Water-soluble stilbene dendrimers
Junpei
Hayakawa
,
Atsuya
Momotake
and
Tatsuo
Arai
*
Department of Chemistry, University of Tsukuba, Tsukuba, Ibaraki, 305-8571, Japan. E-mail: arai@chem.tsukuba.ac.jp; Fax: +81-298-53-6503; Tel: +81-298-53-4315
First published on 26th November 2002
Abstract
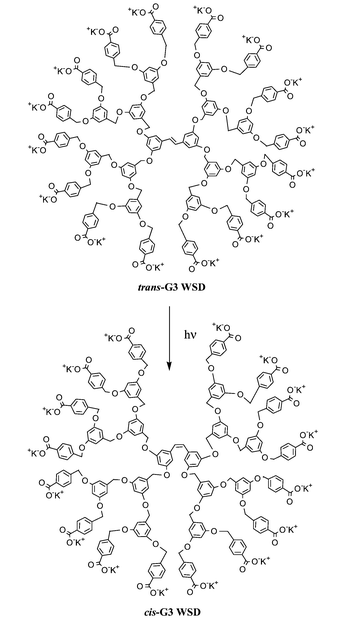
The third generation of novel photo-responsive water-soluble stilbene dendrimer (trans- and cis-G3 WSD) undergoes unusual one-way trans-to-cis isomerization to give 100% of cis isomer at the photostationary state on UV irradiation in water.
The mechanisms of photo-induced cis–trans isomerization of arylethenes such as stilbenes have been widely investigated since the 1960s.1 The photochemical and photophysical behaviour of arylethenes themselves as usual small molecules is well understood and has become an important field due to the large number of their practical and potential applications. However, the photoisomerization of arylethenes with large molecular weights, like dendrimers, is still obscure. In this field, only photoisomerization of a dendrimer with an azobenzene core has been published.2 These dendrimers may have a well-defined structure in which the arylethene or azobenzene core is spatially isolated from the outside by their dendrons. We have recently reported that stilbene dendrimers with benzyl ether type dendrons showed a volume-conserving isomerization mechanism and a macromolecular effect, or a generation effect of the dendrimer, such as energy transfer efficiency from the peripheral dendron to the core stilbene in organic solvents.3 We now report here the first synthesis of photo-responsive water-soluble stilbene dendrimers (Scheme 1) and their photochemical behavior in water, where the core of the higher generation dendrimer should be isolated from the outside water. A water-soluble dendrimer with a photoresponsive core may be a model compound of a photoreversible biomolecule and a photoreversible unimolecular micelle.
 | ||
| Scheme 1 | ||
In order to prepare dendrons containing polar termini, the convergent synthesis of Hawker and Frechet4 was employed.5 Coupling reaction of each generation of dendrons with cis or trans-3,3′,5,5′-tetrahydroxystilbene allowed use of the same alkylation reactions of phenol in the enhanced generation of dendrons. Each generation of dendrimers terminated with a methyl ester group was purified by flash column chromatography (eluting 0–10% ether in CH2Cl2) followed by recrystallization from hexanes–ethyl acetate (ethyl acetate being added to the boiling hexanes until the dendrimer was dissolved). The final hydrolysis of methyl esters was accomplished by potassium hydroxide in a mixed solvent system, then in water,4 followed by dialysis6 to give the potassium salts of stilbene dendrimers [trans- and cis-Gn WSDs (n = 1–3)] quantitatively.†
On UV irradiation at room temperature in 2 × 10−3 M KOH aqueous solution, trans-G3 WSD underwent one-way isomerization to cis-G3 WSD, which was revealed by the change in the absorption spectra. The absorption changes for irradiation of trans-G3 WSD are shown in Fig. 1. The initial absorbance of the trans-stilbene moiety, 0.44, at 330 nm decreased with irradiation time to give a constant value of 0.08. This result indicates the cis isomer composition at the photostationary state to be as high as 100% on irradiation at 330 nm. The same photostationary states were observed on irradiation of cis-G3 WSD. The ratios of trans and cis isomers at the photostationary state were ([t]/[c])pss = 4/96 and 1/99 for G1 and G2 WSDs, respectively. The possible experimental error for the ratio at the photostationary states arising from UV spectrometer is ±0.27%. Unlike 3,3′,5,5′-tetramethoxystilbene,3,7 UV irradiation of trans- and cis-G1 WSD did not give the cyclization product. The photochemical cyclization also did not take place from G2 and G3 WSDs.
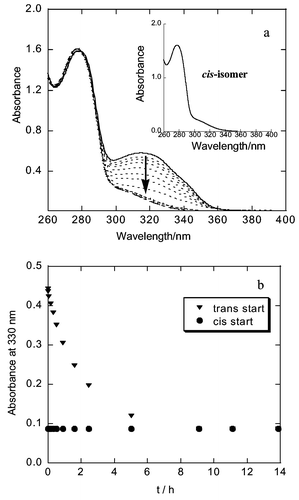 | ||
| Fig. 1 (a) Change of absorption spectrum of trans-G3 WSD on irradiation with 330 nm light in 2 × 10−3M KOH aqueous solution. Inset shows the absorption spectrum of cis-G3 WSD synthesized independently. (b) Time dependence of the absorption change observed at 330 nm. | ||
The observed one-way isomerization is the first clear evidence of the preparation of the less stable cis isomer of stilbenes with 100% efficiency. Usually, photochemical isomerization of stilbenes takes place mutually between cis and trans isomers.3 When one of the phenyl groups is replaced by a large aromatic group like anthracene, one-way cis-to-trans isomerization takes place as an adiabatic process.3 The observation of one-way isomerization in the trans-to-cis direction is unprecedented and is the first clear evidence of a water-soluble dendron in which highly selective isomerization is induced in an unusual direction.
The fluorescence spectra of trans-Gn WSDs in 2 × 10−3 M KOH aqueous solution on excitation at 310 nm where the extinction coefficient of the stilbene core was much larger than that of the dendron, are shown in Fig. 2. Similar fluorescence spectra of trans-Gn WSDs in water were observed on excitation at 280 nm, where the extinction coefficient of the dendron, or the benzyl ether group, was much larger than that of the stilbene core in trans-G3 (data not shown).
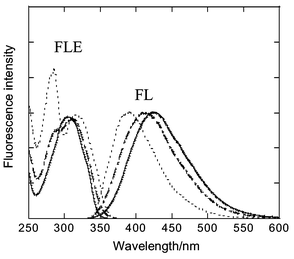 | ||
| Fig. 2 Fluorescence (FL) and fluorescence excitation spectra (FLE) of trans-G1 (solid line), G2 (dot-dash line) and G3 (dotted line) WSDs in 2 × 10−3 M KOH aqueous solutions. The spectra were measured with the absorbance of absorption maximum to be <0.1 and were normalized. | ||
Fluorescence spectra of trans-Gn Me Esters showed slight red shifts in chloroform. Thus, the fluorescence maximum appeared at 377 nm, 376 nm and 387 nm for G1, G2 and G3, respectively. Interestingly, the fluorescence maximum from the stilbene core of trans-Gn WSDs blue-shifted with increasing generations (λmax: 424 nm, 411 nm and 389 nm for G1, G2 and G3, respectively). These results suggest that increasing generation of WSDs decreases the interaction between the stilbene core and water due to their hydrophobic dendron group, which diminishes the stabilization of the excited state of the stilbene core by water. The Stokes shifts of trans-Gn WSDs between the absorption maximum and fluorescence maximum were obtained as 9100, 7600, and 5900 cm−1 for G1, G2 and G3, respectively.
Fluorescence excitation spectra observed at 424 nm, 411 nm and 389 nm for G1, G2 and G3, respectively were similar to the absorption spectra of the corresponding dendrimers, WSDs. This result indicates that a photon absorbed by the surrounding dendron group as well as the stilbene core could induce the fluorescence emission from the stilbene core. The efficiency of energy transfer from the dendrons to the stilbene core was estimated to be 49 and 31% for G2 and G3, respectively.
To the best of our knowledge, this is the first report for the synthesis and photoisomerization of water-soluble dendrimers, where the inner core is hydrophobic and is surrounded by an ionic polar group and may act as a unimolecular micelle. In addition, G3 WSD showed the first example of one-way isomerization from the trans isomer to the cis isomer. Further research into the photochemical properties of WSDs is now under way.
We thank Dr. Ritsuko Nagahata of the Reseach Center of Macromolecular Technology, National Institute of Advanced Industrial Science and Technology (AIST) for MALDI-TOF mass spectroscopy. This work was supported by a Grant-in-Aid for Scientific Research on Priority Areas (417) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of the Japanese Government and by the Asahi Glass Foundation.
Notes and references
- (a) T. Arai and K. Tokumaru, Chem. Rev., 1993, 93, 23–29 CrossRef CAS; (b) J. Saltiel, J. D’Agostino, E. D. Megarity, L. Metts, K. R. Neuberger, M. Wrighton and O. C. Safiriou, in Organic photochemistry, ed. O. L. Chapman, Marcel Dekker, New York, 1973, vol. 3, p. 1 Search PubMed.
- (a) D.-L. Jiang and T. Aida, Nature, 1997, 454–456 CAS; (b) D. M. Junge and D. V. McGrath, Chem. Commun., 1997, 857–858 RSC; (c) S. Li and D. V. McGrath, J. Am. Chem. Soc., 2000, 122, 6795–6796 CrossRef CAS.
- (a) T. Mizutani, M. Ikegami, R. Nagahata and T. Arai, Chem. Lett., 2001, 1014–1015 CrossRef CAS; (b) M. Uda, T. Mizutani, J. Hayakawa, A. Momotake, M. Ikegami, R. Nagahata and T. Arai, Photochem. Photobiol. Search PubMed , in press.
- C. J. Hawker, K. L. Wooley and J. M. Frechet, J. Chem. Soc., Perkin Trans. 1, 1993, 1287–1297 RSC.
- S. M. Grayson and J. M. Frechet, Chem. Rev., 2001, 101, 3819–3867 CrossRef CAS.
- A. Sadamoto, N. Tomioka and T. Aida, J. Am. Chem. Soc., 1996, 118, 3978–3979 CrossRef CAS.
- N. Castel, E. Fischer, K. R. Jabalameli and W. Lutke, J. Photochem. Photobiol., A: Chemistry, 1989, 50, 221–237 Search PubMed.
Footnote |
† trans-G1 WSD (potassium salt): 1H NMR (400 MHz, D2O) δ 7.89 (8H, d, J = 8.5 Hz, ArH), 7.54 (8H, d, J = 8.5 Hz, ArH), 7.11 (2H, bs, CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 6.91 (4H, bs, ArH), 6.67 (2H, bs, ArH), 5.21 (8H, s, CH2Ar). MALDI-TOFMS (acid form): Calc. for C46H36O12Na [M + Na]+: m/z 803.8. Found: 803.3. cis-G1 WSD (potassium salt): 1H NMR (400 MHz, D2O) δ 7.78 (8H, d, J = 6.8 Hz, ArH), 7.22 (8H, bs, ArH), 6.40-6.58 (8H, 3bs, ArH and CH CH), 6.91 (4H, bs, ArH), 6.67 (2H, bs, ArH), 5.21 (8H, s, CH2Ar). MALDI-TOFMS (acid form): Calc. for C46H36O12Na [M + Na]+: m/z 803.8. Found: 803.3. cis-G1 WSD (potassium salt): 1H NMR (400 MHz, D2O) δ 7.78 (8H, d, J = 6.8 Hz, ArH), 7.22 (8H, bs, ArH), 6.40-6.58 (8H, 3bs, ArH and CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 4.74 (8H, bs, CH2Ar). MALDI-TOFMS (acid form): Calc. for C46H36O12Na [M + Na]+: m/z 803.8. Found: 803.1. trans-G2 WSD (acid form): 1H NMR (400 MHz, DMSO-d6) δ 7.93 (16H, d, J = 8.4 Hz, ArH), 7.51 (16H, d, J = 8.4 Hz, ArH), 7.21 (2H, bs, CH CH), 4.74 (8H, bs, CH2Ar). MALDI-TOFMS (acid form): Calc. for C46H36O12Na [M + Na]+: m/z 803.8. Found: 803.1. trans-G2 WSD (acid form): 1H NMR (400 MHz, DMSO-d6) δ 7.93 (16H, d, J = 8.4 Hz, ArH), 7.51 (16H, d, J = 8.4 Hz, ArH), 7.21 (2H, bs, CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 6.87 (4H, bs, ArH), 6.73 (8H, bs, ArH), 6.65 (4H, bs, ArH), 6.56 (2H, bs, ArH), 5.17 (16H, s, CH2Ar), 5.05 (8H, s, CH2Ar). MALDI-TOFMS: Calc. for C106H84O28Na [M + Na]+: m/z 1828.8. Found: 1828.5. cis-G2 WSD (potassium salt): 1H NMR (400 MHz, D2O) δ 7.81 (16H, d, J = 8.5 Hz, ArH), 7.23 (16H, d, J = 8.5 Hz, ArH), 6.30 (4H, d, J = 2.0 Hz, ArH), 6.28 (10H, d, J = 2.0 Hz, ArH and CH CH), 6.87 (4H, bs, ArH), 6.73 (8H, bs, ArH), 6.65 (4H, bs, ArH), 6.56 (2H, bs, ArH), 5.17 (16H, s, CH2Ar), 5.05 (8H, s, CH2Ar). MALDI-TOFMS: Calc. for C106H84O28Na [M + Na]+: m/z 1828.8. Found: 1828.5. cis-G2 WSD (potassium salt): 1H NMR (400 MHz, D2O) δ 7.81 (16H, d, J = 8.5 Hz, ArH), 7.23 (16H, d, J = 8.5 Hz, ArH), 6.30 (4H, d, J = 2.0 Hz, ArH), 6.28 (10H, d, J = 2.0 Hz, ArH and CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 6.24 (2H, t, J = 2.0 Hz, ArH), 6.14 (4H, bs, ArH), 4.47 (24H, bs, CH2Ar). MALDI-TOFMS (acid form): Calc. for C106H84O28Na [M + Na]+: m/z 1828.8. Found: 1828.3. trans-G3 WSD (acid form): 1H NMR (400 MHz, DMSO-d6) δ 7.91 (32H, d, J = 6.6 Hz, ArH), 7.48 (32H, d, J = 6.6 Hz, ArH), 7.20 (2H, s, CH CH), 6.24 (2H, t, J = 2.0 Hz, ArH), 6.14 (4H, bs, ArH), 4.47 (24H, bs, CH2Ar). MALDI-TOFMS (acid form): Calc. for C106H84O28Na [M + Na]+: m/z 1828.8. Found: 1828.3. trans-G3 WSD (acid form): 1H NMR (400 MHz, DMSO-d6) δ 7.91 (32H, d, J = 6.6 Hz, ArH), 7.48 (32H, d, J = 6.6 Hz, ArH), 7.20 (2H, s, CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 6.87 (4H, s, ArH), 6.74–6.53 (38H, m, ArH), 5.11 (32H, bs, CH2Ar), 4.99 (24H, bs, CH2Ar). MALDI-TOFMS: Calc. for C226H180O60Na [M + Na]+: m/z 3878.8. Found: 3877.9. cis-G3 WSD (acid form): 1H NMR (400 MHz, DMSO-d6) δ 7.89 (32H, d, J = 8.5 Hz, ArH), 7.43 (32H, d, J = 8.5 Hz, ArH), 6.63 (16H, bs), 6.58–6.54 (16H, 2bs), 6.50 (12H, bs), 5.03 (32H, bs, CH2Ar), 4.85 (16H, bs, CH2Ar), 4.82 (8H, bs, CH2Ar). MALDI-TOFMS: Calc. for C226H180O60Na [M + Na]+: m/z 3878.8. Found: 3878.0. CH), 6.87 (4H, s, ArH), 6.74–6.53 (38H, m, ArH), 5.11 (32H, bs, CH2Ar), 4.99 (24H, bs, CH2Ar). MALDI-TOFMS: Calc. for C226H180O60Na [M + Na]+: m/z 3878.8. Found: 3877.9. cis-G3 WSD (acid form): 1H NMR (400 MHz, DMSO-d6) δ 7.89 (32H, d, J = 8.5 Hz, ArH), 7.43 (32H, d, J = 8.5 Hz, ArH), 6.63 (16H, bs), 6.58–6.54 (16H, 2bs), 6.50 (12H, bs), 5.03 (32H, bs, CH2Ar), 4.85 (16H, bs, CH2Ar), 4.82 (8H, bs, CH2Ar). MALDI-TOFMS: Calc. for C226H180O60Na [M + Na]+: m/z 3878.8. Found: 3878.0. |
| This journal is © The Royal Society of Chemistry 2003 |
