Formation of an α-ketocarbene by photolysis of aqueous 2-bromophenol
Florent Bonnichona, Claire Richard*a and Gottfried Grabnerb
aLaboratoire de Photochimie Moléculaire et
Macromoléculaire, UMR 6505, Université Blaise Pascal, 63177, Aubière Cedex, France.. E-mail: claire.richard@univ-bpclermont.fr;; Tel: +33 4 73 40 71 42;; Fax: +33 4 73 40 77 00
bInstitut für Theoretische Chemie und Molekulare
Strukturbiologie, Universität Wien, Althanstrasse 14, 1090, Wien, Austria
First published on 14th December 2000
Abstract
The α-ketocarbene 2-oxocyclohexa-3,5-dienylidene (λmax = 360, 375 and 388 nm) is detected upon laser flash photolysis of 2-bromophenol in aqueous solution; its formation is confirmed by photoproduct studies.
para-Substituted halogenophenols have been shown to undergo heterolytic photodehalogenation in polar protic solvents.1–10 The mechanism of this process could be clarified by demonstration of the formation of the carbene 4-oxocyclohexa-2,5-dienylidene (λmax = 370 and 384 nm), which is long-lived enough to be detected by nanosecond laser flash photolysis experiments in aqueous solution.1
This intermediate arises from HCl elimination; its characteristic reactivity is governed by its triplet multiplicity: the addition of oxygen yields the para-benzoquinone O-oxide (λmax = 460 nm) and subsequently para-benzoquinone, and the reduction by H-donor molecules such as alcohols gives rise to a phenoxyl radical (λmax = 385 and 400 nm) and subsequently to phenol. Reactions with water and the halogenophenol itself produce hydroquinone and 5-chloro-2,4′-dihydroxybiphenyl respectively, in agreement with earlier studies.2–5 This mechanism of the photoreactivity of 4-halogenophenols was later confirmed by several studies using transient absorption spectroscopy, EPR, and photoproduct analysis.6–10
In the case ofaqueous 2-chlorophenol (2-ClP) or 2-bromophenol (2-BrP), UV irradiation leads to photocontraction into cyclopentadienic acids and to photohydrolysis.11,12
The ring contraction corresponds to a Wolff rearrangement;13 it is also observed in the photolysis of α-diazoketones.14 The similarity of the two reactions led to the proposal of intermediate formation of the α-ketocarbene, 2-oxocyclohexa-3,5-dienylidene, in both cases.12,14 However, this carbene has not until now been detected, the first intermediate reported in aqueous solution on a nanosecond time scale being the ketene fulvene 6-oxide (λmax = 255 nm), which subsequently adds a water molecule to yield fulvene 6,6-diol (λmax = 295 nm).15,16
The photo-Wolff rearrangement is expected to proceed on the excited singlet surface.13,17 A putative singlet α-ketocarbene arising as an intermediate on the reaction coordinate of ring contraction might have a lifetime in the subnanosecond range, as indicated by time-resolved studies of α-diazocarbonyl compounds.18,19 On the other hand, α-ketocarbenes may have triplet ground states.17 Currently available evidence indicates that the photoinduced dehalogenation of 4-halogenophenols proceeds on the excited triplet surface.1,10 The formation of triplet α-ketocarbenes in the analogous reaction of 2-halogenophenols is therefore conceivable, either preceding ring contraction or in competition to it. A recent photoproduct study of the degradation of 2-ClP in aqueous surfactant solutions indicated that both singlet and triplet pathways may contribute to product formation.20
The transient spectrum obtained by laser flash photolysis of aqueous 2-ClP (Nd∶YAG laser, Quanta-Ray DCR-1, pulse duration 7 ns, λexc = 266 nm) is dominated by the strong and broad absorption of the species resulting from ring contraction,16 which mask a possible contribution from a carbene which, in analogy to that derived from 4-halogenophenols, is expected in the 350–420 nm range. However, careful examination of the transient spectrum in oxygenated solution revealed a very weak band with a maximum around 475 nm (ε × Φ = 11 ± 3 M−1 cm−1), reminiscent of the quinone oxide resulting from addition of O2 to 4-oxocyclohexa-2,5-dienylidene.1 Upon addition of propan-2-ol (0.085 M), an equally weak new species with a two-band absorption (λmax = 380 and 400 nm, ε400 × Φ = 9 ± 3 M−1 cm−1) could be detected, indicating the formation of a phenoxyl radical21 in these conditions.
These results suggested the transient formation of a triplet α-ketocarbene in the photochemistry of aqueous 2-ClP, but a direct proof of its presence was still lacking. Based on the hypothesis that the photoinduced reactions proceed on the triplet surface, it could be argued that 2-BrP, because of its intrinsically faster intersystem crossing, might be better suited as a substrate molecule to detect the carbene.
Laser flash photolysis of aqueous 2-BrP (10−3 M) yielded the ring contraction products, ketene and enol, just as with 2-ClP. However, an additional transient absorbing within the wave length range 350–400 nm (λmax = 360, 375 and 388 nm, Fig. 1, spectrum 1) was also observed. This species decayed by first-order kinetics with kd = 2.5 × 105 s−1. In deoxygenated solutions containing propan-2-ol (0.17 M), it was converted into the phenoxyl radical with a formation rate of 1.6 × 106 s−1 (Fig. 1, spectrum 2). In oxygen-saturated solutions we found again the broad band with maximum around 475 nm (Fig. 1, spectrum 3), which had a formation rate of 9.0 × 106 s−1.
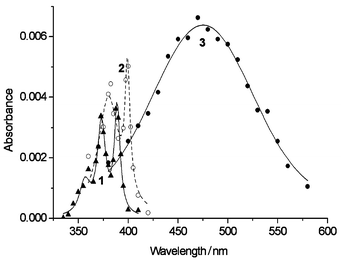 | ||
| Fig. 1 Transient absorption spectra from neutral 2-BrP (10−3 M). Absorbances normalized at P = 1 mJ pulse−1 and A(266) = 1.5. 1: deoxygenated solution, differences between absorbances measured at the pulse end and 16 μs after. 2: deoxygenated solution containing propan-2-ol (0.17 M), absorbances measured 2 μs after the pulse end. 3: oxygen-saturated solution, absorbances measured 1 μs after the pulse end. | ||
As seen in Fig. 2, all these transients derived from monophotonic processes. We deduced from the slopes of the linear relationships of absorbance vs. laser pulse energy the following values of ε × Φ : 90 ± 20 M−1 cm−1 for the end-of-pulse transient at 388 nm, 130 ± 25 M−1 cm−1 for the phenoxyl radical at 400 nm and 175 ± 30 M−1 cm−1 for the transient observed in oxygenated solution at 475 nm.
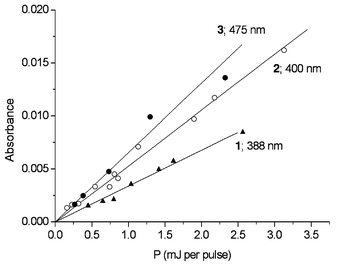 | ||
| Fig. 2 Dependences of transient absorbances on pulse energy. | ||
The complete analogy of the transients shown in Fig. 1 with those observed in the photochemistry of 4-halogenophenols1 prompted us to assign the end-of-pulse transient to the α-ketocarbene formed by dehalogenation of 2-BrP. The reactivity with propan-2-ol and O2 demonstrate the triplet character of this carbene; the 475 nm transient is therefore assigned to the ortho-benzoquinone O-oxide resulting from addition of O2. Based on ε = 3000 M−1 cm−1 for the phenoxyl radical at 400 nm21 and assuming complete conversion of the α-ketocarbene into the phenoxyl radical, values of Φ = 0.04 ± 0.01 and Φ = 0.003 ± 0.001 are found for the quantum yields of carbene formation from 2-BrP and 2-ClP, respectively. The molar extinction coefficient of the α-ketocarbene at 388 nm is estimated as ε = 2100 ± 500 M−1 cm−1.
Product studies were undertaken to confirm the occurrence of an additional reaction pathway in the case of 2-BrP, besides that leading to ring contraction. The substrate (5 × 10−4 M 2-ClP or 2-BrP in neutral aqueous solution) was irradiated at 280 nm with low light intensities and the irradiated solutions were analysed by HPLC (Table 1). Within detection limits, only photocontraction and photohydrolysis are observed in the case of 2-ClP; the reaction is not measurably affected by the presence of O2 or of propan-2-ol (0.017 M). In the case of 2-BrP, the formation of phenol and of three new products eluted after 2-BrP are observed in deoxygenated solutions. One of them, also obtained by bromination of 2,2′-dihydroxybiphenyl, is likely to be 2-bromo-6-(2′-hydroxyphenyl)phenol. In oxygenated solution biphenyls and phenol are not formed and the quantum yield of 2-BrP photolysis is reduced. The addition of propan-2-ol (0.17 M) clearly favours the formation of phenol, decreases the quantum yield of 2-BrP photolysis and inhibits drastically the formation of biphenyls. These results demonstrate that the photoproduct distribution of 2-BrP is indeed different from that of 2-ClP. The presence of biphenyls and of phenol among the photoproducts is indicative of the characteristic triplet carbene reactions, addition to the substrate and H abstraction, in analogy to the photoproducts from 4-halogenophenols.1 Quinoid compounds are absent, which is not surprising in view of the instability of ortho-benzoquinone.
| Conditions | Φd | Φcyclopentadienic Acids | Φpyrocatechol | Other products detected | |
|---|---|---|---|---|---|
| 2-ClP | N2 or O2 | 0.065 ± 0.007 | 0.042 ± 0.008 | 0.012 ± 0.001 | none |
| 2-BrP | N2 | 0.085 ± 0.008 | 0.035 ± 0.007 | 0.009 ± 0.001 | biphenyls and phenol (phis = 0.005) |
| O2 | 0.065 ± 0.007 | 0.035 ± 0.007 | 0.011 ± 0.001 | none | |
| N2, propan-2-ol (0.17 M) | 0.07 ± 0.007 | 0.025 ± 0.005 | 0.009 ± 0.001 | phenol (phis = 0.024) |
To summarize, we have been able to characterize a hitherto unknown triplet α-ketocarbene by means of transient absorption spectroscopy and characteristic reactivity. The yield of this carbene is over ten times higher from 2-BrP than from 2-ClP, in agreement with the hypothesis that intersystem crossing at the molecular level precedes its formation. The relatively long triplet α-ketocarbene lifetime, of the order of microseconds, excludes the possibility of it being an intermediate on the way to ring contraction. It is therefore likely that ring contraction and formation of the triplet carbene are competing reactions, as indicated in Scheme 1.
 | ||
| Scheme 1 | ||
Notes and references
- G. Grabner, C. Richard and G. Köhler, J. Am. Chem. Soc., 1994, 116, 11470 CrossRef CAS.
- P. Boule, C. Guyon and J. Lemaire, Toxicol. Environ. Chem., 1984, 7, 97 Search PubMed.
- K. Omura and T. Matsuura, Tetrahedron, 1971, 27, 3101 CrossRef CAS.
- E. Lipczynska-Kochany and J. R. Bolton, J. Photochem. Photobiol., 1991, 58, 315 Search PubMed.
- K. Oudjehani and P. Boule, J. Photochem. Photobiol., 1992, 68, 363 Search PubMed.
- A. Ouardaoui, C. A. Steren, H. van Willigen and C. Yang, J. Am. Chem. Soc., 1995, 117, 6803 CrossRef CAS.
- A.-P. Y. Durand, R. G. Brown, D. Worrall and F. Wilkinson, J. Photochem. Photobiol., A: Chem., 1996, 96, 35 Search PubMed.
- A. Ouardaoui, D. M. Martino, C. A. Steren and H. van Willigen, Appl. Magn. Reson., 1997, 13, 275 Search PubMed.
- A.-P. Y. Durand, R. G. Brown, D. Worrall and F. Wilkinson, J. Chem. Soc., Perkin Trans. 2, 1998, 365 RSC.
- F. Bonnichon, G. Grabner, G. Guyot and C. Richard, J. Chem. Soc., Perkin Trans. 2, 1999, 1203 RSC.
- C. Guyon, P. Boule and J. Lemaire, Tetrahedron Lett., 1982, 23, 1581 CrossRef CAS.
- C. Guyon, P. Boule and J. Lemaire, Nouv. J. Chim., 1984, 8, 685 Search PubMed.
- H. Meier and K.-P. Zeller, Angew. Chem., 1975, 87, 52 CAS.
- M. Yagihara, Y. Kitahara and T. Asao, Chem. Lett., 1974, 1015 CAS.
- B. Urwyler and J. Wirz, Angew. Chem., Int. Ed. Engl., 1990, 29, 790 CrossRef.
- P. Boule, K. Othmen, C. Richard, B. Szczepanik and G. Grabner, Int. J. Photoenergy, 1999, 1, 49 Search PubMed.
- R. J. McMahon, O. L. Chapman, R. A. Hayes, T. C. Hess and H.-P. Krimmer, J. Am. Chem. Soc., 1985, 107, 7597 CrossRef CAS.
- J. J. M. Vleggaar, A. H. Huizer, P. A. Kraakman, W. P. M. Nijssen, R. J. Visser and C. A. G. O. Varma, J. Am. Chem. Soc., 1994, 116, 11754 CrossRef CAS.
- Y. Chiang, A. J. Kresge and V. V. Popik, J. Am. Chem. Soc., 1999, 121, 5930 CrossRef CAS.
- Z. Shi, M. E. Sigman, M. M. Ghosh and R. Dabestani, Environ. Sci. Technol., 1997, 31, 3581 CrossRef CAS.
- R. H. Schuler and G. K. Buzzard, Int. J. Radiat. Phys. Chem., 1976, 8, 563 Search PubMed.
| This journal is © The Royal Society of Chemistry 2001 |
