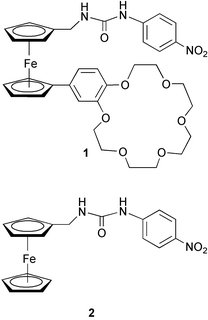
A ditopic ferrocene receptor for anions and cations that functions as a chromogenic molecular switch†
Hidekazu
Miyaji
*,
Simon R.
Collinson‡
,
Ivan
Prokeš
and
James H. R.
Tucker
*
School of Chemistry, University of Exeter, Exeter, UK EX4 4QD. E-mail: j.h.r.tucker@ex.ac.uk
First published on 28th November 2002
Abstract
A ferrocene-based ditopic receptor containing a urea and a benzocrown ether unit shows a remarkable colour switching (ON-and-OFF) function induced by anion and cation recognition.
Chromogenic receptors, compounds that undergo a colour change upon complexation, are an important class of optical device within supramolecular chemistry. A number of different systems are now known that respond to cations1 or anions.2 Here we report a chromoionophore, 1, that contains a binding site for both an anion and a cation. Related ditopic receptors that bind anions and cations are known,3 but we are unaware of tailor-made chromophore-containing receptors of this type. We show how compound 1 can function as a molecular switch by using appropriate combinations of anions and cations in solution to control its colour.
A nitrobenzene-linked urea moiety was chosen as the anion binding site, since previously it has been shown that the absorption maximum of the nitrobenzene unit shifts from the UV to the visible region of the spectrum in organic solvents upon addition of fluoride (as its tetrabutylammonium salt).2,4 A crown-ether was chosen as the second binding site of the receptor due to its well-known cation binding properties. Ferrocene was chosen as the spacer unit primarily because it is relatively easy to introduce different functional groups onto its two cyclopentadienyl (Cp) arms and in addition, its Cp units can rotate like a ball-bearing to encourage the formation of a stable ditopic complex. Compound 1 was synthesised in four steps5 from formylferrocene (see ESI†). Control compound 2 was synthesized from 1-aminomethylferrocene.6 All target compounds were isolated, purified and characterized by the usual methods.
Fig. 1 shows the effect of the addition of F− and then K+ on the spectrum of 1 in acetonitrile (5 × 10−5 M). The absorption maxima associated with the nitrobenzene moiety of the receptor (λ = 304, 332 nm) clearly separates (288, 356 nm) after addition of 10 molar equivalents of F− (as its tetrabutylammonium salt) and at the same time, a new absorbance appears at 472 nm (Fig. 1(b)), resulting in a change in the appearance of the solution from colourless to yellow. The binding constant for a 1∶1 complex between 1 and fluoride in acetonitrile was determined7 from the increase in absorption intensity at 472 nm, providing Ka = 9340 M−1 (error <10%, see ESI†). Interestingly, the addition of K+ reverses the chromogenic process; by the addition of 10 molar equivalents of KPF6 to the solution (PF6− does not complex with 1), the yellow colour induced by the presence of fluoride was now no longer observable and the absorption maximum at 472 nm had disappeared (Fig. 1(c)). The observed binding constant in acetonitrile at 25 °C between K+ and 1 in the presence of 10 molar equivalents of F− was determined from the decrease in the absorption intensity at 472 nm,7 providing Ka = 1460 M−1 (error < 10%, see ESI†).
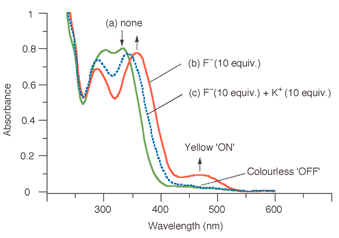 | ||
| Fig. 1 UV-vis spectrum of 1 in CH3CN (5 × 10−5 M) (a) in its free form (b) in the presence of 10 mol. equiv. of F− (c) in the presence of 10 mol. equiv. of F− and 10 mol equiv. of K+. | ||
Fig. 2 shows the colour changes of a solution of 1 in acetonitrile (2.5 × 10−4 M: left to right: none, in the presence of 10 molar equivalents of F−, in the presence of 10 molar equivalents of F− plus 10 molar equivalents of K+). The colour change (optical output) is thus controlled by the ditopic inputs of anion and cation; F− ‘switches on’ the optical output and K+ ‘switches off’ the output.
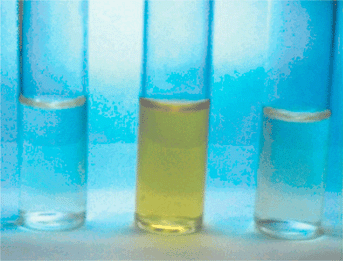 | ||
| Fig. 2 Photograph showing the colour changes of 1 in CH3CN (2.5 × 10−4 M). Left to right: In the absence of ions, in the presence of 10 mol. equiv. of F−, in the presence of 10 mol. equiv. of F− and 10 mol. equiv. of K+. | ||
As a comparison, the order of guest addition was then reversed. As expected, the addition of K+ (10 molar equivalents) to a solution of 1 (5 × 10−5 M) in acetonitrile brought about no colour change, although a small change in absorption intensity was observed. When 10 molar equivalents of F− were then added, the solution remained colourless and only small changes were observed to the spectrum. Therefore K+ appears to inhibit the chromogenic response of the system to F− anions. A convenient way of representing these changes is through a molecular logic gate8 truth table (see ESI†), which gives the logic response of 1 as either ‘ON’ = 1 or ‘OFF’ = 0 in the presence of various combinations of anion and cation at either 0 mol. equiv. (‘input’ = 0) or 10 mol. equiv. (‘input’ = 1). The output is only 1 if (anion, cation) = (1, 0). These logic responses correspond to the INH logic gate that was recently reported in a supramolecular system that used fluorescence as the output and protons (pH control) and oxygen as inputs.8a
NMR studies in CD3CN (17 mM) at 25 °C were carried out in an attempt to understand the binding mode of the complexes (see ESI†). The 1H NMR spectrum of 1 showed that the signals for the two urea protons (9.01 and 6.19 ppm) were considerably more downfield than those for the corresponding control compound 2 at a similar concentration (7.97 and 5.64 ppm respectively), which indicated the presence of intermolecular hydrogen bonds between the urea protons and the oxygen atoms of the benzocrown unit.§ When K+ (10 molar equivalents) was added to a solution of 1, the benzocrown proton signals, in particular those for the ArOCH2CH2O protons, shifted downfield slightly, due to cation complexation by the benzocrown unit. These changes were accompanied by the signals for the urea NH protons moving upfield to 7.72 and 5.44 ppm respectively, due to cation complexation preventing hydrogen bonding from the benzocrown. Interestingly, upon addition of 2 molar equivalents of F− to the solution of the [1·K]+ complex, the urea proton signals shifted downfield slightly to 7.87 and 5.53 ppm, which indicated a weak hydrogen bonding interaction with F−. On the other hand, when F− was added first, the resonances corresponding to the urea-NH protons of 1 disappeared in the presence of 2 molar equivalents of F−. This broadening effect was found in previous studies9 and is consistent with the formation of strong hydrogen bonds between the urea NH protons and F−. When 10 molar equivalents of K+ were added to the solution of the [1·F]− complex, the urea and crown proton signals reverted to the positions observed previously. It is clear from these studies that the bound K+ must interact with the urea arm of the receptor in such a way that the hydrogen bonding interaction between 1 and F− is weakened, possibly because of a K+–F− interaction that pulls electron density away from the urea unit, as found in a similar ditopic system.3e
In order to gain a clearer picture of how the benzocrown unit and K+ affects the chromogenic properties of 1, a UV-vis study was conducted on the control compound 2. In contrast to 1, 2 showed just one absorption band at 336 nm in acetonitrile. After addition of an excess amount of 10 molar equivalents of F− to the solution of 2 (5 × 10−5 M), the peak shifted to 360 nm, and at the same time a new band appeared at 460 nm and the solution turned yellow. Therefore the UV response to fluoride is rather similar in both compounds. Clearly both the higher and lower energy bands are relatively intense in the spectra of 1 and 2 and it seems likely that a combination of these bands causes the observable colour change at concentrations as low as 10−5 M. Whilst the higher energy band is associated with the nitrobenzene unit, the longer wavelength band is in the region of the d–d band of the ferrocene unit. It is known from other studies10 that the complexation of charged species by ferrocene receptors can affect the d–d band, with complexation in general leading to bathochromic shifts and more intense absorptions, as is the case here (uncomplexed 1, λ = 450 nm (shoulder), ε = < 500 M−1 cm−1, [1∶F]− complex, λ = 472 nm (sh), ε = 1740 M−1 cm−1). The binding constant for a 1∶1 complex between 2 and fluoride in acetonitrile was determined from the increase in absorption intensity at 460 nm,7 providing Ka = 9660 M−1 (error <10%, see ESI†). It was interesting that the addition of K+ to a solution of 2 (5 × 10−5 M, containing a ten-fold excess of fluoride) also caused a quenching of the yellow colour. However the observed binding constant between 2 and K+ in the presence of a ten-fold excess of F−, determined from the decrease in the absorption intensity at 460 nm,7 was much smaller (Ka = 230 M−1, error <10%, see ESI†) than that for the ditopic receptor 1. Therefore the benzocrown moiety of 1 must provide a suitable binding site for K+ which stabilises the resulting complex in the presence of F−. These results support the notion that it is the presence of K+ in close proximity to the fluoride binding site of 1 and 2 that causes the colour quenching process.
In conclusion, a ditopic chromoionophore 1 has been synthesized that contains a recognition site for both an anion and a cation. At low (ca. 10−4–10−5 M) concentrations, an observable colour change (optical output, ‘OFF-to-ON’) only occurs in the presence of F− but in the absence of K+. We believe this system provides a useful addition to the range of optical devices that can operate at the molecular level.11
We thank the EPSRC for the award of a PDRA grant (to S. R. C. and H. M.).
Notes and references
- M. Takagi, in Cation Binding by Macrocycles, ed. Y. Inoue and G. W. Gokel, Marcel Dekker, New York, 1990, pp. 465–496 Search PubMed; W. Wasikiewicz, M. Slaski, G. Rokicki, V. Böhmer, C. Schmidt and E. F. Paulus, New J. Chem., 2001, 25, 581 Search PubMed and references therein.
- H. Miyaji, W. Sato, J. L. Sessler and V. Lynch, Tetrahedron Lett., 2000, 41, 1369 CrossRef CAS; H. Miyaji, W. Sato and J. L. Sessler, Angew. Chem., Int. Ed., 2000, 39, 1777 CrossRef CAS; S. Nishizawa, R. Kato, T. Hayashita and N. Teramae, Anal. Sci., 1998, 14, 595 Search PubMed; D. H. Lee, H. Y. Lee, K. H. Lee and J.-I. Hong, Chem. Commun., 2001, 1188 RSC.
- (a) For a recent review, see: G. J. Kirkovits, J. A. Shriver, P. A. Gale and J. L. Sessler, J. Inclusion Phenom. Macrocycl. Chem., 2001, 41, 69 Search PubMed; (b) J. M. Cooper, M. G. B. Drew and P. D. Beer, J. Chem. Soc., Dalton Trans., 2000, 2721 RSC; (c) J. Scheerder, J. P. M. van Duynhoven, J. F. J. Engbersen and D. N. Reinhoudt, Angew. Chem., Int. Ed. Engl., 1996, 35, 109 CrossRef; (d) T. Tozawa, Y. Misawa, S. Tokita and Y. Kubo, Tetrahedron Lett., 2000, 41, 5219 CrossRef CAS; (e) R. Shukla, T. Kida and B. D. Smith, Org. Lett., 2000, 2, 3099 CrossRef CAS.
- C. B. Black, B. Andrioletti, A. C. Try, C. Ruiperez and J. L. Sessler, J. Am. Chem. Soc., 1999, 121, 10438 CrossRef CAS; H. Miyaji and J. L. Sessler, Angew. Chem., Int. Ed., 2001, 40, 154 CrossRef CAS.
- G. Iftime, C. Moreau-Bossuet, E. Manoury and G. G. A. Balavoine, Chem. Commun., 1996, 527 RSC; N. Miyaura and A. Suzuki, Chem. Rev., 1995, 95, 2457 CrossRef CAS; H.-B. Kraatz, J. Organomet. Chem., 1999, 579, 222 CrossRef CAS.
- P. D. Beer and D. K. Smith, J. Chem. Soc., Dalton Trans., 1998, 417 RSC.
- H. A. Benesi and J. H. Hildebrand, J. Am. Chem. Soc., 1949, 71, 2703 CrossRef CAS.
- (a) T. Gunnlaugsson, D. A. MacDonail and D. Parker, Chem. Commun., 2000, 93 RSC and references therein; (b) A. P. de Silva, H. Q. N. Gunaratne and C. P. McCoy, Nature, 1993, 364, 42 CrossRef; (c) G. McSkimming, J. H. R. Tucker, H. Bouas-Laurent and J. P. Desvergne, Angew. Chem., Int. Ed., 2000, 39, 2167 CrossRef CAS and references therein.
- H. Miyaji, P. Anzenbacher, Jr., J. L. Sessler, E. R. Bleasdale and P. A. Gale, Chem. Commun., 1999, 1723 RSC.
- J. D. Carr, S. J. Coles, M. B. Hursthouse and J. H. R. Tucker, J. Organomet. Chem., 2001, 637–639, 304 CrossRef CAS and references therein.
- ‘Molecular machines special issue’, Acc. Chem. Res., ed. J. F. Stoddart, 2001, 34(6), 410–522 Search PubMed; A. P. de Silva, H. Q. N. Gunaratne, T. Gunnlaugsson, A. J. M. Huxley, C. P. McCoy, J. T. Rademacher and T. E. Rice, Chem. Rev., 1997, 97, 1515 Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: synthetic route to 1, 1H NMR spectra of 1, UV-vis titration. See http://www.rsc.org/suppdata/cc/b2/b210227g/ |
| ‡ Present address: School of Chemistry, University of Nottingham, University Park, Nottingham, UK NG7 2RD. |
| § A 1H NMR dilution study in CD3CN revealed that the urea–benzocrown hydrogen-bonding in 1 was intermolecular rather than intramolecular and was not apparent at UV-vis concentrations. |
| This journal is © The Royal Society of Chemistry 2003 |