Guanidinatoaluminum complexes: synthesis, crystal structures and reactivities†
Abstract
Treatment of diethylaminolithium with carbodiimide (CyN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NCy or CyN
NCy or CyN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NC6H5) and dimethylaluminium chloride (AlMe2Cl) in a molar ratio of 1 : 1 : 1 afforded two kinds of guanidinatoaluminum complexes, [{(Et2N)C(NR1)(NR2)}AlMe2] (R1, R2 = Cy (1); R1 = Cy, R2 = C6H5 (2)) and [{(Et2N)C(NCy)(NC6H5)}2AlMe] (3). The reactivities of the mononuclear guanidinatoaluminum complexes (1 and 2) with O2 or carbodiimide were studied. The complexes of [{(Et2N)C(NCy)2}AlMe(μ-OMe)]2 (4) and [{N(C6H5)C(NCy)N(C6H5)C(NEt2)N(Cy)}AlMe2] (5) were produced by introducing dry oxygen or carbodiimide (CyN
NC6H5) and dimethylaluminium chloride (AlMe2Cl) in a molar ratio of 1 : 1 : 1 afforded two kinds of guanidinatoaluminum complexes, [{(Et2N)C(NR1)(NR2)}AlMe2] (R1, R2 = Cy (1); R1 = Cy, R2 = C6H5 (2)) and [{(Et2N)C(NCy)(NC6H5)}2AlMe] (3). The reactivities of the mononuclear guanidinatoaluminum complexes (1 and 2) with O2 or carbodiimide were studied. The complexes of [{(Et2N)C(NCy)2}AlMe(μ-OMe)]2 (4) and [{N(C6H5)C(NCy)N(C6H5)C(NEt2)N(Cy)}AlMe2] (5) were produced by introducing dry oxygen or carbodiimide (CyN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NC6H5) slowly into the solution of the mononuclear guanidinatoaluminum complex (1 or 2). Meanwhile, the addition of O2 or carbodiimide (ArNC
NC6H5) slowly into the solution of the mononuclear guanidinatoaluminum complex (1 or 2). Meanwhile, the addition of O2 or carbodiimide (ArNC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NC6H5, Ar = 2,6-Me2C6H3) to the reaction solution of diethylaminolithium with ArN
NC6H5, Ar = 2,6-Me2C6H3) to the reaction solution of diethylaminolithium with ArN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NC6H5 and AlMe2Cl in a molar ratio of 1 : 1 : 1 yielded [(Et2N)C(NC6H5)(NAr)AlMe(μ-OMe)]2 (6) and [{N(C6H5)C(NAr)N(C6H5)C(NEt2)N(Ar)}AlMe2] (7). In addition, a one-pot reaction of diethylaminolithium with CyN
NC6H5 and AlMe2Cl in a molar ratio of 1 : 1 : 1 yielded [(Et2N)C(NC6H5)(NAr)AlMe(μ-OMe)]2 (6) and [{N(C6H5)C(NAr)N(C6H5)C(NEt2)N(Ar)}AlMe2] (7). In addition, a one-pot reaction of diethylaminolithium with CyN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NCy and AlMe2Cl in a molar ratio of 1 : 1 : 1 in the presence of H2O produced a tetranuclear guanidinatoaluminum complex,
NCy and AlMe2Cl in a molar ratio of 1 : 1 : 1 in the presence of H2O produced a tetranuclear guanidinatoaluminum complex, 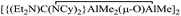 (8), which was also prepared by treatment of complex 1 with equivalent AlMe3 and H2O in 35% yield. All complexes 1–8 were characterized by 1H, 13C NMR spectroscopy and single crystal X-ray diffraction analysis.
(8), which was also prepared by treatment of complex 1 with equivalent AlMe3 and H2O in 35% yield. All complexes 1–8 were characterized by 1H, 13C NMR spectroscopy and single crystal X-ray diffraction analysis.


 Please wait while we load your content...
Please wait while we load your content...