A chiral metallacyclophane for asymmetric catalysis†
Hua
Jiang
,
Aiguo
Hu
and
Wenbin
Lin
*
Department of Chemistry, CB#3290, University of North Carolina, Chapel Hill, NC 27599, USA. E-mail: wlin@unc.edu
First published on 3rd December 2002
Abstract
Chiral metallacyclophanes were self-assembled from cis-(PEt3)2PtCl2 and enantiopure atropisomeric 1,1′-binaphthyl-6,6′-bis(acetylenes) and used in highly enantioselective catalytic diethylzinc additions to aldehydes to afford chiral secondary alcohols.
The design of functional supramolecular assemblies has received intense interest from synthetic and materials chemists.1 Nanoscopic supramolecular assemblies can be expected to provide enhanced performance over their constituent building blocks.2 The last decade has in particular witnessed tremendous progress in the synthesis of metallosupramolecular assemblies.3 These rigid supramolecular assemblies can provide better selectivity in sensory and catalytic applications. Fujita and coworkers have illustrated such advantages by performing cavity-directed synthesis of labile silanol oligomers and stereoselective [2 + 2] photodimerization of olefins.4
We have become interested in chiral supramolecular assemblies for potential applications in enantioselective processes. Our approaches combine rigid bridging ligands derived from 1,1′-bi-2-naphthol (BINOL) and appropriate metallo-corners to generate supramolecular assemblies that bear chiral functionalities. BINOL and its derivatives have been shown to be a ‘privileged’ ligand system for highly enantioselective catalytic processes and chiral separations.5,6 Herein we wish to report the self-assembly and characterization of novel chiral metallacyclophanes [cis-(PEt3)2Pt(L1–3)]2(where L1–3 is enantiopure 6,6′-bis(alkynyl)-1,1′-binaphthalene), and our preliminary results on the application of [cis-(PEt3)2Pt(L3)]2 in highly enantioselective diethylzinc additions to aldehydes to afford chiral secondary alcohols.
Enantiomerically pure atropisomeric bis(acetylenes) L1 and L3 were synthesized by modified literature procedures,7 while L2 was synthesized by treating L3 with acetic anhydride. Treatment of ligands L1 and L2 with one equiv. of cis-Pt(PEt3)2Cl2 in the presence of catalytic amounts of CuCl in diethylamine at room temperature afforded chiral cycles [cis-(PEt3)2Pt(L1)]21 and [cis-(PEt3)2Pt(L2)]22 in 49 and 59% yield, respectively (Scheme 1). Treatment of L3 with one equiv. of cis-Pt(PEt3)2Cl2 under a variety of conditions gave the hydroxy cycle 3 in very low yields (<20%), presumably due to undesired competitive coordination of the dihydroxy groups of L3 to lead to intractable products. Instead, 3 can be obtained in quantitative yield by treating 2 with K2CO3 in a mixture of THF and methanol. Compounds 1–3 have been characterized by 1H, 13C{1H} and 31P{1H} NMR spectroscopy, HR-MS, elemental analysis, and IR, UV–Vis, and circular dichroism (CD) spectroscopies.
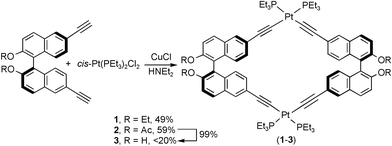 | ||
| Scheme 1 Synthesis of 1–3. | ||
NMR spectra of 1–3 indicated a single ligand environment, consistent with the formation of cyclic species. HR FAB-MS data showed the presence of molecular ions due to dinuclear species for 1–3. The terminal acetylenic C–H stretches of L1–3 at ∼3280 cm−1 disappeared upon the formation of 1–3. The IR spectra of 1–3 exhibit expected C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C stretches at ∼2110 cm−1. All these spectroscopic data are consistent with a cyclic dimeric structure of approximate D2 symmetry. These results are in stark contrast with an earlier report where polymeric compounds were obtained when bis(alkynyl) ligand L1 was treated with trans-Pt(PEt3)2Cl2.7a,8
C stretches at ∼2110 cm−1. All these spectroscopic data are consistent with a cyclic dimeric structure of approximate D2 symmetry. These results are in stark contrast with an earlier report where polymeric compounds were obtained when bis(alkynyl) ligand L1 was treated with trans-Pt(PEt3)2Cl2.7a,8
A single-crystal X-ray diffraction study on compound 3 unambiguously demonstrated the formation of a chiral metallacyclophane.9 Compound 3 crystallizes in chiral monoclinic space group P21.‡ Two cis-Pt(PEt3)2 units are linked by two enantiopure L3 ligands to form a cyclic dinuclear structure (Fig. 1). Both Pt centers adopt slightly distorted square planar geometry with the cis angles around the Pt1 center ranging from 82.4(2) to 101.3(1)° and the cis angles around the Pt2 center ranging from 84.3(2) to 100.3(1)°. The rigid metallacyclophane structure of 3 is characterized by very small dihedral angles between the naphthyl rings within each L3 ligand (62.18 and 73.45°).
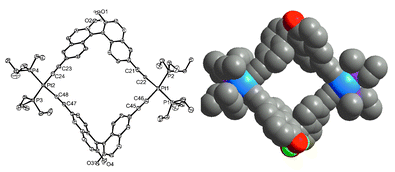 | ||
| Fig. 1 (Left) ORTEP view of metallacyclophane 3. Key bond distances (Å): Pt1–C22 1.983(8), Pt1–C46 2.016(9), Pt1–P1 2.306(2), Pt1–P2 2.310(2), Pt2–C24 1.989(9), Pt2–C48 1.999(8), Pt2–P4 2.314(2), Pt2–P3 2.316(2). (Right) A space-filling model of 3. | ||
The electronic spectra of L1–3 show three major π→π* transitions: the naphthyl π→π* transitions at ∼240 and ∼255 nm and a weak absorption at ∼290 nm due to acetylenic π→π* transition that has been delocalized into naphthyl ring systems. Upon the formation of metallacyclophanes 1–3, a new peak appears at 230–240 nm, which can be assigned to the cis-Pt(PEt3)2 moiety. The naphthyl π→π* transitions and the acetylenic π→π* transition have significantly red-shifted (Fig. 2). Bathochromic shifts are well-established in platinum acetylides, assignable to the mixing of Pt p-orbitals into the acetylenic π→π* bands.10 The π→π* transitions at ∼310 nm in 1–3 thus have significant ligand-to-metal charge transfer (LMCT) character. CD spectra of ligands L1–3 exhibit one major bisignate band corresponding to naphthyl π → π* transitions at ∼245 nm and one minor band at ∼290 nm due to acetylenic π→π* transition. CD spectra of metallacyclophanes 1–3 exhibit a bisignate band at ∼260 nm due to the naphthyl π→π* transitions and an intense band at 320 nm assignable to the acetylenic π→π* transitions, along with a band at ∼230 nm which can be attributed to the chiral arrangment of the PEt3 groups on the Pt centers (Fig. 3). Interestingly, the intensities of the naphthyl π→π* CD bands of coordinated L1–3 in 1–3 have decreased to ∼1/4 of those of free L1–3, probably a consequence of the reduction in their dihedral angles upon the formation of metallacyclophanes.
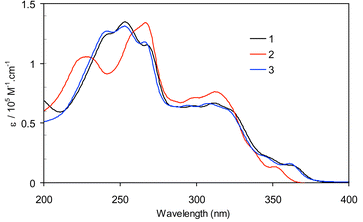 | ||
| Fig. 2 UV-Vis spectra of 1–3 in acetonitrile. | ||
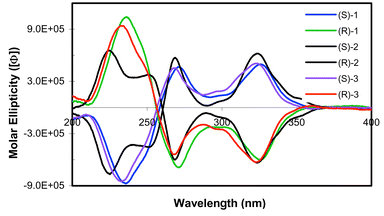 | ||
| Fig. 3 Circular dichroism spectra of 1–3 in acetonitrile. | ||
The presence of chiral dihydroxy groups in 3 has prompted us to examine its utility in asymmetric catalysis. We have carried out prototypical diethylzinc additions to aromatic aldehydes using a combination of 3 and Ti(OiPr)4 as the catalyst (eqn. 1).11 As shown in Table 1, the Ti(IV) complexes of 3 are excellent catalysts for the additions of diethylzinc to 1-naphthaldehyde with 94% ee and >95% conversion at 0 °C. The enantioselectivity has however dropped significantly when other smaller aromatic aldehydes were used as the substrates. This result differs from the performance of BINOL and a BINOL-derived organometallic triangle, both of which have a very broad substrate scope.11 We believe that this difference is a direct consequence of much more rigid structure of 3; the dihedral angles of naphthyl rings in the Ti(IV) catalyst can no vary to accommodate aldehydes of various sizes to give high enantioselectivity. The chiral dihydroxy groups in 3 thus differ from those of BINOL, and may prove useful for mechanistic work owing to their rigid structure.
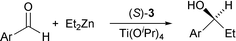 | (1) |
| Aldehyde | Temp./K | Time/h | Conversion (%) | Ee (%) |
|---|---|---|---|---|
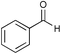
|
r.t.
0 °C |
16
16 |
>95
>95 |
77
84 |
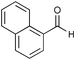
|
r.t.
0 °C |
16
16 |
>95
>95 |
91
94 |
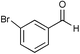
|
r.t.
0 °C |
16
16 |
>95
>95 |
75
78 |
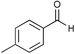
|
r.t.
0 °C |
16
40 |
>95
∼40 |
77
78 |
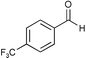
|
r.t.
0 °C |
16
40 |
>95
∼80 |
76
77 |
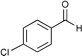
|
r.t.
0 °C |
16
16 |
>95
>95 |
75
78 |
In summary, a family of novel chiral metallacyclophanes has been readily assembled based on robust Pt–acetylide linkages. Metallacyclophane 3 has been used as a chiral ligand for enantioselective catalytic diethyl zinc additions to aromatic aldehydes. Such a supramolecular approach will add a new dimension to the rapidly expanding field of asymmetric catalysis.
We acknowledge financial support from NSF (CHE-0208930). W. L. is an Alfred P. Sloan Fellow, an Arnold and Mabel Beckman Young Investigator, a Cottrell Scholar of Research Corp, and a Camille Dreyfus Teacher–Scholar.
Notes and references
- J.-M. Lehn, Supramolecular Chemistry, Concepts and Perspectives, VCH, New York, 1995. Search PubMed.
- P. H. Dinolfo and J. T. Hupp, Chem. Mater., 2001, 13, 3113 CrossRef CAS.
- (a) S. Leininger, B. Olenyuk and P. J. Stang, Chem. Rev., 2000, 100, 853–907 CrossRef CAS; (b) B. J. Holiday and C. A. Mirkin, Angew. Chem., Int. Ed., 2001, 40, 2022–2043 CrossRef CAS; (c) M. Fujita, Chem. Soc. Rev., 1998, 27, 417–425 RSC.
- (a) M. Yoshizawa, T. Kusukawa, M. Fujita, S. Sakamoto and K. Yamaguchi, J. Am. Chem. Soc., 2001, 123, 10454–10459 CrossRef CAS; (b) M. Yoshizawa, Y. Takeyama, T. Kusukawa and M. Fujita, Angew. Chem., Int. Ed., 2002, 41, 1347–1349 CrossRef CAS.
- (a) L. Pu, Chem. Rev., 1998, 98, 2405 CrossRef CAS; (b) R. Noyori, Angew. Chem. Int. Ed., 2002, 41, 2008 CrossRef CAS.
- S. J. Lee and W. Lin, J. Am. Chem. Soc., 2002, 124, 4554–4555 CrossRef CAS.
- (a) K. Onitsuka, Y. Harada, F. Takei and S. Takahashi, Chem. Commun., 1998, 643–644 RSC; (b) H. Sasai, T. Tokunaga, S. Watanabe, T. Suzuki, N. Itoh and M. Shibasaki, J. Org. Chem., 1995, 60, 7388–7389 CrossRef CAS.
- S. M. Al Qaisi, K. J. Galat, M. Chai, D. G. Ray, P. L. Rinaldi, C. A. Tessier and W. J. Youngs, J. Am. Chem. Soc., 1998, 120, 12149 CrossRef CAS.
- J. W. Steed and J. L. Atwood, Supramolecular Chemistry, Wiley, New York, 2000 Search PubMed.
- V. W.-W. Yam, Acc. Chem. Res., 2002, 35, 555 CrossRef CAS.
- (a) L. Pu and H.-B. Yu, Chem. Rev., 2001, 101, 757 CrossRef CAS; (b) S. J. Lee, A. Hu and W. Lin, J. Am. Chem. Soc., 2002, 124, 12948 CrossRef CAS.
Footnotes |
| † Electonic supplementary information (ESI) available: experimental details and analytical data for 2 and 3, and general procedure for analysis. See http://www.rsc.org/suppdata/cc/b2/b208324h/ |
| ‡ X-Ray single-crystal diffraction data for 3·EtAc·H2O were collected on a Siemens SMART CCD diffractometer. Crystal data: monoclinic, space group P21, a = 13.833(3), b = 15.047(3), c = 17.264(4) Å, β = 92.105(5)°, U = 3591.1(14) Å3, Z = 2, Dc = 1.51 g cm−3, μ(Mo-Kα) = 40.3 cm−1. Least-squares refinement based on 13710 reflections with I > 2σ(I) and 802 parameters led to convergence, with a final R1 = 0.050, wR2 = 0.105, and GOF = 1.03. Flack parameter = −0.02(6). See http://www.rsc.org/suppdata/cc/b2/b208324h/ for crystallographic data in CIF or other electronic format. |
| This journal is © The Royal Society of Chemistry 2003 |
