Metal ion complexation by a new, highly sterically hindered, bowl-shaped carboxylate ligand†
Ferman A.
Chavez
,
Lawrence
Que Jr.
* and
William B.
Tolman
*
Department of Chemistry and Center for Metals in
Biocatalysis, University of Minnesota, 207 Pleasant St. SE, Minneapolis, Minnesota 55455, USA.. E-mail: que@chem.umn.edu; tolman@chem.umn.edu
First published on 15th December 2000
Abstract
A carboxylate encapsulated by arene groups arranged in a bowl-like shape coordinates to Fe(II), Co(II) and Cu(II) to form mononuclear complexes with atypical structures enforced by the extreme steric demands of the ligand.
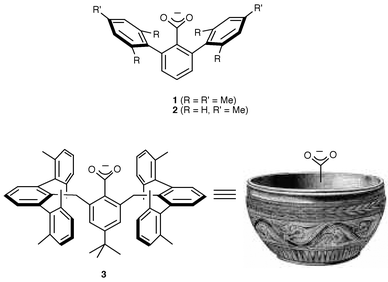
Carboxylate groups (from Asp or Glu side chains or C termini) play key roles as supporting ligands in a diverse array of metalloprotein active sites.1 Such carboxylates are notable for the facility with which they adopt different binding modes,2 in particular during the catalytic cycles of dioxygen-activating mono- and di-iron enzymes.3 In efforts to synthesize models of these metalloprotein active sites, we4 and others5,6 have begun using sterically bulky carboxylate ligands in order to control coordination geometry, mimic the hydrophobic active site environment, and access coordinatively unsaturated species akin to those implicated during enzymatic catalysis. For example, novel biologically relevant structures and reactivity have been discovered for iron complexes of carboxylates 14 and 2,5 which contain arene substituents on the benzoate unit that provide a high degree of hydrophobic ‘shielding’. In expectation of even greater encapsulation of metal sites in model complexes, we targeted 3, a carboxylate derivative of the known irregular ‘bowl-shaped’ 4-tert-butyl-2,6-bis[(2,2″,6,6″-tetramethyl-m -terphenyl-2′-yl)methyl]phenyl (Bmt) fragment that was used to isolate various species BmtX (X = Br, SH, SO, SI, SO2H, AlH3).7 Herein we report the successful synthesis and X-ray crystallographic characterization of BmtCO2H (3-H) and Fe(II), Co(II) and Cu(II) complexes of 3, which adopt atypical structures owing to the extreme steric bulk of the new carboxylate ligand.
The synthesis of BmtCO2H was achieved by lithiation of BmtBr,‡ addition of CO2(g), acidification, and then column chromatography. The product was identified using spectroscopic (ESI†) and X-ray diffraction§ data. Treatment of BmtCO2H with BunLi in thf afforded the lithium carboxylate (BmtCO2Li·2thf) that was isolated as an analytically pure solid for use as the starting material for the preparation of metal complexes.
Reaction of BmtCO2Li·2thf (2 equiv.) with MCl2 in MeOH afforded [M(BmtCO2)2(MeOH)n]·m MeOH [M = Fe(II), Co(II), n = m = 4; M = Cu(II), n = 2, m = 0]. Use of >2 equiv. of the carboxylate yielded the same products, indicating that only two of these bulky ligands may be accommodated. The X-ray crystal structures§ of the Fe and Cu complexes are shown in Figs. 1 and 2, respectively. The Co structure is isomorphous with that of Fe, so only data for the Fe case is presented. Common to all of the complexes is a trans disposition of two BmtCO2– ligands that coordinate in a syn monodentate fashion. The noncoordinating carboxylate oxygen atoms participate in hydrogen bonding with the bound MeOH ligands (Fig. 3). Additional hydrogen bonding occurs in the Fe and Co complexes involving included solvent MeOH molecules. Similar intramolecular hydrogen bonding patterns have been seen in other Fe(II)8 and Cu(II)9 carboxylate complexes and have been suggested to play a role in stabilizing their structures.
![Representation of the X-ray crystal structure of
[Fe(BmtCO2)2- (MeOH)4]·4MeOH as 50%
thermal ellipsoids, with H atoms and the solvent molecules omitted for
clarity. Selected bond distances (Å) and angles (°):
Fe(1)–O(2) 2.125(18), Fe(1)–O(3) 2.162(2), Fe(1)–O(4)
2.091(2), Fe(1)⋯O(1) 3.225(2); O(4)–Fe(1)–O(2) 89.69(8),
O(4)–Fe(1)–O(3) 90.86(10), O(2)–Fe(1)–O(3)
89.30(8).](/image/article/2001/CC/b006647h/b006647h-f1.gif) | ||
| Fig. 1 Representation of the X-ray crystal structure of [Fe(BmtCO2)2- (MeOH)4]·4MeOH as 50% thermal ellipsoids, with H atoms and the solvent molecules omitted for clarity. Selected bond distances (Å) and angles (°): Fe(1)–O(2) 2.125(18), Fe(1)–O(3) 2.162(2), Fe(1)–O(4) 2.091(2), Fe(1)⋯O(1) 3.225(2); O(4)–Fe(1)–O(2) 89.69(8), O(4)–Fe(1)–O(3) 90.86(10), O(2)–Fe(1)–O(3) 89.30(8). | ||
![Representation of the X-ray crystal structure of
[Cu(BmtCO2)2- (MeOH)2]as 50%
thermal ellipsoids, with H atoms omitted for clarity. Selected bond
distances (Å) and angles (°): Cu(1)–O(2) 1.893(2),
Cu(1)–O(3) 1.950(2), Cu(1)⋯O(1) 3.245;
O(2)–Cu(1)–O(3) 91.70(9), O(2)–Cu(1)–O(3a)
88.30(9).](/image/article/2001/CC/b006647h/b006647h-f2.gif) | ||
| Fig. 2 Representation of the X-ray crystal structure of [Cu(BmtCO2)2- (MeOH)2]as 50% thermal ellipsoids, with H atoms omitted for clarity. Selected bond distances (Å) and angles (°): Cu(1)–O(2) 1.893(2), Cu(1)–O(3) 1.950(2), Cu(1)⋯O(1) 3.245; O(2)–Cu(1)–O(3) 91.70(9), O(2)–Cu(1)–O(3a) 88.30(9). | ||
![Coordination spheres of (a)
[Fe(BmtCO2)2(MeOH)4]·4MeOH (showing
two of the MeOH solvent molecules; the other two are highly disordered) and
(b) [Cu(BmtCO2)2(MeOH)2], with hydrogen
bonding interactions indicated by dashed lines. Relevant interatomic
distances (Å): (a) O(1)⋯O(4a) 2.582(3), O(1)⋯O(3)
3.199(3), O(3)⋯O(6b) 2.718(7), (b) O(1)⋯O(3) 2.511(3)
Å.](/image/article/2001/CC/b006647h/b006647h-f3.gif) | ||
| Fig. 3 Coordination spheres of (a) [Fe(BmtCO2)2(MeOH)4]·4MeOH (showing two of the MeOH solvent molecules; the other two are highly disordered) and (b) [Cu(BmtCO2)2(MeOH)2], with hydrogen bonding interactions indicated by dashed lines. Relevant interatomic distances (Å): (a) O(1)⋯O(4a) 2.582(3), O(1)⋯O(3) 3.199(3), O(3)⋯O(6b) 2.718(7), (b) O(1)⋯O(3) 2.511(3) Å. | ||
Mononuclear bis(carboxylato) iron(II) complexes possessing trans carboxylates are rare, 4a,c,10 and [Fe(BmtCO2)2(MeOH)4] is a unique example with an all-oxygen donor set. The Ocarb–Fe–Ocarb angle of 180° presumably results from the tendency of the BmtCO2– ligands to position themselves as far apart as possible. This angle in the other two known bis(carboxylato) iron(II) complexes with trans monodentate carboxylate groups deviates significantly from linearity [168.5(2) and 154.74(8)°].4a,c,10 The small space remaining in the equatorial plane of the octahedral BmtCO2– complex is ideal for accommodation of small donors such as MeOH.
A Cu(II) complex possessing the same donor set as [Cu(BmtCO2)2(MeOH)2] has been reported,9a but in this complex of a functionalized benzoate (‘furosemide’) there are additional weak axial interactions between the Cu(II) center and the carboxylate oxygen atoms from adjacent molecules [Cu–O 2.720(4) Å]. In the BmtCO2– compound, no other potential donor ligand is within bonding distance to the square-planar Cu(II) center due to blocking of the apical site by the carboxylate xylyl groups. The mononuclear structure of the complex contrasts with the familiar dinuclear paddlewheel11 or other common topologies in which carboxylates bridge between multiple Cu(II) centers.12 The discrete structure of [Cu(BmtCO2)2(MeOH)2] is also unusual insofar as many Cu(II) carboxylate complexes which exist as monomers in solution form intermolecular hydrogen bonded extended structures in the solid state.13
In conclusion, we have developed a synthesis of the carboxylate 3 in which the ligating unit is encapsulated in an irregular ‘molecular bowl’. The extreme steric demands of the ligand have been illustrated through characterization of the monomeric Fe(II), Co(II) and Cu(II) complexes comprising two molecules of 3 coordinated trans in a syn monodentate mode. Further studies will explore the potential for the use of 3 and the complexes described herein for accessing unusual structures pertinent to nonheme, carboxylate rich metalloprotein active sites.
Acknowledgements
We thank Dr Katherine Aubrecht and Dr Victor G. Young, Jr., for the structural determination of BmtCO2H (3-H), Jamie Schneider for her efforts in improving the synthesis of BmtBr, and the NIH (GM38767 to L. Q.) for financial support.Notes and references
- R. H. Holm, P. Kennepohl and E. I. Solomon, Chem. Rev., 1996, 96, 2239 CrossRef CAS.
- R. L. Rardin, W. B. Tolman and S. J. Lippard, New J. Chem., 1991, 15, 417 CAS.
- A. L. Feig and S. J. Lippard, Chem. Rev., 1994, 94, 759 CrossRef CAS; L. Que, Jr., in Bionorganic Catalysis, ed. J. Reedijk and E. Bouman, Marcel Dekker, Inc., 1999 Search PubMed; B. J. Wallar and J. D. Lipscomb, Chem. Rev., 1996, 96, 2625 Search PubMed.
- (a) J. R. Hagadorn, L. Que, Jr. and W. B. Tolman, J. Am. Chem. Soc., 1998, 120, 13531 CrossRef CAS; (b) J. R. Hagadorn, L. Que, Jr., W. B. Tolman, I. Prisecaru and E. Münck, J. Am. Chem. Soc., 1999, 121, 9760 CrossRef CAS; (c) J. R. Hagadorn, L. Que, Jr. and W. B. Tolman, Inorg. Chem., 2001, 40, in press. Search PubMed.
- (a) D. Lee and S. J. Lippard, J. Am. Chem. Soc., 1998, 120, 12153 CrossRef CAS; (b) D. Lee, J. D. Bois, D. Petasis, M. P. Hendrich, C. Kres, B. H. Huynh and S. J. Lippard, J. Am. Chem. Soc., 1999, 121, 9893 CrossRef CAS; (c) D. Lee, C. Krebs, B. H. Huynh, M. P. Hendrich and S. J. Lippard, J. Am. Chem. Soc., 2000, 122, 5000 CrossRef CAS.
- S. C. Payne and K. S. Hagen, J. Am. Chem. Soc., 2000, 122, 6399 CrossRef CAS.
- (a) K. Goto, J. Kobayashi and R. Okazaki, Organometallics, 1999, 18, 1357 CrossRef CAS; (b) K. Goto, M. Holler and R. Okazaki, Chem. Commun., 1998, 1915 RSC; (c) K. Goto, M. Holler and R. Okazaki, J. Am. Chem. Soc., 1997, 119, 1460 CrossRef CAS; (d) K. Goto and R. Okazaki, Liebigs Ann./Recueil, 1997, 2393 Search PubMed; (e) K. Goto, M. Holler and R. Okazaki, Tetrahedron Lett., 1996, 37, 3141 CrossRef CAS.
- S. Herold and S. J. Lippard, J. Am. Chem. Soc., 1997, 119, 145 CrossRef CAS; M. M. Morelock, M. L. Good, L. M. Trefonas, R. Majeste and D. G. Karraker, Inorg. Chem., 1982, 21, 3044 CrossRef CAS.
- (a) For examples, see: P. Bontchev, H. Kadum, G. Gochev, B. Evtimova, J. Macicek and C. Nachev, Polyhedron, 1992, 11, 1973 CrossRef; (b) H. Muhonen, Acta Crystallogr., Sect. C, 1983, 39, 536 CrossRef.
- B. Singh, J. R. Long, G. C. Papaefthymiou and P. Stavropoulos, J. Am. Chem. Soc., 1996, 118, 5824 CrossRef CAS; B. Singh, J. R. Long, F. F. deBiani, D. Gatteschi and P. Stavropoulos, J. Am. Chem. Soc., 1997, 119, 7030 CrossRef CAS.
- R. J. Doedens, Prog. Inorg. Chem., 1975, 19, 173 Search PubMed; M. Melnik, Coord. Chem. Rev., 1982, 42, 259 CrossRef CAS.
- G. Psomas, C. P. Raptopoulou, L. Iordanidis, C. Dendrinou-Samara, V. Tangoulis and D. P. Kessissoglou, Inorg. Chem., 2000, 39, 3042 and references cited therein. Search PubMed.
- For example, see: H. Muhonen, Acta Crystallogr., Sect. C, 1983, 39, 536 CrossRef; C. K. Prout, R. A. Armstrong, J. R. Carruthers, J. G. Forrest, P. Murray-Rust and J. C. Rossotti, J. Chem. Soc. A, 1968, 2791 RSC; C. K. Prout, M. J. Barrow and F. J. C. Rossotti, J. Chem. Soc. A, 1971, 3326 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available: detailed procedures for the syntheses of BmtBr, BmtCO2H and BmtCO2Li as well as characterization data for all new compounds. See http://www.rsc.org/suppdata/cc/b0/b006647h/ |
| ‡ The precursor BmtBr was synthesized via the method described in ref. 7(e), but improvements were made that allowed the product to be isolated in higher yield (70% vs. 32%). For detailed procedures for the syntheses of BmtBr, BmtCO2H and BmtCO2Li, see ESI.† |
§ Crystal data for
BmtCO2H (3-H):
C171H174O6, M = 2325.10,
triclinic, space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , a = 13.2288(7),
b = 21.7697(11), c = 26.0770(13) Å,
α = 76.040 (1), β = 82.286(1),
γ = 84.717(1)°, V = 7208(1)
Å3, T = 173(2) K, Z = 2,
μ(Mo-Kα) = 0.062 mm–1, 63469 reflections
measured, 32161 unique (Rint = 0.027) which were used
in all calculations. The final wR(F2) was
0.1298 (all data), R1 = 0.0541. For
[Fe(BmtCO2)2(MeOH)4]·4MeOH:
C122H146O12Fe, M = 1860.24,
monoclinic, space group P21/n, a =
21.1152(13), b = 13.3988(9), c = 21.1719(13) Å,
β = 115.0900(10)°, V = 5424.7(6)
Å3, T = 173(2) K, Z = 2,
μ(Mo-Kα) = 0.198 mm–1, 27850 reflections
measured, 9562 unique (Rint = 0.0481) which were used
in all calculations. The final wR(F2) was
0.1772 (all data), R1 = 0.0558. For
[Cu(BmtCO2)2(MeOH)2]:
C116H122O6Cu, M = 1675.68,
monoclinic, space group P21/n, a =
15.2154(7), b = 11.1085(5), c = 27.7956(12) Å,
β = 97.9700(10)°, V = 4652.6(4)
Å3, T = 173(2), Z = 2,
μ(Mo-Kα) = 0.292 mm–1, 23238 reflections
measured, 8203 unique (Rint) = 0.0439) which were used
in all calculations. The final wR(F2) was
0.1795 (all data), R1 = 0.0598. CCDC 182/1859. See
http://www.rsc.org/suppdata/cc/b0/b006647h/ for crystallographic files in
.cif format. , a = 13.2288(7),
b = 21.7697(11), c = 26.0770(13) Å,
α = 76.040 (1), β = 82.286(1),
γ = 84.717(1)°, V = 7208(1)
Å3, T = 173(2) K, Z = 2,
μ(Mo-Kα) = 0.062 mm–1, 63469 reflections
measured, 32161 unique (Rint = 0.027) which were used
in all calculations. The final wR(F2) was
0.1298 (all data), R1 = 0.0541. For
[Fe(BmtCO2)2(MeOH)4]·4MeOH:
C122H146O12Fe, M = 1860.24,
monoclinic, space group P21/n, a =
21.1152(13), b = 13.3988(9), c = 21.1719(13) Å,
β = 115.0900(10)°, V = 5424.7(6)
Å3, T = 173(2) K, Z = 2,
μ(Mo-Kα) = 0.198 mm–1, 27850 reflections
measured, 9562 unique (Rint = 0.0481) which were used
in all calculations. The final wR(F2) was
0.1772 (all data), R1 = 0.0558. For
[Cu(BmtCO2)2(MeOH)2]:
C116H122O6Cu, M = 1675.68,
monoclinic, space group P21/n, a =
15.2154(7), b = 11.1085(5), c = 27.7956(12) Å,
β = 97.9700(10)°, V = 4652.6(4)
Å3, T = 173(2), Z = 2,
μ(Mo-Kα) = 0.292 mm–1, 23238 reflections
measured, 8203 unique (Rint) = 0.0439) which were used
in all calculations. The final wR(F2) was
0.1795 (all data), R1 = 0.0598. CCDC 182/1859. See
http://www.rsc.org/suppdata/cc/b0/b006647h/ for crystallographic files in
.cif format. |
| This journal is © The Royal Society of Chemistry 2001 |