Qin, Y. Tang, H. Wei, X. Gao, Y. Liu and X. Wang, Enhanced catalytic activity and catalyst stability in cyclohexanone ammoximation by introducing anatase into TS-1 zeolite, Microporous Mesoporous Mater., 2023, 351, 112467 CrossRef CAS
.
- L. Kong, G. Li and X. Wang, Mild oxidation of thiophene over TS-1/H2O2, Catal. Today, 2004, 93, 341–345 CrossRef
.
- K. Chaudhari, D. Srinivas and P. Ratnasamy, Reactive oxygen species in titanosilicates TS-1 and TiMCM-41: an in situ EPR spectroscopic study, J. Catal., 2001, 203, 25–32 CrossRef CAS
.
- T. Blasco, M. Camblor, A. Corma, P. Esteve, J. Guil, A. Martinez, J. Perdigon-Melon and S. Valencia, Direct synthesis and characterization of hydrophobic aluminum-free Ti− beta zeolite, J. Phys. Chem. B, 1998, 102, 75–88 CrossRef CAS
.
- L. L. Noc, O. D. Trong, S. Solomykina, B. Echchahed, C. Bel F. dit Moulin and L. Bonneviot, Stud. Surf. Sci. Catal., 1996, 101, 611 CrossRef
.
- A. Zecchina, S. Bordiga, C. Lamberti, G. Ricchiardi, D. Scarano, G. Petrini, G. Leofanti and M. Mantegazza, Structural characterization of Ti centres in Ti-silicalite and reaction mechanisms in cyclohexanone ammoximation, Catal. Today, 1996, 32, 97–106 CrossRef CAS
.
- S. Bordiga, F. Boscherini, S. Coluccia, F. Genonic, C. Lamberti, G. Leofanti, L. Marchese, G. Petrini, G. Vlaic and A. Zecchina, XAFS study of Ti-silicalite: structure of framework Ti (IV) in presence and in absence of reactive molecules (H2O, NH3), Catal. Lett., 1994, 26, 195–208 CrossRef CAS
.
- M. Boccuti, K. Rao, A. Zecchina, G. Leofanti and G. Petrini, Spectroscopic characterization of silicalite and titanium-silicalite, Stud. Surf. Sci. Catal., 1989, 48, 133–144 CrossRef
.
- J. Klaas, K. Kulawik, G. Schulz-Ekloff and N. I. Jaeger, Comparative spectroscopic study of TS-1 and zeolite-hosted extraframework titanium oxide dispersions, Stud. Surf. Sci. Catal., 1994, 84, 2261–2268 CrossRef CAS
.
- G. Coudurier, C. Naccache and J. C. Vedrine, Uses of ir spectroscopy in identifying ZSM zeolite structure, J. Chem. Soc., Chem. Commun., 1982, 1413–1415 RSC
.
- S. L. Burkett and M. E. Davis, Mechanism of structure direction in the synthesis of Si-ZSM-5: an investigation by intermolecular 1H-29Si CP MAS NMR, J. Phys. Chem., 1994, 98, 4647–4653 CrossRef CAS
.
- T. Zhang, Y. Zuo, M. Liu, C. Song and X. Guo, Synthesis of titanium silicalite-1 with high catalytic performance for 1-butene epoxidation by eliminating the extraframework Ti, ACS Omega, 2016, 1, 1034–1040 CrossRef CAS PubMed
.
- Y. Zuo, M. Liu, M. Ma, Y. Wang, X. Guo and C. Song, Enhanced Catalytic Activity on Post-Synthesized Hollow Titanium Silicalite-1 with High Titanium Content on the External Surface, ChemistrySelect, 2016, 1, 6160–6166 CrossRef CAS
.
- P. Kumar, J. Gupta, G. Muralidhar and T. P. Rao, Acidity studies on titanium silicalites-1 (TS-1) by ammonia adsorption using microcalorimetry, Stud. Surf. Sci. Catal., 1998, 113, 463–472 CrossRef CAS
.
- A. J. de Man and J. Sauer, Coordination, structure, and vibrational spectra of titanium in silicates and zeolites in comparison with related molecules. An ab initio study, J. Phys. Chem., 1996, 100, 5025–5034 CrossRef CAS
.
- M. Camblor, A. Corma and J. Perez-Pariente, Infrared spectroscopic investigation of titanium in zeolites. A new assignment of the 960 cm−1 band, J. Chem. Soc., Chem. Commun., 1993, 557–559 RSC
.
- S. A. Asher, UV resonance Raman spectroscopy for analytical, physical, and biophysical chemistry. Part 2, Anal. Chem., 1993, 65, 201A–210A CrossRef CAS PubMed
.
- P. C. Stair and C. Li, Ultraviolet Raman spectroscopy of catalysts and other solids, J. Vac. Sci. Technol., A, 1997, 15, 1679–1684 CrossRef CAS
.
- C. Li, G. Xiong, Q. Xin, Jk Liu, Pl Ying, Zc Feng, J. Li, W. B. Yang, Y. Z. Wang and G. R. Wang, UV resonance Raman spectroscopic identification of titanium atoms in the framework of TS-1 zeolite, Angew. Chem., Int. Ed., 1999, 38, 2220–2222 CrossRef CAS PubMed
.
- C. Li and P. C. Stair, An advance in Raman studies of catalysts: ultraviolet resonance Raman spectroscopy, Stud. Surf. Sci. Catal., 1996, 101, 881–890 CrossRef CAS
.
- D. T. On, L. Bonneviot, A. Bittar, A. Sayari and S. Kaliaguine, Titanium sites in titanium silicalites: an XPS, XANES and EXAFS study, J. Mol. Catal., 1992, 74, 233–246 CrossRef CAS
.
-
L. Le Noc, T. D. On and S. Solomykina, Characterization of two different framework titanium sites and quantification of extra-framework species in TS-1 silicalites, Elsevier Science, New York, NY (United States), 1996 Search PubMed
.
- Y. Zhai, F. Wang, X. Zhang, G. Lv, Z. Zhu, K. Wang, Z. Xu and L. Jiang, Functional mother liquor reversed titanium species for the green synthesis of anatase-free hollow TS-1 with tunable titanium micro-environment via a kinetic-thermodynamic co-regulatory pathway, Mater. Chem. Front., 2022, 6, 2072–2084 RSC
.
- S. Bordiga, A. Damin, F. Bonino, G. Ricchiardi, A. Zecchina, R. Tagliapietra and C. Lamberti, Resonance Raman effects in TS-1: the structure of Ti (IV) species and reactivity towards H2O, NH3 and H2O2: an in situ study, Phys. Chem. Chem. Phys., 2003, 5, 4390–4393 RSC
.
- C. Lamberti, S. Bordiga, A. Zecchina, G. Vlaic, G. Tozzola, G. Petrini and A. Carati, XAFS, IR, Raman and UV-Vis characterization of framework Ti(IV) species in Ti-silicalites, J. Phys. IV, 1997, 7, C2-851 Search PubMed
.
- G. N. Vayssilov, Structural and physicochemical features of titanium silicalites, Catal. Rev.: Sci. Eng., 1997, 39, 209–251 CrossRef CAS
.
- L. Marchese, T. Maschmeyer, E. Gianotti, S. Coluccia and J. M. Thomas, Probing the titanium sites in Ti−MCM41 by diffuse reflectance and photoluminescence UV−vis spectroscopies, J. Phys. Chem. B, 1997, 101, 8836–8838 CrossRef CAS
.
- L. Marchese, E. Gianotti, V. Dellarocca, T. Maschmeyer, F. Rey, S. Coluccia and J. M. Thomas, Structure–functionality relationships of grafted Ti-MCM41 silicas. Spectroscopic and catalytic studies, Phys. Chem. Chem. Phys., 1999, 1, 585–592 RSC
.
- C. Lamberti, S. Bordiga, D. Arduino, A. Zecchina, F. Geobaldo, G. Spano, F. Genoni, G. Petrini, A. Carati and F. Villain, Evidence of the presence of two different framework Ti (IV) species in Ti−silicalite-1 in vacuo conditions: an EXAFS and a photoluminescence study, J. Phys. Chem. B, 1998, 102, 6382–6390 CrossRef CAS
.
- Y. Wang, L. Li, R. Bai, S. Gao, Z. Feng, Q. Zhang and J. Yu, Amino acid-assisted synthesis of TS-1 zeolites containing highly catalytically active TiO6 species, Chinese J. Catal., 2021, 42, 2189–2196 CrossRef CAS
.
- W. Xu, T. Zhang, R. Bai, P. Zhang and J. Yu, A one-step rapid synthesis of TS-1 zeolites with highly catalytically active mononuclear TiO6 species, J. Mater. Chem. A, 2020, 8, 9677–9683 RSC
.
- J. Wang, Z. Chen, Y. Yu, Z. Tang, K. Shen, R. Wang, H. Liu, X. Huang and Y. Liu, Hollow core–shell structured TS-1@ S-1 as an efficient catalyst for alkene epoxidation, RSC Adv., 2019, 9, 37801–37808 RSC
.
- C. Zhang, G. Wang, Y. Li, G. Wu, H. Guo and Z. Feng, Effect of Phosphates Modification on TS-1 Zeolite and its Catalytic Performance in Epoxidation of Propylene and Hydrogen Peroxide, J. Mol. Catal., 2021, 35, 1–12 CAS
.
- X. Fan, W. Hu, S. Jin, G. Tao, Z. Tang, C. Wang, H. Sun and W. Yang, Effect of P modification on the structure and catalytic performance of Ti-MWW zeolite, Microporous Mesoporous Mater., 2022, 336 Search PubMed
.
- S. Zhang, S. Jin, G. Tao, Z. Wang, W. Liu, Y. Chen, J. Luo, B. Zhang, H. Sun and Y. Wang, The evolution of titanium species in boron-containing Ti-MWW zeolite during post-treatment revealed by UV resonance Raman spectroscopy, Microporous Mesoporous Mater., 2017, 253, 183–190 CrossRef CAS
.
- X. Fang, Q. Wang, A. Zheng, Y. Liu, Y. Wang, X. Deng, H. Wu, F. Deng, M. He and P. Wu, Fluorine-planted titanosilicate with enhanced catalytic activity in alkene epoxidation with hydrogen peroxide, Catal. Sci. Technol., 2012, 2 Search PubMed
.
- X. Fang, Q. Wang, A. Zheng, Y. Liu, L. Lin, H. Wu, F. Deng, M. He and P. Wu, Post-synthesis, characterization and catalytic properties of fluorine-planted MWW-type titanosilicate, Phys. Chem. Chem. Phys., 2013, 15 Search PubMed
.
- M. Tamura, W. Chaikittisilp, T. Yokoi and T. Okubo, Incorporation process of Ti species into the framework of MFI type zeolite, Microporous Mesoporous Mater., 2008, 112, 202–210 CrossRef CAS
.
- J. Xu, Z. Zhang, D. Yu, W. Du, N. Song, X. Duan and X. Zhou, Au/TS-1 catalyst for propylene epoxidation with H2 and O2: Effect of surface property and morphology of TS-1 zeolite, Nano Res., 2023, 16, 6278–6289 CrossRef CAS
.
- T. Maschmeyer, F. Rey, G. Sankar and J. M. Thomas, Heterogeneous catalysts obtained by grafting metallocene complexes onto mesoporous silica, Nature, 1995, 378, 159–162 CrossRef CAS
.
- P. Wu, T. Tatsumi, T. Komatsu and T. Yashima, A novel titanosilicate with MWW structure. I. Hydrothermal synthesis, elimination of extraframework titanium, and characterizations, J. Phys. Chem. B, 2001, 105, 2897–2905 CrossRef CAS
.
- S. Jin, Z. Wang, G. Tao, S. Zhang, W. Liu, W. Fu, B. Zhang, H. Sun, Y. Wang and W. Yang, UV resonance Raman spectroscopic insight into titanium species and structure-performance relationship in boron-free Ti-MWW zeolite, J. Catal., 2017, 353, 305–314 CrossRef CAS
.
- X. Fan, W. Hu, S. Jin, G. Tao, Z. Tang, C. Wang, H. Sun and W. Yang, Effect of P modification on the structure and catalytic performance of Ti-MWW zeolite, Microporous Mesoporous Mater., 2022, 336, 111887 CrossRef CAS
.
- S. Zhang, S. Jin, G. Tao, Z. Wang, W. Liu, Y. Chen, J. Luo, B. Zhang, H. Sun, Y. Wang and W. Yang, The evolution of titanium
species in boron-containing Ti-MWW zeolite during post-treatment revealed by UV resonance Raman spectroscopy, Microporous Mesoporous Mater., 2017, 253, 183–190 CrossRef CAS
.
- J. Zhang, S. Jin, D. Deng, W. Liu, G. Tao, Q. Luo, H. Sun and W. Yang, Insight into the formation of framework titanium species during acid treatment of MWW-type titanosilicate and the effect of framework titanium state on olefin epoxidation, Microporous Mesoporous Mater., 2021, 314, 110862 CrossRef CAS
.
- J. Su, G. Xiong, J. Zhou, W. Liu, D. Zhou, G. Wang, X. Wang and H. Guo, Amorphous Ti species in titanium silicalite-1: Structural features, chemical properties, and inactivation with sulfosalt, J. Catal., 2012, 288, 1–7 CrossRef CAS
.
- D. Bianchi, L. Balducci, R. Bortolo, R. D'Aloisio, M. Ricci, G. Spanò, R. Tassinari, C. Tonini and R. Ungarelli, Oxidation of benzene to phenol with hydrogen peroxide catalyzed by a modified titanium silicalite (TS-1B), Adv. Synth. Catal., 2007, 349, 979–986 CrossRef CAS
.
- F.-Z. Zhang, X.-W. Guo, X.-S. Wang, G. Li, J.-C. Zhou, J.-Q. Yu and C. Li, The active sites in different TS-1 zeolites for propylene epoxidation studied by ultraviolet resonance Raman and ultraviolet visible absorption spectroscopies, Catal. Lett., 2001, 72, 235–239 CrossRef CAS
.
- Q. Guo, K. Sun, Z. Feng, G. Li, M. Guo, F. Fan and C. Li, A Thorough Investigation of the Active Titanium Species in TS-1 Zeolite by In Situ UV Resonance Raman Spectroscopy, Chem. - Eur. J., 2012, 18, 13854–13860 CrossRef CAS PubMed
.
- Y. Wang, H. Yang, G. Hou, B. Zhang, Z. Feng, X. Guo and C. Li, Catalytically active species in bifunctional Aluminum-Containing titanium Silicalite-1 zeolite for ethylene epoxidation, Chem. Eng. Sci., 2023, 282 Search PubMed
.
- N. A. Grosso-Giordano, A. S. Hoffman, A. Boubnov, D. W. Small, S. R. Bare, S. I. Zones and A. Katz, Dynamic reorganization and confinement of TiIV active sites controls olefin epoxidation catalysis on two-dimensional zeotypes, J. Am. Chem. Soc., 2019, 141, 7090–7106 CrossRef CAS PubMed
.
- M. Zhang, Y. Zuo, T. Li, M. Liu, H. Yang and X. Guo, Kinetics simulation of propylene epoxidation over different Ti species in TS-1, AlChE J., 2021, 67, e17261 CrossRef CAS
.
- L. Xu, D.-D. Huang, C.-G. Li, X. Ji, S. Jin, Z. Feng, F. Xia, X. Li, F. Fan and C. Li, Construction of unique six-coordinated titanium species with an organic amine ligand in titanosilicate and their unprecedented high efficiency for alkene epoxidation, Chem. Commun., 2015, 51, 9010–9013 RSC
.
- X. Song, X. Yang, T. Zhang, H. Zhang, Q. Zhang, D. Hu, X. Chang, Y. Li, Z. Chen and M. Jia, Controlling the morphology and titanium coordination states of TS-1 zeolites by crystal growth modifier, Inorg. Chem., 2020, 59, 13201–13210 CrossRef CAS PubMed
.
- F. Geobaldo, S. Bordiga, A. Zecchina, E. Giamello, G. Leofanti and G. Petrini, DRS UV-Vis and EPR spectroscopy of hydroperoxo and superoxo complexes in titanium silicalite, Catal. Lett., 1992, 16, 109–115 CrossRef CAS
.
- G. Petrini, A. Cesana, G. De Alberti, F. Genoni, G. Leofanti, M. Padovan, G. Paparatto and P. Roffia, Deactivation phenomena on Ti-silicalite, Stud. Surf. Sci. Catal., 1991, 68, 761–766 CrossRef CAS
.
- T. Blasco, M. Camblor, A. Corma and J. Perez-Pariente, The state of Ti in titanoaluminosilicates isomorphous with zeolite. beta, J. Am. Chem. Soc., 1993, 115, 11806–11813 CrossRef CAS
.
- Y. Wang, H. Yang, Y. Zuo, D. Tian, G. Hou, Y. Su, Z. Feng, X. Guo and C. Li, New penta- and hexa-coordinated titanium sites in titanium silicalite-1 catalyst for propylene epoxidation, Appl. Catal., B, 2023, 325 Search PubMed
.
- R. Bai, Y. Song, G. Tian, F. Wang, A. Corma and J. Yu, Titanium-rich TS-1 zeolite for highly efficient oxidative desulfurization, Green Energy Environ., 2023, 8, 163–172 CrossRef CAS
.
- J. Zhang, R. Bai, Y. Zhou, Z. Chen, P. Zhang, J. Li and J. Yu, Impact of a polymer modifier on directing the non-classical crystallization pathway of TS-1 zeolite: accelerating nucleation and enriching active sites, Chem. Sci., 2022, 13, 13006–13014 RSC
.
- L. Xu, D.-D. Huang, C.-G. Li, X. Ji, S. Jin, Z. Feng, F. Xia, X. Li, F. Fan, C. Li and P. Wu, Construction of unique six-coordinated titanium species with an organic amine ligand in titanosilicate and their unprecedented high efficiency for alkene epoxidation, Chem. Commun., 2015, 51, 9010–9013 RSC
.
- W. Xu, L. Li, T. Zhang and J. Yu, Tailoring Porosity and Titanium Species of TS-1 Zeolites via Organic Base-assisted Sequential Post-treatment, Chem. Res. Chin. Univ., 2021, 38, 50–57 CrossRef
.
- Z. Liu and R. J. Davis, Investigation of the structure of microporous Ti-Si mixed oxides by X-ray, UV reflectance, FT-Raman, and FT-IR spectroscopies, J. Phys. Chem., 1994, 98, 1253–1261 CrossRef CAS
.
- T. Zhang, X. Chen, G. Chen, M. Chen, R. Bai, M. Jia and J. Yu, Synthesis of anatase-free nano-sized hierarchical TS-1 zeolites and their excellent catalytic performance in alkene epoxidation, J. Mater. Chem. A, 2018, 6, 9473–9479 RSC
.
- R. Khomane, B. Kulkarni, A. Paraskar and S. Sainkar, Synthesis, characterization and catalytic performance of titanium silicalite-1 prepared in micellar media, Mater. Chem. Phys., 2002, 76, 99–103 CrossRef CAS
.
- Y. Qi, C. Ye, Z. Zhuang and F. Xin, Preparation and evaluation of titanium silicalite-1 utilizing pretreated titanium dioxide as a titanium source, Microporous Mesoporous Mater., 2011, 142, 661–665 CrossRef CAS
.
- D. Lin, Q. Zhang, Z. Qin, Q. Li, X. Feng, Z. Song, Z. Cai, Y. Liu, X. Chen and D. Chen, Reversing titanium oligomer formation towards high-efficiency and green synthesis of titanium-containing molecular sieves, Angew. Chem., Int. Ed., 2021, 60, 3443–3448 CrossRef CAS PubMed
.
- J. Xing, D. Yuan, H. Liu, Y. Tong, Y. Xu and Z. Liu, Synthesis of TS-1 zeolites from a polymer containing titanium and silicon, J. Mater. Chem. A, 2021, 9, 6205–6213 RSC
.
- R. Bai, Q. Sun, Y. Song, N. Wang, T. Zhang, F. Wang, Y. Zou, Z. Feng, S. Miao and J. Yu, Intermediate-crystallization promoted catalytic activity of titanosilicate zeolites, J. Mater. Chem. A, 2018, 6, 8757–8762 RSC
.
- C. Liu, J. Huang, D. Sun, Y. Zhou, X. Jing, M. Du, H. Wang and Q. Li, Anatase type extra-framework titanium in TS-1: A vital factor influencing the catalytic activity toward styrene epoxidation, Appl. Catal., A, 2013, 459, 1–7 CrossRef CAS
.
- F. Jin, C.-C. Chang, C.-W. Yang, J.-F. Lee, L.-Y. Jang and S. Cheng, New mesoporous titanosilicate MCM-36 material synthesized by pillaring layered ERB-1 precursor, J. Mater. Chem. A, 2015, 3, 8715–8724 RSC
.
- W. Fan, B. Fan, X. Shen, J. Li, P. Wu, Y. Kubota and T. Tatsumi, Effect of ammonium salts on the synthesis and catalytic properties of TS-1, Microporous Mesoporous Mater., 2009, 122, 301–308 CrossRef CAS
.
- J. Zhang, H. Shi, Y. Song, W. Xu, X. Meng and J. Li, High-efficiency synthesis of enhanced-titanium and anatase-free TS-1 zeolite by using a crystallization modifier, Inorg. Chem. Front., 2021, 8, 3077–3084 RSC
.
- A. Thangaraj, R. Kumar, S. Mirajkar and P. Ratnasamy, Catalytic properties of crystalline titanium silicalites I. Synthesis and characterization of titanium-rich zeolites with MFI structure, J. Catal., 1991, 130, 1–8 CrossRef CAS
.
- M. Sasidharan, P. Wu and T. Tatsumi, Direct Formation of Pinacols from Olefins over Various Titano–Silicates, J. Catal., 2002, 209, 260–265 CrossRef CAS
.
- A.-Y. Geng, G.-Q. Ding, H.-H. She, H.-X. Wang, X.-Q. Li and Y.-L. Zhu, Effect of hydrolysis of tetraethyl orthosilicate (TEOS) on titanium distribution of TS-1 zeolite, J. Fuel Chem. Technol., 2022, 50, 1471–1479 CrossRef CAS
.
- Y. Liu, F. Wang, X. Zhang, Q. Zhang, Y. Zhai, G. Lv, M. Li and M. Li, One-step synthesis of anatase-free hollow titanium silicalite-1 by the solid-phase conversion method, Microporous Mesoporous Mater., 2022, 331, 111676 CrossRef CAS
.
- D. Serrano, R. Sanz, P. Pizarro, A. Peral and I. Moreno, Improvement of the hierarchical TS-1 properties by silanization of protozeolitic units in presence of alcohols, Microporous Mesoporous Mater., 2013, 166, 59–66 CrossRef CAS
.
- A. Wróblewska, J. Tołpa, D. Kłosin, P. Miądlicki, Z. C. Koren and B. Michalkiewicz, The application of TS-1 materials with different titanium contents as catalysts for the autoxidation of α-pinene, Microporous Mesoporous Mater., 2020, 305, 110384 CrossRef
.
- M. Zhang, S. Ren, Q. Guo and B. Shen, Synthesis of hierarchically porous zeolite TS-1 with small crystal size and its performance of 1-hexene epoxidation reaction, Microporous Mesoporous Mater., 2021, 326, 111395 CrossRef CAS
.
- Y. Xue, Y. Xie, H. Wei, Y. Wen, X. Wang and B. Li, Improving the performance of TS-1 catalysts for continuous cyclohexanone ammoximation through controlment of active species distribution, New J. Chem., 2014, 38, 4229–4234 RSC
.
- A. Alba-Rubio, J. Fierro, L. León-Reina, R. Mariscal, J. Dumesic and M. L. Granados, Oxidation of furfural in aqueous H2O2 catalysed by titanium silicalite: Deactivation processes and role of extraframework Ti oxides, Appl. Catal., B, 2017, 202, 269–280 CrossRef CAS
.
- Z. K. Shal, M. Goepel, R. Glaeser and M. Shakeri, Synthesis of TS-1 from supported embryonic to nano-/micro-metersized crystalline particles: The impact of accessibility of Ti species on the catalytic performance, Microporous Mesoporous Mater., 2022, 337, 111900 CrossRef
.
- L. Balducci, D. Bianchi, R. Bortolo, R. D'Aloisio, M. Ricci, R. Tassinari and R. Ungarelli, Direct Oxidation of Benzene to Phenol with Hydrogen Peroxide over a Modified Titanium Silicalite, Angew. Chem., Int. Ed., 2003, 115, 5087–5090 CrossRef
.
- D. Pan, L. Kong, H. Zhang, Y. Zhang and Y. Tang, TS-1 synthesis via subcrystal aggregation: construction of highly active hydrogen-bonded titanium species for alkene epoxidation, ACS Appl. Mater. Interfaces, 2023, 15, 28125–28134 CrossRef CAS PubMed
.
- K. Lin, L. Wang, F. Meng, Z. Sun, Q. Yang, Y. Cui, D. Jiang and F.-S. Xiao, Formation of better catalytically active titanium species in Ti-MCM-41 by vapor-phase silylation, J. Catal., 2005, 235, 423–427 CrossRef CAS
.
- S. Wang, P. Wang, Z. Qin, Y. Chen, M. Dong, J. Li, K. Zhang, P. Liu, J. Wang and W. Fan, Relation of catalytic performance to the aluminum siting of acidic zeolites in the conversion of methanol to olefins, viewed via a comparison between ZSM-5 and ZSM-11, ACS Catal., 2018, 8, 5485–5505 CrossRef CAS
.
- Z. Chen, L. Zhang, Y. Yu, D. Liu, N. Fang, Y. Lin, D. Xu, F. Li, Y. Liu and M. He, TS-1 zeolite with homogeneous distribution of Ti atoms in the framework: synthesis, crystallization mechanism and its catalytic performance, J. Catal., 2021, 404, 990–998 CrossRef CAS
.
- K. Takaishi, T. Yamamoto and M. Matsukata, Synthesis of TS-1 with preferably distributed Ti in depth direction of crystals, Chem. Lett., 2016, 45, 532–534 CrossRef CAS
.
- Y. Wang, Y. Zuo, M. Liu, C. Dai, Z. Feng and X. Guo, The High-Performance Hollow Silicalite-1@ Titanium Silicalite-1 Core-Shell Catalyst for Propene Epoxidation, ChemistrySelect, 2017, 2, 10097–10100 CrossRef CAS
.
- Z. Chen, X. Liu, Y. Yu, Z. Tang, J. Wang, D. Liu, N. Fang, Y. Lin, Y. Liu and M. He, Gripper-like silicon species for efficient synthesis of crystalline metallosilicates with spatially homogeneous heteroatoms in the framework, Chem. Mater., 2021, 33, 4988–5001 CrossRef CAS
.
- D. Lin, Q. Zhang, Z. Qin, Q. Li, X. Feng, Z. Song, Z. Cai, Y. Liu, X. Chen, D. Chen, S. Mintova and C. Yang, Reversing Titanium Oligomer Formation towards High-Efficiency and Green Synthesis of Titanium-Containing Molecular Sieves, Angew. Chem., Int. Ed., 2020, 60, 3443–3448 CrossRef PubMed
.
- Z. Tang, Y. Yu, L. Li, Z. Chen, J. Wang, Y. Liu and M. He, Regulation of framework titanium siting in MWW-type titanosilicates by boron and aluminum incorporation and its effect on the catalytic performance, Microporous Mesoporous Mater., 2019, 287, 93–100 CrossRef CAS
.
- D. Zhou, H. Zhang, J. Zhang, X. Sun, H. Li, N. He and W. Zhang, Density functional theory investigations into the structure and spectroscopic properties of the Ti4+ species in Ti-MWW zeolite, Microporous Mesoporous Mater., 2014, 195, 216–226 CrossRef CAS
.
- Y. Li, W. Guo, W. Fan, S. Yuan, J. Li, J. Wang, H. Jiao and T. Tatsumi, A DFT study on the distributions of Al and Brønsted acid sites in zeolite MCM-22, J. Mol. Catal. A: Chem., 2011, 338, 24–32 CrossRef CAS
.
- Y. Wang, D. Zhou, G. Yang, S. Miao, X. Liu, X. Bao and A. DFT, study on isomorphously substituted MCM-22 zeolite, J. Phys. Chem. A, 2004, 108, 6730–6734 CrossRef CAS
.
- C. E. Ramachandran, H. Du, Y. J. Kim, M. C. Kung, R. Q. Snurr and L. J. Broadbelt, Solvent effects in the epoxidation reaction of 1-hexene with titanium silicalite-1 catalyst, J. Catal., 2008, 253, 148–158 CrossRef CAS
.
- T. Sooknoi and V. Chitranuwatkul, Ammoximation of cyclohexanone in acetic acid using titanium silicalite-1 catalyst: Activity and reaction pathway, J. Mol. Catal. A: Chem., 2005, 236, 220–226 CrossRef CAS
.
Footnote |
| † Yihao Wang and Kaiwei Wang contributed equally to this review. |
| This journal is © the Partner Organisations 2025 |