Ruthenium-catalysed oxidation of alkanes with peracetic acid in trifluoroacetic acid: ruthenium as an efficient catalyst for the oxidation of unactivated C–H bonds
Naruyoshi Komiya, Satoru Noji and Shun-Ichi Murahashi*
Department of Chemistry, Graduate School of Engineering Science, Osaka University, 1-3, Machikaneyama, Toyonaka, Osaka 560-8531, Japan.. E-mail: mura@chem.es.osaka-u.ac.jp
First published on 14th December 2000
Abstract
The role of ruthenium catalysts for the oxidation of alkanes with peracetic acid in trifluoroacetic acid has been confirmed.
During the course of simulation of the functions of cytochrome P-450 enzyme with transition metal complexes, we have found that transition metal-catalysed oxidations of various substrates can be performed highly efficiently.1 In 1994 we reported that ruthenium-catalysed oxidation of alkanes with peracetic acid in ethyl acetate gives the corresponding ketones and alcohols.2 Furthermore, the RuCl3-catalysed oxidation of cyclohexane in TFA proceeds highly efficiently to give cyclohexyl trifluoroacetate and cyclohexanone with high conversion and high selectivity.2 Recently, Moody and O’Connell reported that the oxidation of cyclohexane using urea hydrogen peroxide (UHP) in TFA without a metal catalyst gives cyclohexyl trifluoroacetate,3 which was originally reported by Deno et al.4 They obtained the rate constants (k = 3.3 × 10–5 M−1 s−1) for the oxidation of cyclohexane with UHP in TFA in the presence or absence of RuCl3 catalyst, indicating that the system is not ruthenium dependent. They claimed our system (peracetic acid–Ru) is not ruthenium dependent. However, our system using peracetic acid is quite different from that of UHP oxidation. Here, we describe our ruthenium-catalysed oxidation of alkanes with peracetic acid, which is very fast and an efficient ruthenium dependent reaction. The kinetics show the rate is 105 times faster than previously reported.
As described in our previous paper,2 the oxidation of cyclohexane with peracetic acid in the presence of ruthenium on charcoal (Ru/C) or RuCl3 (1 mol%) catalyst in ethyl acetate gives cyclohexanone along with a small amount of cyclohexanol [eqn. (1)]. No oxidation takes place without a catalyst.
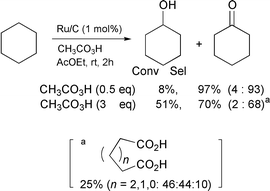 | (1) |
In order to generate more active oxo-metal species which may lead to high conversion and high selectivity for the oxidation of alkanes,5–7 we used TFA. As described in our previous paper,2 the RuCl3-catalysed oxidation of cyclohexane with peracetic acid in a mixture of TFA and CH2Cl2 (5∶1) for 4 h gave cyclohexyl trifluoroacetate and cyclohexanone with 90% selectivity (85∶15) and 90% conversion [eqn. (2)]. However,
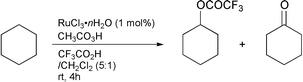 | (2) |
The present catalytic oxidation can be applied to a variety of alkanes. The representative results of the ruthenium-catalysed oxidation of alkanes with peracetic acid in a mixture of TFA and CH2Cl2 are shown in Table 1. Both linear and cyclic alkanes can be converted into the corresponding esters of trifluoroacetic acid along with ketones. The reaction of adamantane gave adamantan-1-ol, which was formed by hydrolysis of 1-adamantyl trifluoroacetate.
| Alkane | Conversionb (%) | Product | Yieldc (%) |
|---|---|---|---|
| a To a mixture of alkane (2.5 mmol), RuCl3 (0.025 mmol), TFA (5 mL) and CH2Cl2 (1 mL) was added dropwise a 30% peracetic acid (5.0 mmol) solution in ethyl acetate over a period of 2 h. After stirring for 2 h, the reaction was quenched by adding 5% aqueous sodium sulfite solution.b Determined by GC based on the starting alkane using an internal standard (acetophenone).c Determined by GC based on the converted alkane.d TFA (5 mL), CH2Cl2 (10 mL) and acetic acid (5 mL) were used as a solvent.e 1-Adamantyl trifluoroacetate was readily hydrolyzed to give adamantan-1-ol during the work up.f The IUPAC name for norbornane is bicyclo[2.2.1]heptane.g Not determined.h 2-:3-Hexyl trifluoroacetate = 45:55.i 2-:3-One = 34:66. | |||
| Cyclohexane | 90 | Cyclohexyl trifluoroacetate | 77 |
| Cyclohexanone | 13 | ||
| Cyclooctane | 81 | Cyclooctyl trifluoroacetate | 40 |
| Cyclooctanone | 10 | ||
| Adamantaned | 70 | Adamantan-1-ole | 89 |
| 2-Adamantyl trifluoroacetate | 9 | ||
| Norbornanef | 90g | exo-2-Norbornyl trifluoroacetate | 61 |
| Hexane | nd | Hexyl trifluoroacetatesh | 24b |
| Hexanonesi | 6b | ||
Kinetic experiments on the reaction of cyclohexane with peracetic acid in TFA were carried out. In the presence of a large excess of cyclohexane, the rate was first-order with respect to the concentration of peracetic acid (Fig. 1). The first-order rate constant increased with an increase in the concentration of RuCl3 (Fig. 2), but the rate constant was independent of the concentration of cyclohexane. The rate law for the ruthenium-catalysed oxidation of cyclohexane with peracetic acid in TFA and CH2Cl2 was expressed by the equation (−d[cyclohexane]/dt = k[RuCl3][CH3CO3H]). The second-order rate constant was determined to be k = 5.4 M−1 s−1. In contrast, the rate constant for the oxidation of cyclohexane without a metal catalyst is very small (k < 10−6 s−1). These results clearly show that the oxidation of cyclohexane is catalysed by RuCl3 catalyst dramatically.
![Pseudo-first-order rate plot of the ruthenium-catalysed oxidation of
cyclohexane with peracetic acid in the presence of TFA. Solvent,
TFA–CH2Cl2–AcOEt = 5∶1∶1;
[RuCl3] = 7.1 × 10−4 M;
[CH3CO3H] = 1.4 × 10−2 M;
[cyclohexane] = 3.6 × 10−1 M; 20 °C;
kobsd = 3.9 × 10−3
s−1.](/image/article/2001/CC/b006869l/b006869l-f1.gif) | ||
| Fig. 1 Pseudo-first-order rate plot of the ruthenium-catalysed oxidation of cyclohexane with peracetic acid in the presence of TFA. Solvent, TFA–CH2Cl2–AcOEt = 5∶1∶1; [RuCl3] = 7.1 × 10−4 M; [CH3CO3H] = 1.4 × 10−2 M; [cyclohexane] = 3.6 × 10−1 M; 20 °C; kobsd = 3.9 × 10−3 s−1. | ||
![Dependence of the first-order rate constants kobsd
on the concentration of RuCl3. Solvent,
TFA–CH2Cl2–AcOEt = 5∶1∶1;
[CH3CO3H] = 1.4 × 10−2 M;
[cyclohexane] = 3.6 × 10−2 M; 20 °C.](/image/article/2001/CC/b006869l/b006869l-f2.gif) | ||
| Fig. 2 Dependence of the first-order rate constants kobsd on the concentration of RuCl3. Solvent, TFA–CH2Cl2–AcOEt = 5∶1∶1; [CH3CO3H] = 1.4 × 10−2 M; [cyclohexane] = 3.6 × 10−2 M; 20 °C. | ||
Intermolecular deuterium isotope effect
(kH/kD) of the
ruthenium-catalysed oxidation of
cyclohexane–cyclohexane-d12 in TFA was
determined to be 2.9, which is smaller than the value (6.8) obtained from
the same oxidation in ethyl acetate, indicating that a more reactive
species is involved in the oxidation in TFA. The oxidation can be
rationalized by assuming the mechanism which involves oxo-ruthenium
species. The reaction of LnRuIII with
peracetic acid would give
LnRuIIIOOC(O)CH3 which undergoes
heterolytic cleavage8 to give the
LnRuV![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) O species. Hydrogen abstraction
with the oxo-ruthenium species and recombination would give an alcohol.
Under the reaction conditions, an alcohol can be converted into either the
corresponding trifluoroacetate or ketone. Actually, control experiments
show that cyclohexanol is readily converted to a mixture of cyclohexyl
trifluoroacetate and cyclohexanone under the reaction conditions.9 The ruthenium-catalysed oxidation with peracetic
acid is quite different from the oxidation with hydrogen peroxide in TFA,
where electrophilic reaction of perfluoroacetic acid is involved. It is
noteworthy that cyclohexanone has never been obtained from the reaction of
H2O2–TFA.3,4
O species. Hydrogen abstraction
with the oxo-ruthenium species and recombination would give an alcohol.
Under the reaction conditions, an alcohol can be converted into either the
corresponding trifluoroacetate or ketone. Actually, control experiments
show that cyclohexanol is readily converted to a mixture of cyclohexyl
trifluoroacetate and cyclohexanone under the reaction conditions.9 The ruthenium-catalysed oxidation with peracetic
acid is quite different from the oxidation with hydrogen peroxide in TFA,
where electrophilic reaction of perfluoroacetic acid is involved. It is
noteworthy that cyclohexanone has never been obtained from the reaction of
H2O2–TFA.3,4
Acknowledgements
In summary, the oxidation of alkanes with peracetic acid in TFA proceeds efficiently in the presence of ruthenium catalyst to give the corresponding trifluoroacetic acid ester.Notes and references
- (a) S.-I. Murahashi, Angew. Chem., Int. Ed. Engl., 1995, 34, 2443 CrossRef CAS; (b) S.-I. Murahashi and N. Komiya, in Biomimetic Oxidations Catalyzed by Transition Metal Complexes, ed. B. Meunier, Imperial College Press, London, 2000, pp. 563–611. Search PubMed.
- S.-I. Murahashi, Y. Oda, N. Komiya and T. Naota, Tetrahedron Lett., 1994, 35, 7953 CrossRef CAS.
- C. J. Moody and J. L. O’Connell, Chem. Commun., 2000, 1311 RSC.
- N. C. Deno, E. J. Jedziniak, L. A. Messer, M. D. Meyer, S. G. Stroud and E. S. Tomezsko, Tetrahedron, 1977, 33, 2503 CrossRef CAS.
- T.-C. Lau and C.-K. Mak, J. Chem. Soc., Chem. Commun., 1995, 943 RSC.
- A. Sen, Acc. Chem. Res., 1998, 31, 550 CrossRef CAS.
- I. I. Moiseev, in Transition Metal Catalysed Reactions, ed. S.-I. Murahashi and S. G. Davies, Blackwell Science, Oxford, 1999, pp. 343–373. Search PubMed.
- W. A. Lee and T. C. Bruice, J. Am. Chem. Soc., 1985, 107, 513 CrossRef CAS.
- S.-I. Murahashi, T. Naota, Y. Oda and N. Hirai, Synlett, 1995, 733 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2001 |
