Novel, stereoselective tricyclization of a dienyne by titanium aryloxide centers†
Richard A.
Himes
,
Phillip E.
Fanwick
and
Ian P.
Rothwell
*
Department of Chemistry, Purdue University, 1393 Brown Building, West Lafayette, IN, 47907-1393, USA. E-mail: rothwell@purdue.edu; Fax: 765-494-0239; Tel: 765-494-5473
First published on 28th November 2002
Abstract
Titanium centers supported by aryloxide ligation mediate the tricyclization of a dienyne via intramolecular insertion of an olefin into the titanium–vinyl bond of a titanacyclopent-2-ene.
The metal-mediated bicyclization of enynes has proven to be a powerful synthetic method.1 Group 4 metal complexes facilitate the construction of unsymmetrical cyclic compounds via this methodology2 and promote further functionalization via insertion of carbon monoxide,3 isocyanides,4 and aldehydes.5 However, no report exists of ring expansion via insertion of olefins by Group 4 metal complexes.
As part of our continuing studies of the formation and reactivity of titanacyclic compounds supported by aryloxide ligands we have sought routes to titanacyclopent-2-enes for comparison with metallocene analogues. During this work we have discovered the tricyclization of dienynes, a reaction that appears to proceed via intramolecular insertion of olefin into the titanium–vinyl bond of an unobserved titanacyclopent-2-ene intermediate.6,7
Enynes may be synthesized in two steps from dibromoalkanes.8 The alkynyl proton may be substituted (Scheme 1) with silyl or alkyl groups in moderate to high yield via deprotonation by nBuLi and subsequent quenching with an appropriate chlorosilane9 or primary haloalkane.‡ Bicyclization of 1-trimethylsilyl-6-hepten-1-yne (2) according to the route in Scheme 1 affords a titanacyclopent-2-ene (3) in 64% yield as a yellow powder. This compound does not undergo ring expansion in the presence of excess 1-hexene even at elevated temperatures.
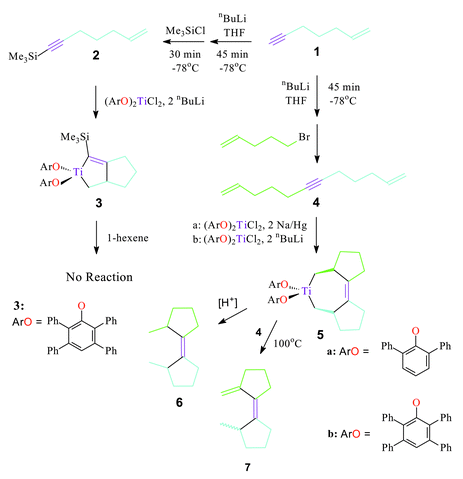 | ||
| Scheme 1 Reaction pathways | ||
Observed as a byproduct in the synthesis of 6-hepten-1-yne (1), 1,11-dodecadien-6-yne (4) may be prepared in 40% yield following the procedure outlined in Scheme 1.§ Aryloxide-supported titanium centers will react with 4 to form a tricyclic titanacyclohept-3-ene (5). The 2,6-diphenylphenoxide derivative (5a) was isolated in greater purity and higher yield (67%) as an orange solid following recrystallization from hot benzene. Slow cooling affords crystals suitable for X-ray diffraction analysis.
Fig. 1 shows the solid-state structure of 5a. Seven-membered titanacyclic rings are scarce.10 The structure of 5a is the first reported for a titanacycloheptene.11 The geometry is pseudo-tetrahedral about the metal center. The bond lengths Ti–C(16), Ti–C(26), Ti–O(3), and Ti–O(4) are within expected limits, though the O(3)–Ti–O(4) angle is opened and the C(16)–Ti–C(26) angle pinched compared to other structurally characterized aryloxide-supported titanacycles.11 The molecule exists as a single isomer with the titanacyclic ring adopting the cis conformation.
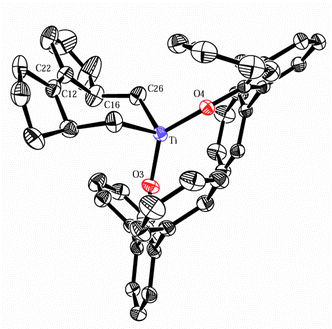 | ||
| Fig. 1 ORTEP view of 5a. Selected bond distances (Å) and angles (°): Ti–O(3) 1.821(2), Ti–O(4) 1.815(2), Ti–C(16) 2.067(4), Ti–C(26) 2.073(4), C(12)–C(22) 1.322(5), O(3)–Ti–O(4) 132.30(11), C(16)–Ti–C(26) 97.41(16), O(3)–Ti–C(16) 104.73(15), O(3)–Ti–C(26) 108.11(14), O(4)–Ti–C(16) 104.48(13), O(4)–Ti–C(26) 104.51(14). | ||
The solid-state structure and stereochemistry are retained in solution, as confirmed by NMR spectroscopy. In the 1H NMR spectrum, the product has a distinct upfield resonance (5a: δ −0.06 ppm; 5b: δ −0.47 ppm) appearing as a sharp doublet. 1H-1H COSY correlations allow unambiguous assignment of the upfield peak to the pseudo-equatorial protons on C(16) and C(26). The protons are coupled (2J = 10.5–10.8 Hz) to the corresponding axial protons. Fig. 1 demonstrates that the pseudo-equatorial protons are in position to experience diamagnetic anisotropy from the phenyl substituents of the aryloxides. The cis conformation is also confirmed by the appearance in the 13C NMR spectrum of two sets of aryloxide peaks, including two well resolved resonances for the ipso Ti–O–C carbons of the inequivalent phenoxides.
Reactive titanium centers are also supplied by precursor titanacycles such as 8 (Scheme 2). The presence of substrate (4) induces retrocyclization of 8 to release 1,7-octadiene. Clean formation of 5b follows. This reaction may be monitored by 1H NMR. Conversion to 5b occurs rapidly at 100 °C, slowly at room temperature, but at no point can intermediates be observed. We hypothesize a mechanism involving intermediate formation of titanacyclopent-2-ene (9) followed by rapid intramolecular insertion of olefin into the titanium–vinyl bond (Scheme 2).
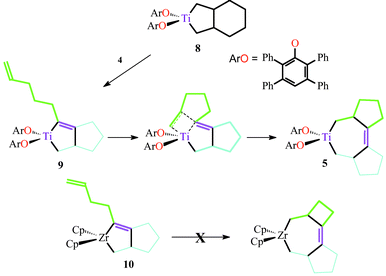 | ||
| Scheme 2 Intramolecular insertion of olefin to give tricyclized product. | ||
Formation of 9 has some precedent. Negishi et al. have reported the in situ synthesis of a zirconacyclopent-2-ene (10) from 1,10-undecadien-5-yne.3 Insertion of olefin does not occur due to the strain of the resulting fused cyclobutane, the electronics of the cyclopentadienyl zirconium center, or a combination of these factors. The reactivity of 10 toward free olefins was not investigated.
Why this mechanism precludes formation of the trans isomer cannot currently be explained. Preference for the cis conformation may arise from the steric demands of the aryloxide ligands. To test this theory, experiments are being conducted with less sterically hindered metal centers.
Simple reactivity of 5 has been investigated. Hydrolysis of the metallacycle yields a novel cyclopentylidene-cyclopentane 6 (Scheme 1). It may be isolated by exposure of a C6D6 solution of 5 to ambient atmosphere followed by preparative-scale TLC. Only one isomer is isolated. Compound 5 also displays thermal reactivity. The metallacycle is unstable at high temperatures (75–100 °C) over long periods. 5a appears to disproportionate into tetraphenoxy titanium and unknown organic products. However, both derivatives of 5 undergo catalysis in the presence of excess dienyne. 1H NMR studies show 5 converts several equivalents of 4 into organic products. We believe the main product to be 7, which forms via β-H abstraction and elimination. However, GC/MS identifies at least five additional C12 and C24 catalytic products. Further elucidation of this reaction will be communicated in due course.
In conclusion, we have synthesized a novel aryloxide-supported titanacyclohept-3-ene via tricyclization of a dienyne. We believe this transformation proceeds through an unprecedented insertion of olefin into the titanium–vinyl bond of a titanacyclopent-2-ene. In addition, the tricyclized organometallic product shows interesting reactivity and synthetic usefulness in forming novel organic molecules by hydrolysis and thermal catalysis. Investigations into further reactivity and all aspects of the chemistry discussed herein are currently underway.
Notes and references
- See the following, and references therein: (a) E.-i. Negishi, in Comprehensive Organic Synthesis,, ed. B. M. Trost, Pergamon, Oxford, 1991,vol. 5, p.1163 Search PubMed; (b) B. M. Trost, Angew. Chem., Int. Ed. Engl., 1995, 34, 259 CrossRef CAS; (c) N. E. Schore, Chem. Rev., 1988, 88, 1081–1119 CrossRef CAS.
- See, for example: (a) S. L. Buchwald and R. B. Nielsen, Chem. Rev., 1988, 88, 1047 CrossRef; (b) R. D. Broene and S. L. Buchwald, Science, 1993, 261, 1696; (c) T. V. RajanBabu, W. A. Nugent, D. F. Taber and P. J. Fagan, J. Am. Chem. Soc., 1988, 110, 7128 CrossRef CAS; (d) E.-i. Negishi, S. J. Holmes, J. M. Tour and J. A. Miller, J. Am. Chem. Soc., 1985, 107, 2568 CrossRef CAS; (e) F. Sato, H. Urabe and S. Okamoto, Chem. Rev., 2000, 100, 2835 CrossRef; (f) H. Urabe, T. Hata and F. Sato, Tetrahedron Lett., 1995, 36, 4261–4264 CrossRef CAS.
- E.-i. Negishi, S. J. Holmes, J. M. Tour, J. A. Miller, F. E. Cederbaum, D. R. Swanson and T. Takahashi, J. Am. Chem. Soc., 1989, 111, 3336 CrossRef CAS.
- S. C. Berk, R. B. Grossman and S. L. Buchwald, J. Am. Chem. Soc., 1994, 116, 8593 CrossRef CAS.
- H. Urabo and F. Sato, J. Org. Chem., 1996, 61, 6756 CrossRef CAS.
- For insertion of olefins into titanacyclopent-3-ene, see: (a) J. E. Hill, P. E. Fanwick and I. P Rothwell, Organometallics, 1991, 10, 3428 CrossRef CAS; (b) J. E. Hill, G. J. Balaich, P. E. Fanwick and I. P. Rothwell, Organometallics, 1993, 12, 2911 CrossRef CAS; (c) G. J. Balaich, J. E. Hill, S. A. Waratuke, P. E. Fanwick and I. P. Rothwell, Organometallics, 1995, 54, 14265.
- For insertions of olefins into titanacyclopenta-2,4-dienes, see: E. S. Johnson, G. J. Balaich and I. P. Rothwell, J. Am. Chem. Soc., 1997, 119, 7685 Search PubMed.
- (a) Synthesis of ω-bromo-1-enes: G. A. Kraus and K. Landgrebe, Synthesis, 1984, 885 Search PubMed; (b) Synthesis of ω-ene-1-yne : W. Novis Smith and O. F. Beumel, Synthesis, 1974, 441 Search PubMed.
- M. Shipman, H. R. Thorpe and I. R. Clemens, Tetrahedron, 1998, 54, 14265 CrossRef CAS.
- Titanacycloheptanes formed by reaction of dilithio- or bis-Grignard reagent with Cp2TiCl2 have been reported : L. M. Engelhardt, Wing-Por Leung, R. I. Papasergio, C. L. Raston, P. Twiss and A. H. White, J. Chem. Soc., Dalton Trans., 1987, 2347 Search PubMed.
- Hetero-titanacycloheptanes have been synthesized. See: M. G. Thorn, J. E. Hill, S. A. Waratuke, E. S. Johnson, P. E. Fanwick and I. P. Rothwell, J. Am. Chem. Soc., 1997, 119, 8630 Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: synthesis of compounds 1–6 and spectroscopic data for 1–3. See http://www.rsc.org/suppdata/cc/b2/b209889j/ |
| ‡ Attempts to synthesize an organometallic compound by reaction of 1 with titanium–aryloxide centers failed. Other researchers have reported difficulty in the cyclization reaction of terminal enynes.2,3 Our system gives a trisubstituted (1-pentenyl)benzene arising from cyclotrimerization as the only isolable product. |
§ Selected spectroscopic data. For 4: 1H NMR (C6D6): δ 5.73–5.60 (m, 2 H), 5.02–4.91 (m, 4 H), 2.08–2.01 (m, 8 H), 1.46 (quin, 4 H); 13C (CDCl3): δ138.24, 115.14, 80.25, 33.04, 28.57, 18.51. HRMS calcd. for C12H17 [M − H]: 161.1330, found: 161.1325. For 5a: 1H NMR (C6D6): δ 7.47–6.89 (aromatics), 2.84 (dd, 2 H), 2.01–1.85 (m, 4H), 1.58 (t, J = 12 Hz, 2H), 1.50–1.31 (m, 6H), 1.29–1.21 (m, 4H), 0.86–0.82 (m, 4H), −0.062 (d, J = 10.5 Hz, 2H); 13C NMR (C6D6): δ 160.23, 159.78 (Ti–O–C); 137.17, 103.13 (TiCH2), 43.52, 38.71, 31.90, 24.52. For 5b: 1H NMR (C6D6): δ 7.39–6.85 (aromatics), 2.85 (dd, 2 H), 2.06–1.82 (m, 4H), 1.50 (t, 2H), 1.46–1.32 (m, 6H), 1.22 (m, 4H), 0.87 (m, 4H), −0.47 (d, J = 10.8 Hz, 2H); 13C NMR (C6D6): δ 160.77 (Ti–O–C; 2 nearly overlapping peaks), 142.46, 104.64 (TiCH2), 43.76, 38.83, 32.02, 24.51. For 6: 1H NMR (C6D6): δ 2.67–2.63 (m, 2 H), 2.14–2.10 (m, 4 H), 1.74–1.57 (m), 1.39–1.35 (m), 1.06 (d, J = 6.9 Hz, 6 H), 0.92–0.89 (m). HRMS calcd. for C12H20: 164.1565. Found: 164.1558.Crystal data for 5a: TiC48H44O2, M = 700.78, triclinic, space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) (no. 2), a = 11.9946(4), b = 11.7706(4), c = 13.7377(6) Å, α = 73.1412(15), β = 77.5151(14), γ = 84.8318(15)°, V = 1811.51(18) Å3, Dc = 1.285 g cm−3, Z = 2, T = 150 K. Of the 8326 unique reflections collected (5 ≤
θ
≤ 28°) with Mo-Kα (λ = 0.71073 Å), the 8313 with Fo2 > 2.0 σ(Fo2) were used in the final least-squares refinement to yield R = 0.085 and RW = 0.179. CCDC 197519. See http://www.rsc.org/suppdata/cc/b2/b209889j/ for crystallographic data in CIF or other electronic format (no. 2), a = 11.9946(4), b = 11.7706(4), c = 13.7377(6) Å, α = 73.1412(15), β = 77.5151(14), γ = 84.8318(15)°, V = 1811.51(18) Å3, Dc = 1.285 g cm−3, Z = 2, T = 150 K. Of the 8326 unique reflections collected (5 ≤
θ
≤ 28°) with Mo-Kα (λ = 0.71073 Å), the 8313 with Fo2 > 2.0 σ(Fo2) were used in the final least-squares refinement to yield R = 0.085 and RW = 0.179. CCDC 197519. See http://www.rsc.org/suppdata/cc/b2/b209889j/ for crystallographic data in CIF or other electronic format |
| This journal is © The Royal Society of Chemistry 2003 |
