Chirality of photopolymerized organized supramolecular polydiacetylene films†
Xin
Huang
and
Minghua
Liu
*
Laboratory of Colloid and Interface Science, Center for Molecular Science, Institute of Chemistry, the Chinese Academy of Sciences, Beijing, 100080, P. R. China. E-mail: liumh@iccas.ac.cn; Fax: 0086-10-62569564; Tel: 0086-10-62569563
First published on 26th November 2002
Abstract
Photopolymerized organized molecular films of polydiacetylene showed chirality although the monomeric amphiphilic diacetylene was achiral.
Chirality plays an important role in materials science and biological systems.1 Continuous interest has been devoted to the synthesis of chiral molecules through covalent bonds, and much attention has also been paid to the chirality of supramolecular systems over the past decades. At a supramolecular level, the chirality of the system can be obtained through the non-covalent interaction between the component chiral molecules or even through a unique arrangement of achiral molecules.2,3 The latter case is especially interesting since macroscopic chirality can be realized from achiral molecules without any chiral auxiliaries.
Polydiacetylene (PDA), one of the most investigated polymers, exhibited many potential applications as biosensors, pathogenic agents, and functional materials.4–6 So far, although supramolecular assemblies of amphiphilic diacetylenes and their photopolymerization have been widely investigated, little has been reported on the chirality of PDA.7 In this communication, we report the chirality of photopolymerized PDA Langmuir and Langmuir–Blodgett (LB) films from an achiral amphiphilic diacetylene derivative. The effect of metal ions in the subphase on the chirality of the PDA films has also been investigated.
The amphiphilic diacetylene derivative used in this work was tricosa-10,12-diynoic acid (TDA, CH3(CH2)9C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–C
C–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C(CH2)8COOH). TDA can be easily spread at the air/water interface to form a stable monolayer. Addition of metal ions into the subphase can influence the monolayer behaviour. These properties were similar to those previously reported for diacetylene derivatives.8,9
C(CH2)8COOH). TDA can be easily spread at the air/water interface to form a stable monolayer. Addition of metal ions into the subphase can influence the monolayer behaviour. These properties were similar to those previously reported for diacetylene derivatives.8,9
The TDA monolayers formed on a water surface, or the subphase containing metal ions could be deposited onto solid substrates.‡ Upon photoirradiation, the colourless TDA LB films turned into blue or red depending on the conditions. Fig. 1 shows the UV-vis spectra of the LB films deposited at 20 mN m−1 from water and the subphase containing 0.01 M Cu(NO3)2. The film from water surface showed absorption at 640 nm (blue film), while that from Cu(NO3)2 appeared at 535 nm (red film). These spectral changes are similar to those reported for the other amphiphilic diacetylene derivative, suggesting polymerization in the LB films.6–9
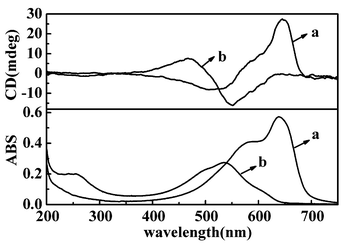 | ||
| Fig. 1 UV-vis and CD spectra of LB films after photopolymerized on quartz plate (a) from pure water (b) from Cu(NO3)2. | ||
When the polymerized PDA LB films were subjected to circular dichroism (CD) measurement, it is interesting to note that the PDA LB films showed an obvious Cotton effect, although the monomer TDA itself was achiral.§ In the case of PDA from water surface, positive and negative Cotton effects appeared at 645 and 515 nm respectively, with a cross over at 560 nm. In the case of Cu(II) ion, negative and positive Cotton effects were observed at 460 and 545 nm respectively, with a crossover at 500 nm. Both of the CD signals split at the shoulder absorption band of the blue and red films.
TDA can be polymerized not only in transferred LB films, but also on water surface. We compressed the monolayer to 20 mN m−1 and photoirradiated the monolayer in situ for 5 minutes. The polymerized film could be deposited onto solid substrate. It was found that this kind of LB film also showed chirality. Fig. 2 showed the UV-vis and CD spectra of the films transferred from the in situ polymerized PDA monolayers on water and the subphase containing Cu(II) ion. Red films could be obtained as those in previous reports.8,9 The films showed CD signals in the corresponding absorption band. It was further found that although the in situ polymerized PDA film on water or aqueous Cu(NO3)2 always showed significant CD signals, the sign of the CD signal could be opposite in different batches.
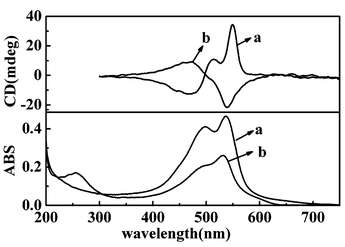 | ||
| Fig. 2 UV-vis and CD spectra of LB films deposited from in situ photopolymerized PDA film (a) from pure water (b) from Cu(NO3)2. | ||
It is well-known that PDA is formed through a typical topochemical reaction. The main chains of the polymer are π-conjugated backbones consisting of alternating double and triple bonds (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CR1–C
CR1–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–CR2
C–CR2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ). The formation of the conjugated backbone is very sensitive to the environment and two kinds of colour (blue and red) can appear depending subtly on the external environment such as pH, temperature, mechanical stress, and solvent.4–6 In our case, we have further found that besides these properties, the films showed chirality. In order to elucidate the chirality of the PDA films, we measured the transmission electron microscopy (TEM) of the in situ polymerized PDA films from water surface, as shown in Fig. 3. In Fig. 3(A) right-handed double helical structures could be clearly observed and regular lined patterns were also seen. At the higher magnification TEM images shown in Fig. 3(B), it could be further verified that these lines were also helical. It is suggested that it is this helical structure that caused the macroscopic chirality of the PDA in the interfacial films. It has been reported that polyisocyanate can form a stiff helical backbone due to competition between electronic and steric factors and the polyisocyanate can show chirality although the monomer is achiral.10 This suggests that a similar case occurred in PDA films, although we cannot exactly verify this at the present stage.
). The formation of the conjugated backbone is very sensitive to the environment and two kinds of colour (blue and red) can appear depending subtly on the external environment such as pH, temperature, mechanical stress, and solvent.4–6 In our case, we have further found that besides these properties, the films showed chirality. In order to elucidate the chirality of the PDA films, we measured the transmission electron microscopy (TEM) of the in situ polymerized PDA films from water surface, as shown in Fig. 3. In Fig. 3(A) right-handed double helical structures could be clearly observed and regular lined patterns were also seen. At the higher magnification TEM images shown in Fig. 3(B), it could be further verified that these lines were also helical. It is suggested that it is this helical structure that caused the macroscopic chirality of the PDA in the interfacial films. It has been reported that polyisocyanate can form a stiff helical backbone due to competition between electronic and steric factors and the polyisocyanate can show chirality although the monomer is achiral.10 This suggests that a similar case occurred in PDA films, although we cannot exactly verify this at the present stage.
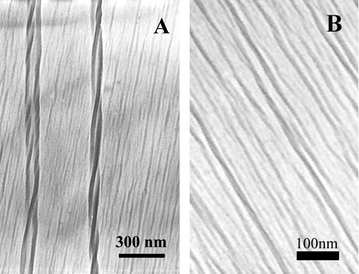 | ||
| Fig. 3 TEM of PDA films in situ polymerized on pure water (negative staining with uranyl acetate was performed to enhance the image quality). | ||
In principle, the chance of the backbone forming the right or left-handed helical structure is equal. However, the experimental results revealed that a chiral LB film was indeed formed. This indicates that in the organized molecular films of PDA, one handed polymer was predominantly formed. That is to say, the symmetry could be easily broken through the interfacial polymerization. In the formation of such a helical polymer, every unit will act cooperatively and a small helical sense in the starting unit may yield a considerable larger excess of helical sense, thus causing a macroscopic chirality. In the in situ polymerized PDA film, the break in symmetry occurred by chance due to the flexibility of the monolayer. Therefore, we could get an opposite CD signal in different batches. These findings are similar to the case of polyisocyanate.10 In the ex situ polymerized Cu(II)-PDA LB films, however, the sign of the CD signals was always the same. This suggested that a certain helical structure was predominantly formed in the LB films on a quartz substrate. This is reasonable because some inorganic crystals are reported to select the chirality.11
It is further noted that metal ions in the subphase can affect the monolayer formation. Although both the monolayers and LB films from the subphase containing metal ions such as Cu(II), Ag(I), Ni(II), Cd(II), Ca(II), and Zn(II) could be photopolymerized, only in the case of Cu(II) did we get the chiral PDA films. This suggested that all these metal ions except Cu(II) block the propagation of the polymer backbone in a helical sense. Amphiphilic diacetylene derivatives with a carboxylic group were reported to form salts with metal ions such as Ag(I), Ca(II) and Cd(II) etc.,12 whilst coordinating with metal ions such as Cu(II), Zn(II) etc. This could be seen from the differences in the π–A isotherms where phase transition regions could be observed in the case of metal ions such as Cu(II) Zn(II) and Ni(II) (see supporting information†). Copper ion differs from the others because of the Jahn–Teller effect. The tetracoordinated copper(II) ion is in a planar square while tetracoordinated zinc(II) and nickel(II) are in a tetrahedron structure in this system. The planar-tetracoordinated copper could keep the same conformation as PDA on the water surface. It was these differences that caused the Cu(II) ion to show a particularity in forming chiral PDA film.
In conclusion, a chiral PDA film can be formed through the photopolymerization of organized molecular films. Metal ions, except the Cu(II) ion, in the subphase can hinder the formation of chiral films.
This work was supported by the Outstanding Youth Fund (No. 20025312), National Science Foundation of China (No. 29992583, 20273078), the Major State Basic Research Development Program (G2000078103, 2002CCA03100) and the Fund of the Chinese Academy of Sciences.
Notes and references
- N. Berova, K. Nakanishi and R. W. Woody, Circular Dichroism Principles and Applications, second edition, Wiley-VCH, 2000 Search PubMed.
- J. M. Ribó, J. Crusats, F. Sagués, J. Claret and R. Rubires, Science, 2001, 292, 20.
- M . Wang, G. L. Silva and B. A. Armitage, J. Am. Chem. Soc., 2000, 122, 9977 CrossRef CAS.
- D. H. Charych, J. O. Nagy, W. Spevak and M. D. Bednarski, Science, 1993, 261, 585 CAS.
- Z. Ma, J. Li, M. Liu, J. Cao, Z. Zou, J. Tu and L. Jiang, J. Am. Chem. Soc., 1998, 120, 12678 CrossRef CAS.
- Y. Lu, Y. Yang, A. Sellinger, M. Lu, J. Huang, H. Fan, R. Haddad, G. Lopez, A. R. Burns, D. Y. Sasaki, J. Shelnutt and C. J. Brinker, Nature, 2001, 410, 913 CrossRef CAS.
- A. F. Drake, P. Udvarhelyi, D. J. Ando, D. Bloor, J. S. Obhi and S. Mann, Polymer, 1989, 30, 1063 CrossRef CAS.
- P. J. Werkman, R. H. Wieringa, E. J. Vorenkamp and A. J. Schouten, Langmiur, 1998, 14, 2119 Search PubMed.
- D. Y. Sasaki, R. W. Carpick and A. R. Burns, J. Colloid Interface Sci., 2000, 229, 490 CrossRef CAS.
- N. V. Lukasheva, S. Niemelä, I. M. Neelov, A. A. Darinskii, F. Sundholm and R. Cook, Polymer, 2002, 43, 1527 CrossRef CAS.
- P. Cintas, Angew. Chem., Int. Ed., 2002, 41, 1139 CrossRef CAS.
- B. Tieke, G. Wegner, D. Naegel and H. Ringsdorf, Angew. Chem., Int. Ed. Engl., 1976, 15, 764 CrossRef.
Footnotes |
| † Electronic supplementary information (ESI) available: surface pressure–area isotherms. See http://www.rsc.org/suppdata/cc/b2/b210032k/ |
| ‡ Monolayers of TDA were formed by spreading a chloroform solution (ca. 1× 10−3 M) onto a water surface or aqueous subphase containing metal ions (1 × 10−2 M). The UV-vis light was 254 nm, 20 W and the distance of the unpolymerized film center from the light was 20 cm. All the experiments were carried out in the dark. |
| § The possible effect of linear dichroism (LD) of the film was removed by rotating the film plate around the incident light: C. Spitz, S. Dähne, A. Ouart and H. W. Abraham, J. Phys. Chem. B, 2000, 104, 8664. |
| This journal is © The Royal Society of Chemistry 2003 |
