A theoretical study on the second-order nonlinear optical properties of Pt(ii) bis-acetylide complexes: substituent and redox effects†
Abstract
Density functional theory studies on the geometric and electronic structures, UV-vis absorption spectra, and second-order nonlinear optical (NLO) properties of four-coordinate Pt(II) bis-acetylide complexes, cis-[Pt(CNtBu)(ADC)(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CR)2]
CR)2] 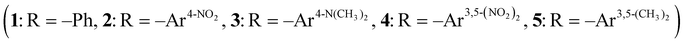 , have been employed. The effects of ligand variation and the single electron redox process on the structures and NLO response of complexes have also been investigated. It shows that the variations of the ligand and electron have little effect on the geometries of the complexes, but there is a significant effect on their electronic structures and NLO responses. The introduction of a single –NO2 group in acetylide ligands increases the first hyperpolarizability of complex 12 times, while one electron lost in five complexes enhances the first hyperpolarizability 496 times at the most. Both methods are considered effective ways for improving the NLO response of Pt(II) bis-acetylide complexes. Based on the analysis of the electronic and optical properties of fifteen studied complexes, the increase of NLO response is mainly ascribed to strong oscillator strengths, lower electron transition energy, and well-directed effective charge transfer. This work reveals some underlying relationships between the NLO responses and electronic structures of complexes, which is helpful for the design and synthesis of high-performance NLO materials of Pt(II) bis-acetylide complexes.
, have been employed. The effects of ligand variation and the single electron redox process on the structures and NLO response of complexes have also been investigated. It shows that the variations of the ligand and electron have little effect on the geometries of the complexes, but there is a significant effect on their electronic structures and NLO responses. The introduction of a single –NO2 group in acetylide ligands increases the first hyperpolarizability of complex 12 times, while one electron lost in five complexes enhances the first hyperpolarizability 496 times at the most. Both methods are considered effective ways for improving the NLO response of Pt(II) bis-acetylide complexes. Based on the analysis of the electronic and optical properties of fifteen studied complexes, the increase of NLO response is mainly ascribed to strong oscillator strengths, lower electron transition energy, and well-directed effective charge transfer. This work reveals some underlying relationships between the NLO responses and electronic structures of complexes, which is helpful for the design and synthesis of high-performance NLO materials of Pt(II) bis-acetylide complexes.



 Please wait while we load your content...
Please wait while we load your content...