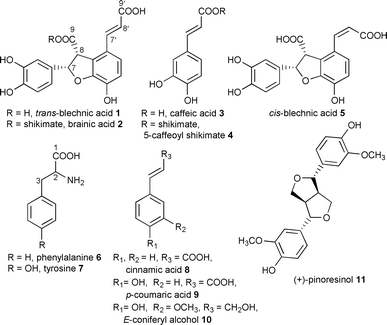
Stereoselective phenolic coupling in Blechnum spicant: formation of 8–2′ linked (−)-cis-blechnic, (−)-trans-blechnic and (−)-brainic acids†
Chang-Zeng Wang, Laurence B. Davin and Norman G. Lewis*
Institute of Biological Chemistry, Washington State University, Pullman, USA WA 99164-6340. E-mail: lewisn@wsu.edu;; Tel.: 509-335-2682;; Fax: 509-335-8206
First published on 15th December 2000
Abstract
In vivo administration experiments using stable (13C) and radio (14C) labeled precursors provide further evidence for vascular plant proteins engendering specific but distinct phenolic coupling modes, i.e. in this case for stereoselective 8–2′ coupling leading to the optically active lignans, (–)-blechnic and (–)-brainic acids.
The ferns (Pteridophytes) are evolutionary forerunners of the gymnosperms and angiosperms. They contain various phenolic natural products which may represent some of the earliest examples in planta of control of stereoselective phenolic coupling. For example, Blechnum spicant (deer fern) and B. orientale1 accumulate the unusually linked, optically active, 8–2′ lignans, (−)-blechnic 1 and (−)-brainic 2 acids.
Gymnosperm and angiosperm lignans are frequently derived from stereoselective 8–8′ coupling of two monolignol (hydroxycinnamyl alcohol) radicals,2 and the recent discovery of dirigent proteins (Latin: dirigere, to guide or to align) and their corresponding genes gave a new perspective into how free radical coupling is controlled in plants in vivo.3–5 There have been no reports on the stereoselective control of other coupling modes, and hence establishing the mechanism of 8–2′ linked lignan formation in the Blechnaceae would be instructive.
The MeOH extract of B. spicant contains as its principal metabolites, caffeic acid 3, 5-caffeoyl shikimate 4, (−)-trans-blechnic acid 1 and (−)-brainic acid 2. An unknown compound 5 was also isolated, which on standing in open solution (in CH3CN or H2O) over 72 h, was converted into (−)-trans-blechnic acid 1. Compound 5 ([α]D −128°, c 0.58, MeOH) had a molecular formula of C18H14O8 as evidenced from its HR-MS (381.7373 [M+Na]+); furthermore, comparison of its 1H and 13C NMR spectral data with those of (−)-blechnic acid (−)-1 revealed that both structures were very similar, except for a cis-double bond in 5 [1H NMR: 6.74 (1H, d, J = 12.7 Hz, H-7′), 5.88 (1H, d, J = 12.7 Hz, H-8′); 13C NMR: 139.7 (C-7′) and 120.2 (C-8′)] vs. the trans double bond in blechnic acid 1 [1H NMR: 7.57 (1H, d, J = 16.1 Hz, H-7′), 6.27 (1H, d, J = 16.1 Hz, H-8′); 13C NMR: 143.3 (H-7′) and 117.8 (H-8′)]. Correlations (or connectivities) for compound 5 were determined by 2D-NMR (1H-1H COSY, HMQC, HMBC) spectroscopic analyses conducted at low temperature (−25°C), and the configuration of the cis-double bond was further confirmed by both NOE experiments and a comparison to that of (−)-trans-blechnic acid 1.
The precursor relationships to the optically active 8–2′ linked lignans was examined through deployment of radiolabeled Phe 6, Tyr 7, cinnamic 8, p-coumaric 9 and acetic acids as potential precursors over extended durations (Table 1). Thus, caffeic acid 3, 5-caffeoyl shikimate 4, (−)-cis-blechnic 5, (−)-trans-blechnic 1 and (−)-brainic 2 acids, were individually isolated with the relative total radiochemical incorporation for each estimated by liquid scintillation counting (see Table 1). Significantly, a rapid incorporation into (−)-cis-blechnic acid 5 (∼3%) was noted within 8 h, relative to that of (−)-trans-blechnic 1 and (−)-brainic 2 acids (⩽0.1%). This indicated that (−)-cis-blechnic acid 5 was the initial coupling product, since it was apparently further metabolized into (−)-trans-blechnic 1 and (−)-brainic 2 acids based on the trends of total incorporation noted for 1 and 2 which approached c. 1–2 and 3–4% over 48–84 h, respectively. A less likely interpretation is that 5 might be a shunt metabolite and not directly involved in the formation of 1 and 2. For each time frame examined, the relative incorporation into (−)-brainic acid 2 was higher than that into (−)-blechnic acid 1, suggesting that cis-blechnic acid 5 was more effectively channeled into (−)-brainic acid 2 rather than into (−)-blechnic acid 1. In a somewhat analogous manner, both [3-14C]cinnamic 8, and [2-14C]p-coumaric 9 acids served as precursors, whereas neither [U-14C]tyrosine 7 nor [2-14C]NaOAc were incorporated into any of the various metabolites of B. spicant examined, (data not shown).
| Potential precursor administered (radio-activity) | Metabolic period (h) | Caffeic acid 3 (% incor- poration) | 5-Caffeoyl shikimate 4 | (−)-cis- Blechnic acid 5 | (−)- trans- Blechnic acid 1 | (−)- Brainic acid 2 | |
|---|---|---|---|---|---|---|---|
| A | [U-14C] Phenylalanine 6 (200 μL, 148 kBq, 18.5 GBq nmol−1) | 4 | 1.2 | 1.7 | 1.1 | <0.1 | <0.1 |
| 8 | 2.3 | 3.4 | 3.0 | <0.1 | 0.1 | ||
| 12 | 2.3 | 2.5 | 2.6 | 0.1 | 0.4 | ||
| 18 | 2.0 | 3.2 | 3.9 | 0.5 | 1.4 | ||
| 24 | 2.9 | 4.1 | 2.3 | 0.6 | 1.6 | ||
| 30 | 2.9 | 5.1 | 2.7 | 0.7 | 1.6 | ||
| 36 | 5.0 | 4.7 | 2.0 | 0.7 | 1.9 | ||
| 48 | 4.5 | 5.3 | 1.4 | 1.1 | 2.9 | ||
| 84 | 2.8 | 3.3 | 1.3 | 2.1 | 4.2 | ||
| B | [3-14C] Cinnamic acid 8 (200 μL, 185 kBq, 1.89 GBq nmol−1) | 24 | 2.9 | 6.9 | 3.5 | 2.0 | 1.5 |
| 50 | 3.8 | 5.6 | 1.0 | 1.1 | 1.4 | ||
| 84 | 2.7 | 5.6 | 1.2 | 1.5 | 1.2 | ||
| C | [2-14C] p-Coumaric acid 9 (200 μL, 148 kBq, 6.07 GBq nmol−1) | 24 | 1.0 | 3.0 | 1.4 | 0.5 | 0.7 |
L-[3-13C], [2-13C] and [1-13C] phenylalanine 6 (1.0 mM) were next individually administered to B. spicant fronds for 5 days, with the resulting (−)-brainic 2 and (−)-trans-blechnic 1 acids isolated by preparative μBondapak C18 column HPLC and subjected to 13C-NMR spectroscopic analyses (see Fig. 1a–d). Only the data for (−)-brainic acid 2 are presented. Fig. 1a shows the natural abundance NMR spectrum and the corresponding assignments for (−)-brainic 2 acid; these are based on 2-D NMR spectroscopic analyses (HMQC, HMBC and 1H-1H COSY) and previous assignments by Wada et al.1 The (−)-brainic 2 acid obtained following administration of [1-13C]Phe 6 displayed carbon-13 enriched resonances for C-9 and C-9′ at 170.5 and 170.6 ppm, respectively (Fig. 1b). In a similar manner, administration of [2-13C]Phe 6 gave enhanced signals for the C-8 and C8′ resonances of (−)-brainic 2 acid at 55.4 and 118.2 ppm (Fig. 1c), whereas with [3-13C]Phe 6, the corresponding enhanced signals at C-7 and C7′ were at 88.0 and 142.7 ppm (Fig. 1d). Comparable results were observed for (−)-trans-blechnic acid 1 biosynthesis (see ESI†).
![13C-NMR spectra of (a) natural abundance (−)-brainic
2 acid, as well as (−)-brainic acids 2 obtained
following administration of (b) [1-13C], (c) [2-13C]
and (d) [3-13C]phenylalanine 6 (1 mM) to B.
spicant fronds for 5 days; all spectra were recorded under identical
conditions.](/image/article/2001/CC/b008174o/b008174o-f1.gif) | ||
| Fig. 1 13C-NMR spectra of (a) natural abundance (−)-brainic 2 acid, as well as (−)-brainic acids 2 obtained following administration of (b) [1-13C], (c) [2-13C] and (d) [3-13C]phenylalanine 6 (1 mM) to B. spicant fronds for 5 days; all spectra were recorded under identical conditions. | ||
We propose that the 8–2′ linked lignans, (−)-blechnic 1 and (−)-brainic 2 acids, result from oxidative coupling of caffeic acid 3 as depicted in Scheme 1. Based on the trends of relative incorporations into 5, 1 and 2, this conversion is likely to result via stereoselective coupling of two molecules of either cis- or trans-caffeic acid 3 to first afford (−)-cis-blechnic acid 5. Less likely, stereoselective coupling could involve p-coumaric acid 9 with subsequent hydroxylation of the aromatic ring to ultimately afford the corresponding catechols 1 and 2. In any case, by comparison to the dirigent mediated stereoselective bimolecular coupling of coniferyl alcohol 10 affording (+)-pinoresinol 11, it is tempting to suggest that the formation of the blechnates 1 and 2 may involve a comparable process.4,5 This will be established in the future by isolation and characterization of the relevant proteins and enzymes involved in the biosynthesis of (−)-trans-blechnic acid 1 and its derivatives in B. spicant; nevertheless both 8–8′ and 8–2′ linked phenylpropanoid coupling products are under full proteinaceous control thereby stipulating the outcome of stereoselective coupling.
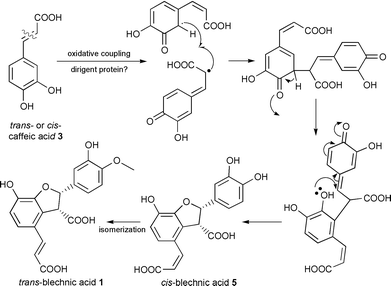 | ||
| Scheme 1 Proposed stereoselective coupling of caffeic acid 3 leading to (−)-trans-blechnic acid 1. | ||
Acknowledgements
The authors thank the USDOE (DE-FG03-97ER20259) and the NSF (MCB-9976684), as well as the L. B. and D. Cullman and G. T. Hargrove Center for Land Plant Adaptation.Notes and references
- H. Wada, T. Kido, N. Tanaka, T. Murakami, Y. Saiki and C. M. Chen, Chem. Pharm. Bull., 1992, 40, 2099 Search PubMed.
- N. G. Lewis and L. B. Davin, Lignans: Biosynthesis and Function, in Comprehensive Natural Products Chemistry, Vol. 1, ed. Sir D. H. R. Barton, K. Nakanishi and O. Meth-Cohn, Elsevier, London, pp 639-712. Search PubMed.
- L. B. Davin and N. G. Lewis, An. Acad. Bras. Cienc. Suppl. 3, 1995, 67, 363 Search PubMed.
- L. B. Davin, H. B. Wang, A. L. Crowell, D. L. Bedgar, D. M. Martin, S. Sarkanen and N. G. Lewis, Science, 1997, 275, 362 CrossRef CAS.
- D. R. Gang, M. A. Costa, M. Fujita, A. T. Dinkova-Kostova, H. B. Wang, V. Burlat, W. Martin, S. Sarkanen, L. B. Davin and N. G. Lewis, Chem. Biol., 1999, 6, 143 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: 13C-NMR spectra of (a) natural abundance (−)-trans-blechnic 1 acid, as well as (−)-trans-blechnic acids 1 obtained following administration of (b) [1-13C], (c) [2-13C] and (d) 3-13C]-phenylalanine 6 (1 mM) to B. spicant fronds for 5 days; all spectra were recorded under identical conditions. The relative carbon-13 enrichments noted were higher for (−)-brainic acid 2 than for (−)-trans-blechnic 1 due in large part to the much higher endogeneous levels of the latter in B. spicant. See http://www.rsc.org/suppdata/cc/b0/b008174o/ |
| This journal is © The Royal Society of Chemistry 2001 |