A novel layered oxysulfide intergrowth compound Sr4Mn2Cu5O4S5 containing a fragment of the α-Cu2S antifluorite structure
Nicolas
Barrier
and
Simon J.
Clarke
*
Inorganic Chemistry Laboratory, Department of Chemistry, University of Oxford, South Parks Road, Oxford, UK OX1 3QR. E-mail: simon.clarke@chem.ox.ac.uk; Fax: 44 1865 272690; Tel: 44 1865 272600
First published on 2nd December 2002
Abstract
Sr4Mn2Cu5O4S5 contains manganese oxide sheets separated by unusual antifluorite-type Cu3S3 layers in which copper(I) ions are distributed over three- and four-coordinate sites in a similar fashion to in α-Cu2−xS and suggestive of high two-dimensional copper ion mobility.
Ternary manganese oxides are of great importance.1 Perovskites, pyrochlores and Ruddlesden-Popper (R–P) phases with a range of MnIII/IV ratios often exhibit the phenomenon of giant magnetoresistance and have a range of composition-dependent crystallographic, magnetic and electronic properties.
Oxysulfides contain anions with different sizes and chemical requirements which order crystallographically, often resulting in layered structures. Phases reminiscent of the R–P oxides, in which perovskite-type oxide layers are separated by Cu2S22− anti-PbO-type puckered layers, have been reported including Sr2MnCu2O2S22 (MnII) containing MnO2 sheets alternating with Cu2S2 layers, Sr2MnCuO3S3 (MnIII) containing double layers of corner-linked MnO5 pyramids separated by Cu2S2 layers and Sr4Mn3Cu2O7.5S23 (MnIII) which contains triple oxide layers, similar to those in the n = 3 R–P MnIV manganate Sr4Mn3O10. Here we demonstrate that more sulfide-rich materials containing thicker sulfide layers are accessible and report the structure, from single crystal X-ray diffraction measurements of Sr4Mn2Cu5O4S5, the first of these complex intergrowth compounds to contain a copper sulfide antifluorite-type layer of approximate stoichiometry Cu3S3 which, along with Cu2S2 layers, separates MnO2 layers. This antifluorite layer resembles the Cu4S3 layers in the sulfides KCu4S3,4 and the homologous series TlCu2nSn+15 but is highly defective and the Cu+ ions may be modelled as disordered over two sites with large displacement ellipsoids, suggesting that they are mobile within the copper sulfide slabs. Such an antifluorite layer with copper ion disorder closely resembles a fragment of the high-temperature antifluorite fast copper ion conductor α-Cu2−xS.6
A ground, compacted mixture of MnO2, SrO, SrS, Sr and Cu2S† in the ratio 2∶1∶1∶1∶1 was contained within a 10 mm diameter alumina crucible sealed under vacuum within a silica tube and heated for 18 days at 1050 °C with the intention of synthesising 1.5 g of Sr3Mn2Cu2O5S2, (cf. Sr3Fe2Cu2O5S2.7) Energy dispersive analysis of X-rays (EDX)† showed that the ratios Sr∶Mn∶Cu∶S in the black, shiny crystals extracted from the pellet were either 2∶1.5∶1∶1.1 or 2∶1∶2.4∶2.6 (estimated standard deviations of 10% on these values.) Reactants were handled in a dry box; the products were air stable.
Single crystal X-ray diffraction measurements‡ confirmed that the crystals poorer in copper and sulfur were Sr4Mn3Cu2O7.5S2.3 The lattice parameters and body centred tetragonal symmetry of the other crystals identified as Sr4Mn2Cu5O4S5 (a = 4.0157(1), c = 39.995(1)) suggested a new layered structure. Structure solution using Direct Methods yielded a structure (Fig. 1) in which MnO2 sheets are separated alternately by the familiar Cu2S2 layers and by unusual antifluorite-type layers of ideal stoichiometry Cu4S3 which may be constructed by fusing two of the Cu2S2 layers together. Subsequent syntheses using reaction mixtures richer in Cu2S resulted in a reproducibly higher yield of Sr4Mn2Cu5O4S5, however reactions carried out using the appropriate stoichiometric mixture of reactants have not enabled preparation of this material as a single phase—the major products being Sr2MnCu2O2S22 occurring with copper sulfide, the title phase and other, as yet unidentified, products.
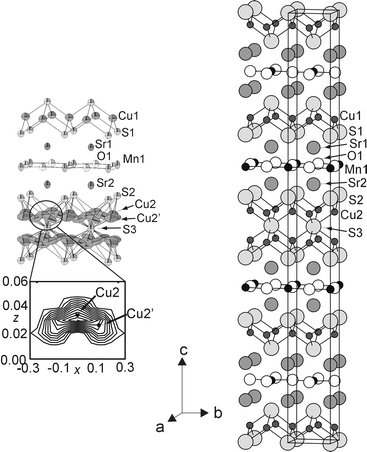 | ||
| Fig. 1 Right: Idealised crystal structure of Sr4Mn2Cu5O4S5 showing Cu2S2 layers and defective Cu4S3 antifluorite-type layers separating MnO2 layers. Left: A portion of the structure derived from single crystal X-ray diffraction (90% displacement ellipsoids). Cu2 is modelled as a split site (Cu2 and Cu2′) which accounts for the Fourier map of the observed electron density due to copper ions in the antifluorite-type sulfide layers (blow-up region). | ||
Refinement against single crystal diffraction data of a model in which a single Cu2 atom was located, as in KCu4S3,4 or TlCu4S3,5 in tetrahedral sites in the antifluorite-type copper sulfide layers produced an isotropic atomic displacement parameter (ADP) for this atom which was four times that of the similarly-coordinated Cu1 atom located in the Cu2S2 single sulfide layers. Subsequent refinement of the occupancy of Cu2 to around 0.75 and refinement of anisotropic ADPs for all atoms led to an unacceptably high R-factor (R(F2)) of 0.109 and a residual electron density of 7.87 e Å−3 located 0.79 Å from the Cu2 position. An observed Fourier map (Fig. 1), indicated that the copper ions in the antifluorite-type sulfide layers could be modelled using two sites. Refinement using this model with all ADPs anisotropic and occupancies of all three Cu sites refined produced an R-factor (R(F2)) of 0.038 and a positive residual electron density of 1.74 e Å−3 located 0.72 Å from Mn1. The displacement ellipsoids of Cu2 and Cu2′ (Fig. 1) are highly anisotropic and reproduce the electron density distribution obtained in the observed Fourier map. The composition derived from refinement of the structure against single crystal diffraction data is consistent with that derived from EDX analysis and the stoichiometry of the title compound is Sr4Mn2Cu4.6(2)O4S4.92(1). In the anti-PbO-type single copper sulfide layer, the Cu1 site is not fully occupied and the formulation is Cu1.61(1)□0.39(1)S2, where □ represents a cation vacancy. In the antifluorite-type copper sulfide layer the composition departs from the ideal Cu4S3 stoichiometry, observed in KCu4S3, which would arise from full occupancy of all the available tetrahedral sites and refines to Cu3.0(1)□1.0(1)S2.92(1)□0.08(1) ≈‘Cu3S3’ (□ represents cation and anion vacancies). The structure is composed of layers arranged in the sequence (MnO2)(Cu1.6S2)(MnO2)(Cu3S3) and separated by Sr2+ ions located in eight-coordinate (4 × S and 4 × O) sites.
| Atom | Site | x | y | z | Occ. |
U
eq/Å2
×100 |
|---|---|---|---|---|---|---|
| a 293 K, Space group I4/mmm, a = 4.0157(1) Å, c = 39.995(1) Å, Z = 2. Sr4Mn2Cu4.6(2)O4S4.92(1), M = 974, Dc= 5.02 g cm−3. | ||||||
| Sr1 | 4e | 0.5 | 0.5 | 0.31654(2) | 1 | 1.28(2) |
| Sr2 | 4e | 0.5 | 0.5 | 0.59752(2) | 1 | 1.46(2) |
| Mn1 | 4e | 0 | 0 | 0.64055(3) | 1 | 1.29(3) |
| Cu1 | 4d | 0.5 | 0 | 0.25 | 0.804(6) | 2.08(4) |
| Cu2 | 8g | 0 | 0.5 | 0.0342(2) | 0.24(2) | 3.4(3) |
| Cu2′ | 16n | 0.157(3) | 0.5 | 0.0259(2) | 0.25(1) | 4.8(2) |
| O1 | 8g | 0.5 | 0 | 0.64170(9) | 1 | 1.44(8) |
| S1 | 4e | 0 | 0 | 0.28461(5) | 1 | 1.40(4) |
| S2 | 4e | 0 | 0 | 0.42945(5) | 1 | 1.61(4) |
| S3 | 2a | 0.5 | 0.5 | 0.5 | 0.92(2) | 2.4(1) |
The crystallographic copper ion disorder in the antifluorite-type sulfide layers is qualitatively similar to that in the fast ion conducting α- and β-forms of Cu2S.6,8 The high-temperature phase α-Cu2−xS (x = 0.05(4))6 has a modification of the antifluorite structure type in which the mobile copper ions are displaced towards the four trigonal sites located at the faces of the ideal tetrahedral sites and may be modelled, following the results of single crystal neutron diffraction measurements, with anharmonic displacement ellipsoids.6 In Sr4Mn2Cu5O4S5, the antifluorite-type sulfide layers represent a highly copper-deficient two-dimensional fragment of α-Cu2−xS in which the copper ions are displaced from their ideal tetrahedral positions towards the two trigonal sites on the two faces of a CuS4 tetrahedron which point towards the central plane of the layers. In Sr4Mn2Cu5O4S5 the Cu2 atoms are modelled using split sites (Fig. 1) with anisotropic displacement ellipsoids similar in size to those determined for the mobile copper ions in α- and β-Cu2S6,8 and the mobile silver ions in LaAgOS,9 suggesting that the copper ions in Sr4Mn2Cu5O4S5 may be similarly mobile in two dimensions. The structure of the antifluorite-type copper sulfide layer in Sr4Mn2Cu5O4S5 contrasts with those in KCu4S34 and TlCu2nSn+15 in which the copper ions have displacement ellipsoids which are isotropic and very similar in size to those of the Cu1 site in Sr4Mn2Cu5O4S5.
The Cu1–S1 distances of 2.439(1) Å are similar to those found in the Cu2S2 layers of related phases2,3,7 (e.g. Cu–S 2.446(3) Å in Sr2MnCu2O2S2.2) The Cu2–S distances for the analogous tetrahedral sites of the antifluorite-type sulfide layer (Cu2–S2 2.480(5) Å; Cu2–S3 2.429(5) Å) are on average (2.45(3) Å) similar to the Cu–S distances in the idealised α-Cu2S structure (2.437 Å). The Cu2′ sites have a similar triangular coordination by sulfide (Cu2′–S2 2.26(1) Å; Cu2′–S3 2.346(5) Å × 2) to the actual Cu sites in α-Cu2-xS6 (2.333 Å × 3).
The Mn1–O1 distances of 2.0084(1) Å (× 4) are similar to those in the Mn2+-containing compound Sr2MnCu2O2S22 (2.0009 Å × 4) and the mean of the Mn1–S1 (2.993(2) Å) and Mn1–S2 (2.800(2) Å) distances at 2.897 Å is very similar to the two identical Mn–S distances of 2.876 Å in Sr2MnCu2O2S2,2 and substantially longer than would be expected for Mn–S bonding distances. Bond valence calculations,10 applied tentatively to this structure with few free crystallographic parameters,11 and assuming Cu+ and S2−, suggest a manganese oxidation state of +2.4, consistent with the X-ray derived composition: Sr4Mn2Cu4.6(2)O4S4.92(1) (i.e. Mn +2.6(1)). The similarity of the manganese coordinations in Sr4Mn2Cu5O4S5 and Sr2MnCu2O2S2 suggests that in both, the manganese oxidation state is +2, which would require holes in the copper chalcogenide layers, as in the metals KCu4S3,4 TlCu2nSn+15 and the layered oxyselenide Bi2YO4Cu2Se2.12 Electrical conductivity measurements on Sr4Mn2Cu5O4S5 are hampered by the existance only of small single crystals. Preliminary measurements on a non-oriented crystal (dimensions 0.03 × 0.01 × 0.01 cm) using a two-probe arrangement with In/Ga eutectic contacts revealed resistivities of 5(1) Ω cm at 290 K and 7(1) Ω cm at 100 K, suggesting semiconducting behaviour. Further investigation of Sr4Mn2Cu5O4S5 and related compounds is in progress.
We thank the UK EPSRC (grant GR/N18758) for funding. S. J. C. thanks the Royal Society for further financial support.
Notes and references
- B. Raveau, A. Maignan, C. Martin and M. Hervieu, Chem. Mater., 1998, 10, 2641 CrossRef CAS; M. A. Subramanian, B. H. Toby, A. P. Ramirez, W. J. Marshall, A. W. Sleight and G. H. Kwei, Science, 1996, 273, 81 CAS; P. D. Battle and M. J. Rosseinsky, Curr. Opin. Solid State Mater. Sci., 1999, 4, 1 63 CrossRef CAS.
- W. J. Zhu and P. H. Hor, J. Solid State Chem., 1997, 130, 319 CrossRef CAS.
- W. J. Zhu and P. H. Hor, J. Solid State Chem., 2000, 153, 26 CrossRef CAS.
- D. R. Brown, J. A. Zubieta, P. A. Vella, J. T. Wrobleski, T. Watt, W. E. Hatfield and P. Day, Inorg. Chem., 1980, 19, 1945 CrossRef CAS.
- K. Klepp, H. Boller and H. Völlenkle, Monatsh. Chem., 1980, 111, 727 CAS; R. Berger and L. Eriksson, J. Less-Common Met., 1990, 161, 165 Search PubMed; R. Berger, J. Less-Common Met., 1989, 147, 141 Search PubMed.
- M. Oliveria, R. K. McMullan and B. J. Wuensch, Solid State Ionics, 1988, 28–30, 1332 Search PubMed.
- W. J. Zhu and P. H. Hor, J. Solid State Chem., 1997, 134, 128 CrossRef CAS.
- R. J. Cava, F. Reidinger and B. J. Wuensch, Solid State Ionics, 1981, 5, 501 Search PubMed.
- D. Wilmer, J. D. Jorgensen and B. J. Wuensch, Solid State Ionics, 2000, 136–137, 961 Search PubMed.
- A. S. Wills and I. D. Brown, VaList, CEA, France, 1999. Program available from ftp://ftp.ill.fr/pub/dif/valist/.
- I. D. Brown, J. Solid State Chem., 1989, 82, 122 CrossRef CAS.
- J. S. O. Evans, E. B. Brogden, A. L. Thompson and R. L. Cordiner, Chem. Commun., 2002, 2002, 912 RSC.
- A. Altomare, M. C. Burla, M. Camalli, G. L. Cascarano, C. Giacovazzo, A. Guagliardi, A. G. G. Moliterni, G. Polidori and R. Spagna, J. Appl. Crystallogr., 1999, 32, 115 CrossRef CAS.
- W. R. Busing and H. A. Levy, Acta Crystallogr., 1957, 10, 180 CrossRef CAS.
- G. M. Sheldrick, http://shelx.uni-ac.gwdg.de/SHELX/index.html.
Footnotes |
| † MnO2 (Aldrich 99.98%), S (Alfa 99.9995%); SrO prepared by decomposing SrCO3 (Alfa 99.994%) at 900 °C under 2 × 10−2 mbar, SrS prepared by reacting SrCO3 with CS2 (Aldrich 99.9%) carried by flowing argon at 900 °C for 12 h. Cu2S prepared by reacting Cu (Alfa 99.999%) with S at 700 °C for 7 days in an evacuated silica tube. Elemental analysis on single crystals was performed using a JEOL JSM-840A scanning electron microscope with an Oxford Instruments ISIS300 energy dispersive X-ray analyser. |
| ‡ Single crystal XRD data were collected on a 0.06 × 0.06 × 0.04 mm3 shiny black crystal using a Nonius Kappa CCD diffractometer: Mo-Kα radiation (λ = 0.71073 Å); angular range 2 ⩽ 2θ ⩽ 70°; 6854 reflections measured (452 independent reflections with F2 > 2σ(F2)). Structure solution (Direct Methods): SIR97;13 absorption correction: Gaussian integration method based on the crystal shape,14 (μ = 26.47 mm−1; max./min. transmission = 0.44/0.26). Refinement: SHELXL9715 (R1 = 0.038, wR2 = 0.092 for F2 >2σ(F2)). CCDC 195021. See http://www.rsc.org/suppdata/cc/b2/b209747h/ for crystallographic data in CIF or other electronic format. |
| This journal is © The Royal Society of Chemistry 2003 |
