Ferromagnetic interactions in the first dicubane-type complex involving cyanate ligand: [Co4(dpk-OH)2(dpk-OMe)2(NCO)4]
M. Gotzone
Barandika
a,
Zurine
Serna
b,
Roberto
Cortés
*a,
Luis
Lezama
b,
M. Karmele
Urtiaga
c,
M. Isabel
Arriortua
c and
Teófilo
Rojo
*b
aDepartamento de Química Inorgánica, Fac. Farmacia, UPV/EHU, Aptdo. 450, Vitoria, E-01080, Spain.. E-mail: qipcomor@lg.ehu.es
bDepartamento de Química Inorgánica, Fac. Ciencias, UPV/EHU, Aptdo. 644, E-48080, Bilbao, Spain
cDepartamento de Mineralogía-Petrología, Fac. Ciencias, UPV/EHU, Aptdo. 644, E-48080, Bilbao, Spain
First published on 15th December 2000
Abstract
The ligands cyanate and di-2-pyridyl ketone (dpk) are used to construct a tetranuclear cobalt(II) complex involving μ1,1-cyanate bridges: [Co4(dpk-OH)2(dpk-OMe)2(NCO)4], which shows a dicubane-type structure, with two missing vertices, and having ferromagnetic interactions.
A considerable ongoing research has been directed at the preparation of molecule-based magnets over recent years. In this area, great activity has been focused on obtaining nanoscale magnets in which each microcrystal behaves as a single domain. A way to deal with the preparation of nanomagnets concerns single molecules having ground electronic states with a large number of unpaired electrons.1 In relation to the ligands for the preparation of those clusters, a variety of blocking organic compounds has been used, most of them providing oxo-bridges between metallic centers.2
An efficient way for the generation of interactions between metallic centers concerns the use of pseudohalide ligands. Among these, azide has been undoubtedly one of the most interesting magnetic coupling species found so far in molecular magnetism.3 One of the latest findings found for this ligand is its incorporation into cubane-type systems and those exhibiting μ1,1-azido bridges have been observed to be ferromagnetic.4 The cyanate pseudohalide is a more unusual ligand, its chemistry and bonding properties have been the subject of several studies5 which focussed upon its ability to coordinate to metals, and its ability and adaptability as a bridging ligand.6 It shows preference for the end-on bridging mode, through the nitrogen atom. In this kind of bridging, the cyanate ligand has been shown to be able to mediate ferromagnetic interactions.7
As mentioned above, in most of the clusters for nanomagnets, the intermetallic connections take place through oxo-bridges. In this way, the coordination behavior of di-2-pyridyl ketone (dpk) as a ligand has also attracted much attention.8 Its hydrolyzed derivatives, namely, the gem-diol9 and its respective mono- and di-anions, are potential chelating or chelating-bridging ligands. In fact, a number of mononuclear10 and oligomeric polynuclear11 complexes derived from these ligands have been isolated and crystallographically characterized. Solvolysis reactions are also observed to occur in contact with other solvents thus widening the possibilities in structural arrangement.
For the indicated reasons above, the simultaneous use of dpk and cyanate ligands could be expected to enhance the formation of intermetallic bridges. Thus, taking into account these considerations, this work reports on a ferromagnetic tetranuclear cobalt(II) system of formula [Co2(dpkOH)(dpkOMe)(NCO)2]2, the structural core of which can be described as that of a ‘dicubane’ having two missing vertices. Very few examples of cobalt(II) dicubanes are known12 and no cobalt–μ1,1-cyanate polynuclear compound has been characterized structurally and magnetically to date. Furthermore, as far as we are aware, this is the first example of a ‘cubane-type’ system involving cyanate ligands.
Reaction of dpk, Co(NO3)2·4H2O and KNCO yielded,† after evaporation, a brown-red crystalline material, the composition of which was consistent with the formula [Co2(C23H20N4- O4)(NCO)2] 1. The X-ray crystal structure determination of 1 (Fig. 1)‡ revealed that it consists of centrosymmetric tetranuclear units in which the cobalt(II) ions are connected through the ligands NCO, dpkOH, and dpkOMe (the latter tworesulting from solvolysis of the dpk ligand) to give a face-shared dicubane-like coordination core containing two missing vertices (Fig. 1).
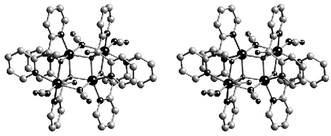 | ||
| Fig. 1 Stereoview of the tetranuclear structure of compound 1. Sizes of spheres is in the order Mn > C > N > O. Hydrogen atoms have been omitted for clarity. | ||
The Co(II) ions exhibit slightly distorted octahedral [CoN3O3] and [CoN4O2] environments, with the Co–N distances ranging from 2.024(6) (dpk) to 2.146(4) Å (cyanate) and the Co–O distances ranging from 2.025(3) to 2.244(3). The EO cyanate bridges form an angle with the metal of 101.8(2)°, while the Co–O–Co angles range from 97.1(1) to 100.6(2)°. The Co…Co distance through the combined cyanate and oxo bridges is 3.283(2) Å, while those corresponding to the oxo-bridges alone are 3.144(1) and 3.238(2) Å.
The temperature dependence of the magnetic susceptibility χm of 1 has been investigated in the range 4–300 K. The product χmT continuously increases upon cooling and its value, per 4 Co atoms, at room temperature is 11.7 cm3 K mol−1, which is larger than the 7.5 cm3 K mol−1 expected for four isolated spin-only S = 3/2 ions. This larger value is the result of contributions to the susceptibility from orbital angular momentum at high temperatures, which produces effective moments per cobalt atom in the range 4.7–5.2 μB.13 A maximum of 18.3 cm3 K mol−1 appears at 12 K. After this maximum, χmT decreases [Fig. 2(a)]. This behavior is characteristic of a system exhibiting intramolecular ferromagnetic interactions; while the sudden decrease must be associated to spin–orbit coupling and/or the existence of intermolecular interactions. It is not possible to fit the behavior of an array of four high-spin CoII centers given current theory, even if the centers are orbitally degenerate. Besides, in the present compound the environment of the Co(II) centers which are in distorted octahedra, probably removes the degenereracy. Taking into account that, even for the simplest of the possible Co(II) tetranuclear systems (i.e. a square planar cluster with a single J value14), fitting has not been carried out, the extreme difficulty of modeling a four-J system (see Scheme 1) for this cation that should include aspects such as spin–orbit coupling, magnetic anisotropy, orbital degeneracy, etc, should be appreciated.
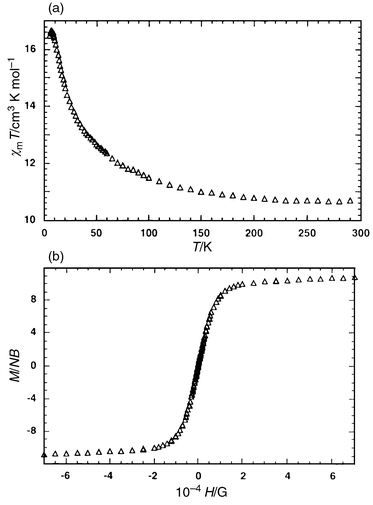 | ||
| Fig. 2 (a) Thermal variation of χmT for 1, per tetranuclear unit. (b) Representation of the magnetization vs. magnetic field at 5 K. | ||
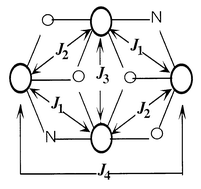 | ||
| Scheme 1 | ||
In order to gain more information on 1, we investigated the variation of the magnetization M vs. the applied magnetic field H, in the field range 0 < H/T < 7 at 5 K and results are shown in Fig. 2(b), where it can be observed that the magnetization reaches the saturation value expected for a tetranuclear ferromagnetic cobalt(II) compound, i.e.Nβg/2 (≈ 12). Further studies are being undertaken to thoroughly characterize this system.
The ferromagnetic behavior of this compound can be explained by the existence of end-on cyanate bridges, with angles close to 100°, which gives rise to moderately strong ferromagnetic interactions.7 In addition, the oxo bridges in the compound also promote ferromagnetic interactions, as has been observed in related cubane oxo-bridged compounds.11b
In conclusion, the combination of dpk and cyanate ligands has been shown to be a good strategy for obtaining ‘cubane-like’ systems and related nickel, cobalt and manganese systems are now under investigation. The resulting compound 1 shows a tetranuclear molecular structure of dicubane type which is unprecedented for cyanate ligand. The bridging ligands connecting the cobalt(II) ions provide global ferromagnetic exchange interactions.
Acknowledgements
Fundings by the Basque Government (grant PI 99/53), the University of the Basque Country (grant UPV 130310-EB201/1998), and the Dirección General de Enseñanza Superior-MEC (Spain) (grant PB97-0637) are gratefully acknowledged. Z. E. S. thanks the University of the Basque Country for a doctoral fellowship.Notes and references
- D. P. Goldberg, A. Caneschi, C. D. Delfs, R. Sesoli and S. J. Lippard, J. Am. Chem. Soc., 1995, 117, 5789 CrossRef CAS; A. K. Powell, S. L. Heath, D. Gatteschi, L. Pardi, R. Sessoli, G. Spina, F. D. Giallo and F. J. Pieralli, J. Am. Chem. Soc., 1995, 117, 2491 CrossRef CAS; A. L. Barra, A. Caneschi, D. Gatteschi and R. Sessoli, J. Am. Chem. Soc., 1995, 117, 8855 CrossRef CAS.
- S. L. Castro, Z. Sun, C. M. Grant, J. C. Bellinger, D. N. Hendrickson and G. J. Christou, J. Am. Chem. Soc., 1998, 120, 2365 CrossRef CAS.
- D. M. Duggan and D. N. Hendrickson, Inorg. Chem., 1973, 12, 2422 CrossRef CAS; J. Commarmond, P. Plumeré, J. M. Lehn, Y. Agnus, R. Louis, R. Weiss, O. Kahn and I. Morgenstern-Badarau, J. Am. Chem. Soc., 1982, 104, 6330 CrossRef; P. Chaudhuri, M. Guttmann, D. Ventur, K. Weighardt, B. Nuber and J. Weiss, J. Chem. Soc., Chem. Commun., 1985, 1618 RSC; R. Cortés, L. Lezama, F. A. Mautner and T. Rojo, in Molecule-Based Magnetic Materials, ed. M. M. Turnbull, T. Sugimoto and L. K. Thomson, ACS Book Series, Washington, 1996, pp. 187–200; Search PubMed; J. Ribas, M. Monfort, R. Vicente, A. Escuer, R. Cortés, L. Lezama and T. Rojo, Coord. Chem. Rev., 1999, 193–195, 1027 and references therein. Search PubMed.
- M. A. Halcrow, J. C. Huffman and G. Christou, Angew. Chem., Int. Ed. Engl., 1995, 34, 889 CrossRef CAS; Z. E. Serna, L. Lezama, M. K. Urtiaga, M. I. Arriortua, M. G. Barandika, R. Cortés and T. Rojo, Angew. Chem., Int. Ed., 2000, 39, 344 CrossRef CAS.
- J. L. Bursmeister, Coord. Chem. Rev., 1968, 3, 225 CrossRef; J. Kohout, M. Havastijova and J. Gazo, Coord. Chem. Rev., 1987, 27, 141 CrossRef.
- A. H. Norbury, Adv. Inorg. Chem. Radiochem., 1975, 17, 232 Search PubMed; A. H. Golub, H. Kohler and V. V. Slopenko, Chemistry of Pseudohalides, Elsevier, Amsterdam, 1986, p. 239. Search PubMed.
- M. I. Arriortua, R. Cortés, J. L. Mesa, L. Lezama, T. Rojo and G. Villeneuve, Transition Met. Chem., 1988, 13, 371 Search PubMed.
- J. A. R. Navarro, M. A. Romero, M. J. Salas, M. Quirós and E. R. T. Tiekink, Inorg. Chem., 1997, 36, 4988 CrossRef CAS; M. C. Feller and R. Robson, Aust. J. Chem., 1968, 21, 2919 CAS; A. Basu, T. G. Kasar and N. Y. Sapre, Inorg. Chem., 1998, 27, 4539; A. Basu, A. R. Saple and N. Y. Sapre, J. Chem. Soc., Dalton Trans., 1987, 1797 RSC.
- N. Okabe and M. Koizumi, Acta Crystallogr., Sect. C., 1998, 54, 288 CrossRef; R. Faure, H. Loiseleur and G. Thomas-David, Acta Crystallogr., Sect. B, 1973, 29, 1890 CrossRef CAS.
- G. Annibale, L. Canovese, L. Cattalini, G. Natile, M. Biagini-Cingi, A. Manotti-Lanfredi and A. Tiripicchio, J. Chem. Soc., Dalton Trans., 1981, 2280 RSC; S. Wang, J. W. Richardson, S. J. Briggs, R. A. Jacobson and W. P. Jensen, Inorg. Chim. Acta, 1986, 111, 67 CrossRef CAS; M. L. Tong, G. Yang, X. M. Chen and S. W. Ng, Acta Crystallogr., Sect. C, 1998, 54, 217 CrossRef.
- (a) V. Tangoulis, S. Paschalidou, E. G. Bakalbassis, S. P. Perlepes, C. P. Raptopoulou and A. Terzis, Chem. Commun., 1996, 1297 RSC; (b) A. Tsohos, S. Dionissopoulou, C. P. Raptopoulou, A. Terzis, E. G. Bakalbassis and S. P. Perlepes, Angew. Chem., Int. Ed., 1999, 34, 983 CrossRef CAS.
- J. A. Bertrand, E. Fujita and D. G. VanDeerVeer, Inorg. Chem., 1979, 18, 230 CrossRef CAS; R. M. Buchanan, B. J. Fitgerald and C. G. Pierpont, Inorg. Chem., 1979, 18, 3439 CrossRef CAS.
- R. L. Carlin, Magnetochemistry, Springer-Verlag, New York, 1986. Search PubMed.
- O. Waldmann, J. Hassmann, P. Müller, G. S. Hanan, D. Volkmer, U. S. Schubert and J. M. Lehn, Phys. Rev. Lett., 1997, 78, 3390 CrossRef CAS; O. Waldmann, J. Hassmann, P. Müller, D. Volkmer, U. S. Schubert and J. M. Lehn, Phys. Rev. B, 1998, 58, 3277 CrossRef CAS.
- G. M. Sheldrick, SHELX-97, Program for structure solution and refinement, University of Göttingen, Germany, 1997..
Footnotes |
| † Experimental procedure: reaction of a methanolic solution (10 cm3) containing dpk (1 mmol) with a aqueous solution (10 cm3) containing both Co(NO3)2·4H2O (1 mmol) and KNCO (1 mmol) yielded, after two weeks evaporation, a brown crystalline material 1. Yield 63%. Elemental analysis. Calc. for C25H20N6O6Co2: C, 48.56; H, 3.26; N, 13.59; Co, 19.06. Found: C, 48.13; H, 3.15; N, 13.22; Co, 19.06%. |
| ‡ Crystal data: C25H20N6O6Co2, M = 618.33, monoclinic, space group P21/c (no. 14), a = 13.039(1), b = 12.303(1), c = 19.365(1) Å, β = 122.57(1)°, V = 2618.0(4) Å3, Z = 4, Dc = 1.569 g cm−3, 2θmax = 60°, T = 293 K, ω–2θ scans; Lorentzian, polarization, and extinction corrections were made, μ(Mo-Kα) = 13.2 cm−1, 14672 reflections measured, 6653 unique (Rint = 0.1483) all included in the refinement; structure solution by direct methods; 353 parameters refined R1(Fo) = 0.058 [for 6653 reflections with I > 2σ(I)]; wR2(Fo2)= 0.098 (all data); quality of fit 0.80. Max./min. residual peaks in the final difference map 0.531/−0.605 e Å−3. Diffraction data were collected on an Enraf-Nonius CAD-4 diffractometer. The structure was solved by direct methods and refined with SHELXL-97.14 All non-H atoms were refined anisotropically. CCDC 182/1849. See http://www.rsc.org/suppdata/cc/b0/b008256m/ for crystallographic files in .cif format. |
| This journal is © The Royal Society of Chemistry 2001 |
