A valence isomer of a dialane
John D. Gorden, Charles L. B. Macdonald and Alan H. Cowley*
Department of Chemistry and Biochemistry, The University of Texas at Austin, Austin, Texas 78712, USA.. E-mail: cowley@mail.utexas.edu
First published on 19th December 2000
Abstract
The compound (η5-C5Me5)Al→Al(C6F 5)3, which is the first valence isomer of a dialane, has been prepared by treatment of [Al(η5-C5Me5)]4 with Al(C6F5)3 and characterized by X-ray crystallography and NMR spectroscopy.
Compounds with aluminium–aluminium bonds are attracting considerable recent attention. The simplest such compounds are the dialanes, R2AlAlR2, and a number of these have now been structurally authenticated.1 It occurred to us that valence isomers of dialanes, viz. RAl→AlR3, might be capable of existence if the appropriate substituents were employed. DFT calculations2 on the prototypical dialane, H2AlAlH2, revealed that the valence isomer HAl→AlH3, is less stable than H2AlAlH2 by 9.17 kcal mol−1. However, replacement of one of the dialane hydride substituents by cyclopentadienide inverted this order and (η5-C5H5)Al→AlH31 is more stable than the dialane (η2-C5H5)(H)Al→AlH2 by 10.79 kcal mol−1. In view of the foregoing, [Al(η5-C5Me5)]4 [65 mg, 0.40 mmol of Al(η5-C5Me5) units]3 was treated with Al(C6F5)3·PhCH3 4 (250 mg, 0.40 mmol) in 30 mL of toluene at 25 °C. After being stirred for 4 h at 25 °C, the yellow reaction mixture was heated to 50 °C for 30 min. Upon cooling to 25 °C, the reaction mixture was filtered and the solvent and volatiles were removed from the filtrate to afford a dark amber oil from which yellow crystalline (η5-C5Me5)Al→Al(C6F 5)32 (220 mg, 80% yield, mp 131–133 °C) deposited over a period of 24 h. The mass spectral data† for 2 are consistent with the proposed dialane isomer formulation. The presence of (η5-C5Me5)Al and Al(C6F5)3 moieties in 2 is evident from the 1H, 13C, and 19F NMR spectroscopic data,† noting however that the equivalence of the C5Me5 ring carbon and Me resonances could be due to the well known fluxional behaviour of cyclopentadienyl–aluminium systems.5 The 27Al NMR spectrum of 2 comprises singlet resonances at δ −115.7 and 106.9. Given that the 27Al chemical shifts for the model compound 1, as computed by the GAIO method,2b,6 are δ −107.9 and 109.0 for the (η5-C5Me5)Al and AlH3 centres, respectively, analogous assignments have been made for 2.† Further support for the proposed assignments stems from the experimentally observed 27Al chemical shifts for monomeric (η5-C5Me5)Al (δ −150)7 and Al(C6F5)3·arene [δ 52 (benzene); δ 61 (toluene)].4 The overall trend of 27Al chemical shifts is consistent with the transfer of electron density from the alanediyl to the Al(C6F5)3 fragment upon formation of the Al→Al donor acceptor bond of 2.
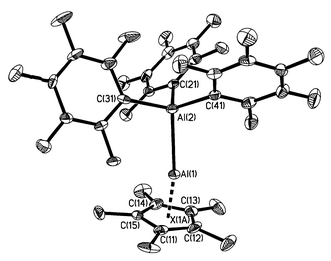 | ||
| Fig. 1 Thermal ellipsoid plot (30% probability level) for (η5-C5Me5)Al→Al(C6F 5)32. Selected bond lengths (Å) and bond angles (°): Al(2)–Al(1) 2.591(2), Al(1)–X(1A) 2.591(8), Al(1)–C(11) 2.172(7), Al(1)–C(12) 2.162(6), Al(1)–C(13) 2.165(7), Al(1)–C(14) 2.200(7), Al(1)–C(15) 2.189(6), Al(2)–C(21) 1.982(7), Al(2)–C(31) 1.999(7); Al(2)–C(41) 1.997(7); Al(2)–Al(1)–X(1A) 170.1(3), C(21)–Al(2)–C(41) 111.0(3), C(21)–Al(2)–C(31) 108.5(3), C(41)–Al(2)–C(31) 113.5(3), C(21)–Al(2)–Al(1) 104.1(2), C(41)–Al(2)–Al(1) 111.2(2), C(31)–Al(2)–Al(1) 108.0(2). | ||
The foregoing spectroscopic conclusions were confirmed by X-ray crystallography.‡ Compound 2 crystallizes in the C2/c space group with Z = 8; the solid state consists of individual molecules of the dialane isomer and there are no unusually short intermolecular contacts. The pentamethylcyclopentadienyl substituent is attached in an η5 fashion and the ring centroid–Al–Al moiety deviates only modestly from linearity [170.1(3)°]. The Al–Al bond length in 2 [2.591(3) Å] is shorter than those in the dialanes {(Me3Si)2CH}4Al2 [2.660(1) Å],1a {2,4,6-Pri3- C6H2}4Al2 [2.647(3) Å],1b and {But3Si}4Al2 [2.751(2) Å]1c but identical to that in [RIAl–AlClR] {R = [(Me3Si)2C(Ph)C(Me3- Si)N]) [2.593(2) Å]}1d within experimental error. The average Al(1)–C bond length of 2.178(7) Å [Al–centroid 1.810(8) Å] is considerably shorter than those reported for Al(η5-C5Me5) [2.388(7) Å]8 and [Al(η5-C5Me5)]4 (2.344 Å, av. Al–centroid 2.011 Å).7 Such a shortening is anticipated as the partially antibonding aluminium ‘lone pair’ orbital of Al(η5-C5Me5) is transformed into the donor–acceptor bond with the concomitant development of positive and negative charges on the aluminium centres.9 The same trend is evident for other group 13 (η5-C5Me5)M→acceptor complexes10 and is true for both main-group and transition element acceptors.
In conclusion, we have prepared (η5-C5Me5)Al→Al(C6F 5)3, a valence isomer of a dialane. This compound also features the first example of an Al→Al donor acceptor bond.
Acknowledgements
We are grateful to the National Science Foundation, Robert A. Welch Foundation, and the National Academy of Sciences, through Sigma Xi, The Scientific Research Society for financial support.Notes and references
- (a) For a review, see: W. Uhl, Angew Chem., Int. Ed. Engl., 1993, 32, 1386 CrossRef see also:; (b) R. J. Wehmschulte, K. Ruhlandt-Senge, M. M. Olmstead, H. Hope, B. E. Sturgeon and P. P. Power, Inorg. Chem., 1993, 32, 2983 CrossRef CAS; (c) N. Wiberg, K. Amelunxen, T. Blank, H. Nöth and J. Knizek, Organometallics, 1998, 17, 5431 CrossRef CAS; (d) K. S. Klimek, C. Cui, H. W. Roesky, M. Noltemeyer and H.-G. Schmidt, Organometallics, 2000, 19, 3085 CrossRef CAS.
- B3LYP: (a) A. D. Becke, J. Chem. Phys., 1993, 98, 5648 CrossRef CAS; (b) A. D. Becke, Phys. Rev. A, 1988, 38, 3098 CrossRef CAS; (c) C. Lee, W. Yang and R. G. Parr, Phys. Rev. B, 1988, 37, 785 CrossRef CAS; (d) S. H. Vosko, L. Wilk and M. Nusair, Can. J. Phys., 1980, 58, 1200 CrossRef CAS All DFT calculations were performed using the Gaussian 94 (revision B2) suite of programs. All-electron basis sets were used for C, H [6-31G(d)] and the group 13 elements [6-31 + G(d])..
- Prepared according to the method of S. Schulz, H. W. Roesky, H. J. Koch, G. M. Sheldrick, D. Stalke and A. Kuhn, Angew. Chem., Int. Ed. Engl., 1993, 32, 1729 CrossRef.
- G. S. Hair, A. H. Cowley, R. A. Jones, B. G. McBurnett and A. Voigt, J. Am. Chem. Soc., 1999, 121, 4922 CrossRef CAS.
- P. J. Shapiro, Coord. Chem. Rev., 1999, 189, 1 CrossRef CAS.
- R. Ditchfield, Mol. Phys., 1974, 27, 789 CAS; K. Wolinski, J. F. Hinton and P. Pulay, J. Am. Chem. Soc., 1990, 122, 8251 CrossRef BP86: J. P. Perdew, Phys. Rev. B, 1986, 33, 8822 CrossRef.
- C. Dohmeier, D. Loos and H. Schnöckel, Angew. Chem., Int. Ed. Engl., 1996, 35, 129 CrossRef CAS.
- A. Haaland, K.-G. Martinsen, S. A. Shlykov, H. V. Volden, C. Dohmeier and H. Schnöckel, Organometallics, 1995, 14, 3116 CrossRef CAS.
- C. L. B. Macdonald and A. H. Cowley, J. Am. Chem. Soc., 1999, 121, 12113 CrossRef CAS.
- J. D. Gorden, A. Voigt, C. L. B. Macdonald, J. S. Silverman and A. H. Cowley, J. Am. Chem. Soc., 2000, 122, 950 CrossRef CAS.
Footnotes |
| † 2: HRMS (CI, CH4) calc. for C28H15Al2F15m/z 690.0565; found 690.0572. 1H NMR (499.35 MHz, 295 K, C6D6) δ 1.49 (s, 15H, C5Me5). 13C{1H} NMR (125.69 MHz, 295 K, C6D6) δ 149.99 (d, o-C6F5, 1JCF 224 Hz), 141.83 (d, p-C6F5, 1JCF 239 Hz), 137.34 (d, m-C6F5, 1JCF 226 Hz), 129.28 (s, ipso-C6F5), 115.94 [s, C5(CH3)5], 8.44 [s, C5(CH3)5]. 19F NMR (469.81 MHz, 295 K, C6D6) δ −122.03 (s, m-C6F5), −153.19 (s, p-C6F5), 161.77 (s, o-C6F5). 27Al NMR (130.25 MHz, 295 K, C6D6) δ 106.9 [br, (C6F5)3AlAlC5Me5 , w1/2 6122 Hz], −115.7 [s, (C6F5)3AlAlC5Me5 ]. |
| ‡ Crystal data for 2:
C28H15Al2F15, monoclinic, space
group C2/c, a = 30.635(6), b =
9.814(2), c = 20.236(4) Å, β = 111.10(3),
V = 5676(2) Å3, Z = 8,
Dc = 1.616 g cm−3,
μ(Mo-Kα) = 0.220 mm−1. A suitable single
crystal of 2 was covered with mineral oil and mounted on a
Nonius-Kappa CCD diffractometer at 123 K. A total of 8481 independent
reflections were collected in the range 5.96 < 2θ <
50.20° using Mo-Kα radiation (λ = 0.71073
Å). Of these, 3815 were considered observed [I >
2.0σ(I)] and were used to solve (direct methods)
and refine (full matrix, least squares on F2) the
structure of 2; R = 0.0767, wR2 = 0.1944. CCDC 182/1856. See http://www.rsc.org/suppdata/cc/b0/b007341p/ for crystallographic files in .cif format |
| This journal is © The Royal Society of Chemistry 2001 |
