Unusual products from CO/ethene reactions catalysed by β-ketophosphine and related complexes of rhodium
Ruth A. M.
Robertson
a,
Andrew D.
Poole
b,
Marc J.
Payne
b and
David J.
Cole-Hamilton
*a
aSchool of Chemistry, University of St. Andrews, St. Andrews, Fife, Scotland, UK KY16 9ST. E-mail: djc@st-and.ac.uk
bBP, Salt End, Hull, UK HU12 8DS
First published on 15th December 2000
Abstract
Using rhodium complexes of tertiary phosphines with carbonyl groups β to the P atom, ethene and CO react in methanol to give products involving increased chain growth (octane-3,6-dione, methyl 4-oxohexanoate) compared with PEt3 complexes and unsaturated products (methyl propenoate, penten-3-one and 1-methoxypentan-3-one from addition of methanol to penten-3-one); mechanistic studies suggest that the ligand carbonyl group prevents coordination of the keto group in the growing chain.
Reactions between ethene and CO in methanol generally give perfectly alternating polyketones or methyl propanoate (MP), with the best catalysts being based on palladium complexes of mono- or ditertiary phosphines,1,2 although we have recently reported that highly electron rich rhodium phosphine complexes can give high selectivities to pentan-3-one (DEK), with the two extra H atoms required being derived from methanol, which forms methyl formate (MF).3 We presented evidence that the selectivity to pentan-3-one (DEK) arose because of binding of the keto-oxygen atom in the growing chain to the rhodium atom to give an η2-3-oxopentyl intermediate. Since this complex has 18e, it more readily protonates and reductively eliminates pentan-3-one than undergoing further insertion to give chain growth (Scheme 1).3
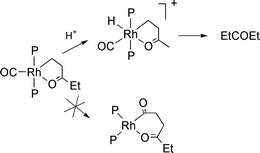 | ||
| Scheme 1 Proposed role of an η2-3-oxopentyl intermediate in determining the selectivity of ethene carbonylation to pentan-3-one catalysed by Rh/PEt3 complexes.3 | ||
One of our interests is in the production of CO/C2H4 oligomers for use as low-volatility solvents containing relatively high oxygen content, so we were interested in the possibility of encouraging chain growth and hence of preventing the formation of the η2-3-oxopentyl intermediate. We, therefore, synthesised a range of phosphines which themselves contain carbonyl groups β to the P atom in the hope that these carbonyl groups might compete with coordination of the keto group in the growing chain and encourage chain growth.
The ligands shown in Table 1 were
synthesised by the reaction of R2PH (R = Et, But, Cy)
with the appropriate bromo compound, R′COCH2Br. (R′
= Ph, Et, OEt), followed by removal of HBr with base (Scheme 2). Catalytic reactions were then carried
out, synthesising the active catalyst in situ from the ligand and
[Rh(acac)(CO)2] (Hacac = pentane-2,4-dione). The results of
these reactions are shown in Table 1 and
indicate that, apart from the complex derived from
But2PCH2C(O)Ph, which does not give an
active catalyst, catalysts based on these ligands show quite different
selectivities compared with those involving PEt3. In particular,
chain growth to octane-3,6-dione (OD) and methyl 4-oxohexanoate (M4OH) has
become significant and the unsaturated products, penten-3-one (EVK) and
methyl propenoate (MA) are observed. A further product,
1-methoxypentan-3-one (1M3P)†
is a major product. We have shown in separate experiments that this is
formed by addition of methanol to penten-3-one (EVK) in an uncatalysed
reaction under the experimental conditions employed. The selectivity to
medium chain products (![[greater than or equal, slant]](https://www.rsc.org/images/entities/char_2a7e.gif) 7 chain atoms) can be as high as 57.6%, whereas
with PEt3 the selectivity to pentan-3-one (DEK) can be >80%
with only traces of medium chain products being formed. Similar results to
those for the β-ketophosphines are obtained (Table 1) using other ligands with O γ to the
P atom such as Me2PCH2P(O)Me2, or
Et2PCH2CH2OMe, but not with N in this
position; Et2PCH2CH2NEt2
behaves more like PEt3, although the rate is lower.
7 chain atoms) can be as high as 57.6%, whereas
with PEt3 the selectivity to pentan-3-one (DEK) can be >80%
with only traces of medium chain products being formed. Similar results to
those for the β-ketophosphines are obtained (Table 1) using other ligands with O γ to the
P atom such as Me2PCH2P(O)Me2, or
Et2PCH2CH2OMe, but not with N in this
position; Et2PCH2CH2NEt2
behaves more like PEt3, although the rate is lower.
| Ligand | MA | MP | EVK | DEK | 1M3P | M4OH | OD | Total turnover | MCP(%) |
|---|---|---|---|---|---|---|---|---|---|
| a [Rh(acac)(CO)2] (0.1 mmol), phosphine (0.4 mmol), CO (35 bar), ethene (35 bar), methanol (10 cm3), 110 °C, 24 h. Amounts expressed as catalyst turnovers. b 2 equivalents of ligand used, i.e. (0.2 mmol). MA (methyl propenoate, methyl acrylate), MP (methyl propanoate), EVK (penten-3-one, ethyl vinyl ketone), DEK (pentan-3-one, diethyl ketone), 1M3P (1-methoxypentan-3-one), M4OH (methyl 4-oxohexanoate), OD (octane-3,6-dione), MCP (medium chain products, 7+ atoms in backbone). | |||||||||
| Et2PCH2C(O)Ph | 4.4 | 4.4 | 2.0 | 24.0 | 12.5 | 2.4 | 9.0 | 58.7 | 40.7 |
| Et2PCH2C(O)Et | 6.7 | 7.8 | 3.1 | 23.4 | 12.0 | 2.6 | 7.1 | 62.7 | 34.6 |
| Et2PCH2C(O)OEt | 12.2 | 6.8 | 3.7 | 38.2 | 14.3 | 5.8 | 9.7 | 90.7 | 32.9 |
| Et2PCH(Me)C(O)Me | 7.2 | 6.9 | 1.4 | 39.7 | 6.7 | 2.5 | 3.1 | 67.5 | 18.2 |
| Cy2PCH2C(O)Ph | — | 4.0 | — | 0.5 | 1.2 | 2.0 | 0.7 | 8.4 | 46.4 |
| But2PCH2C(O)OEt | — | 2.1 | 1.7 | 7.7 | 11.2 | — | 4.4 | 27.1 | 57.6 |
| But2PCH2C(O)Ph | — | — | — | — | — | — | — | 0 | — |
| Et2PC2H4OMe | 4.1 | 15.0 | 1.5 | 37.3 | 17.8 | 3.0 | 9.2 | 87.9 | 34.1 |
| Et2PC2H4NEt2 | — | 26.9 | trace | 41.1 | 3.3 | — | 4.4 | 75.7 | 10.2 |
| Me2PCH2P(O)Me2 | — | 10.7 | 0.9 | 3.5 | 3.7 | — | 2.9 | 21.7 | 30.4 |
| Me2PCH2P(O)Me2b | 4.4 | 5.1 | 3.4 | 24.9 | 13.4 | 2.9 | 14.3 | 68.4 | 44.7 |
 | ||
| Scheme 2 Sythesis of β-ketophosphine ligands. R″ = H, R = Et, R′ = Ph, OEt, Et; R″ = H, R = But, R′ = Ph, OEt; R″ = H, R = Cy, R′ = Ph; R″ = Me, R = Et, R′ = Me. Reagent: i, NaOH. | ||
We have also carried out the reaction using PhCOCH2PEt2 in CD3OD and find that the methyl groups of pentan-3-one (DEK) and of methyl propanoate (MP) contain 0 or 1 D atoms.‡ This contrasts with reactions involving PEt3,3 where multiple deuteriation of the methyl groups of pentan-3-one (DEK) is observed.
These results point to the conclusion that, in these systems, the carbonyl group in the growing chain does not coordinate, presumably because the coordination site is blocked by the carbonyl group β to the phosphine (Scheme 3). Intermediate A in Scheme 3 is an 18e complex, so may protonate and reductively eliminate pentan-3-one (DEK). Alternatively, CO may insert leading to chain growth. We have shown that multiple D incorporation into the methyl group of pentan-3-one (DEK) in the Rh/PEt3 catalysed reactions carried out in CD3OD occurs via β-H abstraction in the η2-3-oxopentyl intermediate to give an enolate. A similar β-H abstraction in the η1-3-oxopentyl intermediate, B in Scheme 3, would lead to penten-3-one (EVK) bound only through the double bond and it appears that this decoordinates to give free penten-3-one (DEK), rather than undergoing reversible C–H bond breakage and multiple D incorporation.
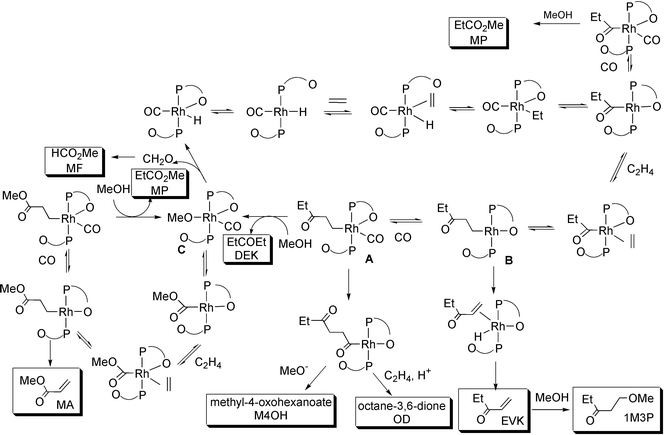 | ||
Scheme 3
Proposed mechanism of ethene carbonylation catalysed by
Rh/β-ketophosphine (P O) complexes. The products shown in boxes
have been observed (Table 1), but the
assignments of the metal containing intermediates are tentative. O) complexes. The products shown in boxes
have been observed (Table 1), but the
assignments of the metal containing intermediates are tentative.
| ||
The formation of methyl propenoate (MA) is also of considerable interest, not only because it indicates that acrylates can be products from CO/C2H4 reactions under non-oxidative conditions, but also because it indicates that a carbomethoxy mechanism1 is operating in addition to the hydride mechanism which is responsible for the other products. This suggests that CO insertion into the Rh–OMe bond competes with β-H abstraction and CO can insert in the 18e complex (C in Scheme 3). Acrylates can be products of CO/alkene reactions in the presence of oxygen.5,6
In conclusion, all of the products obtained from the reaction of CO with ethene in methanol in the presence of rhodium complexes containing phosphine ligands with a carbonyl β to the P atom can be explained as in Scheme 3 if one carbonyl group in the phosphine is coordinated to the rhodium.
Acknowledgements
We thank BP and the EPSRC for funding (R. A. M. R.) and Dr E. Ditzel and Mr P. Howard for helpful discussions.Notes and references
- E. Drent, J. A. M. van Broekhoven and P. H. M. Budzelaar, in Applied Homogeneous Catalysis with Organometallic Compounds, ed. B. Cornils and W. A. Herrmann, VCH, Weinheim, 1996, vol. 1, p. 333 and references therein. Search PubMed.
- E. Drent and P. H. M. Budzelaar, J. Organomet. Chem., 2000, 593–594, 211 CrossRef CAS.
- R. A. M. Robertson, A. D. Poole, M. Payne and D. J. Cole-Hamilton, J. Chem. Soc., Dalton Trans., 2000, 1817 RSC.
- I. N. Nazarov and I. V. Torgov, Chem. Abstr., 1948, 42, 7736a Search PubMed.
- D. M. Fenton and K. L. Olivier, US Pat., 3 349 119, 1967..
- W. Gaenzler and K. Kabs, US Pat., 3 907 882, 1975..
Footnotes |
| † Since this product has seven atoms in the chain and contains two O atoms (bp 62 °C at 24 Torr),4 it may be a suitable component of a low volatility solvent mixture, so is included when calculating the percentage of medium-chain products produced in the reaction. |
| ‡ The methylene groups contain from 0 to 2 D atoms on account of post-reaction exchange with the solvent.3 |
| This journal is © The Royal Society of Chemistry 2001 |
