Twenty-five years of conducting polymers
First published on 25th March 2003
Abstract
A quarter of a century ago, Chemical Communications published a seminal paper called “Synthesis of Electrically Conducting Organic Polymers: Halogen Derivatives of Polyacetylene, (CH)x”.1 A new field of chemistry was born.
 | ||
| Plate1 Hideki Shirakawa, Alan MacDiarmid and Alan Heeger | ||
Three backgrounds merge
Shirakawa recalls that he was always interested in polymers ever since he was a child when his mother used to wrap his lunch box containing hot cooked rice in a sheet of PVC instead of the traditional Japanese cotton furoshiki. He noted that the sheet retained its box-like shape as a result of the heat from the food. Shirakawa was later able to pursue that interest in polymer properties and synthesis at the Tokyo Institute of Technology. In 1966, after receiving his doctorate, he had the opportunity to work on the polymerisation mechanism of acetylene using Ziegler–Natta catalysts.2Heeger’s background was quite different. Working at the University of Pennsylvania, he, like many condensed-matter physicists at the beginning of the 1970s, was interested in the metal–insulator transition, particularly in one-dimensional systems. He started working on the physics of materials like TTF–TCNQ (tetrathiafulvalene–7,7,8,8-tetra-p-quinodimethane). This charge-transfer salt stacks “like poker chips” to form a quasi-one-dimensional conductor mediated by π electrons. Heeger then became intrigued by another chain-like metallic material, the sulfur nitride polymer (SN)x which he felt might be a route into an exciting new area, that of polymers with strong electronic properties.3
 | ||
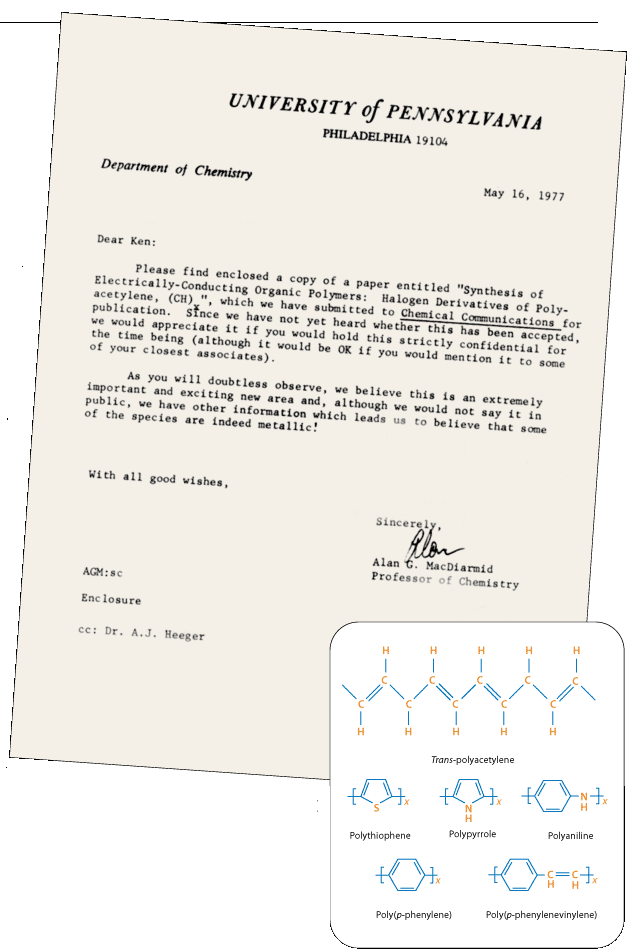
| Plate2 The original 1977 ChemComm paper. | ||
Heeger decided to consult Alan MacDiarmid about making (SN)x. Mac-Diarmid was in the chemistry department at Penn, and had had a longstanding interest in sulfur nitride chemistry, having studied the bright gold compound S4N4 and its derivatives during his MSc in New Zealand (MacDiarmid admits to being drawn to colour in chemistry). S4N4 is the precursor for (SN)x. However, the collaboration nearly didn’t get off the ground. The researchers recall how when they first met to discuss the project, Heeger enthused about a new metallic-like material he called (SN)x but MacDiarmid had thought he meant Snx (tin!) and could not at first understand what all the excitement was.
Meanwhile in Japan, Shirakawa was dealing with his own communication problem. A visiting Korean researcher had asked if he could prepare polyacetylene, but instead of obtaining the expected black powder, the result was a bright, glistening silvery film. Shirakawa was mystified but eventually realised that a misunderstanding had led the student to add molar quantities of the catalyst instead of millimolar amounts – a thousand times as much! It resulted not in the formation of the black powder but in a silvery film on the walls of the reaction vessel which had been wetted by the catalyst solution. It had apparently accelerated the rate of reaction a thousand times. A fortuitous combination of a unique Ziegler–Natta catalyst soluble in organic solvents, Ti(OBu)4–Et3Al, a bizarre experimental mistake, and the fact that polyacetylene is insoluble in just about everything had conspired to produce this promising metallic-looking material.
The real breakthrough was yet to come. MacDiarmid was visiting Tokyo Institute of Technology in 1975 and found himself chatting to the Japanese researcher over a cup of green tea after a lecture. MacDiarmid showed him a sample of the golden (SN)x and Shirakawa showed him the silver (CH)x. Mutual interest established, MacDiarmid invited Shirakawa to spend a year at Penn to work further on the polymer with himself and Heeger. MacDiarmid notes the insight of the programme officer, Ken Wynne at the US Office of Naval Research, in funding the visit.
Infrared measurements of the film treated with a trace amount of halogen had already led Shirakawa to believe that this might have caused “a drastic change in the electronic states of polyacetylene”. MacDiarmid and Heeger had previously found that adding bromine to (SN)x increased its conductivity tenfold. The trio therefore decided to expose high quality films of trans-polyacetylene to bromine vapour at room temperature and measure the resulting conductivity using a four-probe method. They were amazed to find that the conductivity increased by 10 million times in a few minutes – the change was so rapid that it wrecked the electronics of the measuring instrument! Iodine and AsF5 also produced large increases. Today, the best samples of polyacetylene doped in this way can achieve a conductivity similar to that of copper.
 | ||
| Plate3 The significance of a conducting organic polymer was realised immediately (above). Some conducting polymers (right). | ||
The researchers immediately realised the potential of their discovery, as is shown by the excerpt above from MacDiarmid’s letter to Ken Wynne. As well as the practical prospect of cheap, lightweight electronic devices coming to mind, polyacetylene presented a new theoretical model for studying conduction mechanisms and the metal–insulator transition in organic materials. Polyacetylene could be converted from an insulator, to a semiconductor, to a full metal, depending on the concentration of dopant. Heeger says, however, that it took “some courage to propose the existence of a metal–insulator transition in an organic polymer”.4
Here arose, perhaps, one of the first examples of the scientific cultural gap between chemists and physicists becoming apparent. They used languages, both based on quantum mechanics but with a different historical development, to describe the same phenomena. Chemists thought of conducting polyacetylene in terms of a conjugated system of overlapping π orbitals which is oxidised by the halogen to give a radical cation, and a halogen anion partner. Physicists, using the language of condensed matter theory, talked about hole creation as an electron is removed from the top of the valence band of polyacetylene – the process of p-doping (the reverse is n-doping, the polymer can also be reduced by an alkali metal). Conduction can be described in terms of the hole migrating along the chain in the guise of various complex electronic states, or quasi-particles, such as polarons, bipolarons or solitons.5
Heeger recalls that he and MacDiarmid were well aware of the emerging communication problems, and they made a point of meeting up on Saturday mornings to learn each other’s language and discuss ideas. “They were one of those little gems of scientific creativity I’ll never forget,” says MacDiarmid. Since those early days, the rapid growth in the study of complex materials with unusual electronic properties, such as the superconducting cuprates or organic ferromagnets, has resulted in a rewarding scientific synergy between the physics and chemistry communities. “However, even today, I still see misunderstandings between physicists and chemists at meetings,” comments Heeger.
Polyacetylene revealed fascinating properties: it could be p and n-doped via redox reactions thus opening up the possibility of molecular transistors; the doping could be carried out electrochemically to create a battery; and the doped material showed intriguing optical properties – colour changes or changes from transparency to opacity – which were to herald an important application for electronic polymers in the future. Polyacetylene also had major drawbacks, however: it was unstable in air, and insoluble in solvents so was unprocessable.
Other conducting polymers
Naturally, interest soon turned to other conjugated polymers such as poly(p-phenylene), polypyrrole, polythiophene and polyaniline, and their derivatives, which showed similar behaviour but in many cases were stable and processable. The field took off, with academic groups, and electronics and chemical companies around the world, starting research programmes. Over the past 25 years, electronic-polymer R&D has spawned a huge variety of applications from antistatic coatings to light-emitting diodes. | ||
| Plate4 A display built using polymer LEDs made at UNIAX (now DuPont Displays), permission granted by A. J. Heeger. | ||
Shirakawa, MacDiarmid and Heeger all continued to work on conducting polymers, and their subsequent research projects amply demonstrate the current breadth of the field. When Shirakawa returned to Japan, he pursued the characterisation of polyacetylene. He had already shown that the film consisted of entangled microfibrils, and set about preparing uniaxially oriented films he felt were needed to study the physical and chemical properties of a one-dimensional conductor. The first approach was to stretch the films mechanically but later, together with Kotaro Araya of Hitachi, he discovered a more direct method in which the films were oriented in the presence of a nematic liquid crystal phase. This could be done under shear flow conditions or with the aid of a magnetic field.6,7 Iodine-doped polyacetylene films prepared in this way showed considerably enhanced anisotropic conductivity. More recently, one of Shirakawa’s collaborators, Kazuo Akagi, suggested using a chiral nematic liquid crystal solvent.8 The result was a spiral form of the polymer. Since linear polyacetylene behaves as a molecular wire, helical polyacetylene can be considered to offer potential as a molecular solenoid.
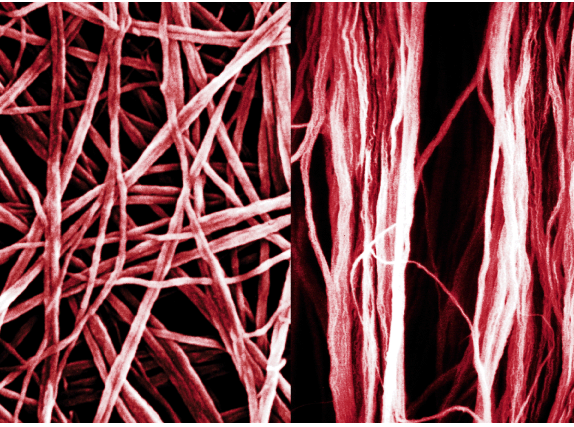 | ||
| Plate5 (left) A scanning electron micrograph of polyacetylene fibrils. (From K. Araya, A. Mukoh, T. Narahara, H. Shirakawa, Synth. Met., 1986, 14, 199, Fig. 3b). (right) Scanning electron micrograph of highly oriented polyacetylene fibrils synthesised by the liquid-crystal polymerisation method under a magnetic field. (From K. Akagi, S. Katayama, H. Shirakawa, K. Araya, Mukoh, T. Narahara, Synth. Met., 1987, 17, 241). | ||
Meanwhile, Heeger moved to the University of California at Santa Barbara, collaborating there with chemist Fred Wudl. Realising the importance of working with processable polymers, he turned his attention to polythiophene and derivatives such as poly(iso-p-thionaphthene), with the objective of probing how to tune electronic properties via the molecular structure. By 1987, Heeger’s team had made the first inroads into device applications – making a diode by casting a polythiophene from solution onto electrodes. In 1990, Heeger and colleagues started a company, UNIAX, to take the conducting polymer technology towards commercialisation.
At around the same time, the field received a major boost when Richard Friend and Andrew Holmes working with Jeremy Burroughes, Paul Burn and Donal Bradley at the University of Cambridge, UK, showed that polymers such as poly(phenylenevinylene) luminesce when a voltage is applied to a thin film between two metallic electrodes. This led to the first polymer light-emitting diodes. Shortly thereafter, Heeger’s group also demonstrated polymer LEDs with high efficiencies, and they went on to develop the first working displays. Subsequent work demonstrated that polymer LEDs can emit light in a variety of colours. Emissive displays fabricated from polymer LEDs will be introduced as products in cell phones and personal digital assistants (PDAs) in 2003. The first commercial display is an electric shaver, as used by James Bond in the latest movie Die Another Day. Full colour polymer displays are under active development by several companies. The great commercial advantage of such polymer devices is that the active luminescent semiconducting material can be processed from solution. Using three different polymers with red, green and blue emission, full colour displays can be fabricated using ink-jet printing.9
Another project, started in the 1990s, was to develop a solar cell based on a composite of a semiconducting polymer and C60.10 When a photon is absorbed, an electron is transferred from the excited state of the polymer onto the fullerene with very high efficiency. An interpenetrating network enables the separated charges to be collected at the electrodes. This polymer-based photovoltaic technology is under development for possible commercial application in university and industrial laboratories in Europe, America and Asia. Heeger’s latest interest is modifying the same semiconducting materials so that they are water soluble and can be used as sensors for identifying proteins or DNA sequences – the first biological application in the field.11
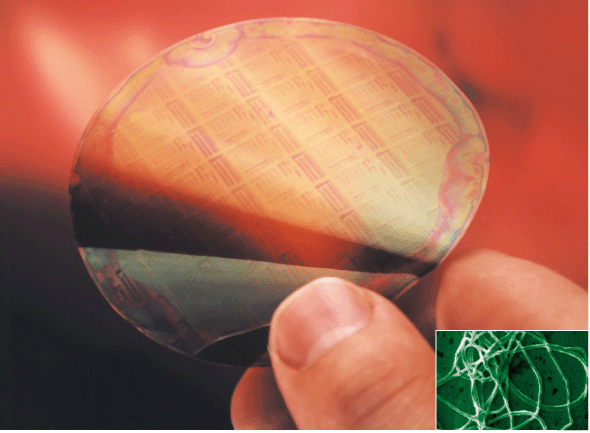 | ||
| Plate6 A flexible, semitransparent plastic chip using conducting polyaniline and containing a 27 millimetre integrated circuit which still operates when sharply bent (Philips Research). (inset) A polyaniline fibre with an average diameter of 139 nanometres. | ||
MacDiarmid’s attention has turned to one of the oldest known synthetic organic polymers, polyaniline, first reported in about 1832 in the Journal of the Chemical Society, and his group started a major programme on the polymer in 1985. MacDiarmid points out that it’s easy to synthesise (“I’ve helped many high-school students make it,” he says). Adding a dilute acid and an oxidising agent to aniline results in a “very beautiful dark green precipitate of metallic polyaniline”. The polymer forms a range of oxidation states, but the doping is mediated by protonation rather than electron transfer as in the case of other conducting polymers.12 MacDiarmid points out that polyaniline has turned out to be one of the most extensively commercialised electronic polymers, often blended or chemically combined with other industrial plastics to obtain the most desirable features. It’s used, for example, in electromagnetic shielding, and when dispersed in paint as an anti-rust agent.
MacDiarmid believes that polyaniline and other electronic polymers will play an important role in the emerging area of nanoscience. Using a novel electrostatic method, his group can fabricate electrically conducting nanofibres of a polyaniline blend, roughly 100 molecules across.12 The aim is to investigate low-dimensional phenomena that might lead to nano electronic devices. The other area that interests him is that of cheap, flexible, throwaway electronic circuits based on plastic and paper.12Conducting polymers are ideal for this purpose since they can be deposited on a substrate very simply by techniques such as the one developed by MacDiarmid and colleagues called ‘line patterning’. A conventionally printed circuit on plastic or paper is exposed to a solution containing a conducting polymer which then deposits preferentially on the bare areas of the substrate to create the circuit. The insulating pattern can then be chemically removed. Philips Research in the Netherlands has already developed polyaniline plastic chips for use as readable barcode labels in supermarkets.
All this work shows just how the field of conducting polymers has matured over the past 25 years. In the next 25, we can expect to see many more commercial applications, as well as new synthetic and fabrication methods. The area has brought together physicists, theorists, chemists, materials scientists and engineers, and is likely to involve biologists as medical science provides an obvious target for devices based on lightweight plastics. Conducting polymers will continue to have an impact on the technologies of the future.
Nina Hall
The author thanks Professors Heeger, MacDiarmid and Shirakawa for their help with this article.
Notes and references
- H. Shirakawa, E. J. Louis, A. G. MacDiarmid, C. K. Chiang and A. J. Heeger, J. Chem. Soc., Chem. Commun., 1977, 578 RSC.
- T. Ito, H. Shirakawa and S. Ikeda, Polymer J. (Tokyo), 1974, 12, 11 Search PubMed.
- C. K. Chiang, M. J. Cohen, A. F. Garito, A. J. Heeger, C. M. Mikulski and A. G. MacDiarmid, Solid State Commun., 1976, 18, 1451 CrossRef CAS.
- C. K. Chiang, C. R. Fincher, Y. W. Park, A. J. Heeger, H. Shirakawa, E. J. Louis, S. C. Grau and A. G. MacDiarmid, Phys. Rev. Lett., 1977, 39, 1098 CrossRef CAS.
- A. J. Heeger, S. Kivelson, J. R. Schrieffer and W. P. Su, Rev. Mod. Phys., 1988, 60, 781 CrossRef CAS.
- K. Araya, A. Mukoh, T. Narahara and H. Shirakawa, Chem. Lett., 1984, 7, 1141.
- K. Akagi, S. Katayama, H. Shirakawa, K. Araya, A. Mukoh and T. Narahara, Synth. Met., 1987, 17, 241 CAS.
- K. Akagi, G. Piao, S. Kaneko, K. Sakamaki, H. Shirakawa and M. Kyotani, Science (Washington D.C.), 1998, 282, 1683 Search PubMed.
- J. M. Shaw and P. M. Seidler, IBM J. Res. Dev., 2001, 45, 3 Search PubMed.
- G. Yu, J. Gao, J. C. Hummelen, F. Wudl and A. J. Heeger, Science (Washington D. C.), 1995, 270, 1789 Search PubMed.
- L. Chen, D. W. McBranch, H.-L. Wang, R. Helgeson, F. Wudl and D. G. Whitten, Proc. Natl. Acad. Sci. U. S. A., 1999, 96, 12287 CrossRef CAS.
- A. G. MacDiarmid, Angew. Chem., Int. Ed., 2001, 40, 2581 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2003 |
