Synthetic hosts via molecular imprinting—are universal synthetic antibodies realistically possible?
Steven C.
Zimmerman
* and
N. Gabriel
Lemcoff
Department of Chemistry, 600 S. Mathews Ave., University of Illinois, Urbana, IL 61801, USA. E-mail: sczimmer@uiuc.edu; Fax: +1 217 244 9919; Tel: +1 217 333 6655
First published on 29th July 2003
Abstract
Of the many ways to make synthetic hosts, one of the most appealing involves molecular imprinting. In the commonest approach monomer units assemble around or are attached to a template (imprint) molecule and then linked together using a cross-linking agent. Template removal ideally leaves cavities within the molecularly imprinted polymer (MIP) that possess a shape and functional group complementarity to the imprint molecule allowing its tight and selective uptake. This review highlights some recent advances in the synthesis of MIPs (often called “synthetic antibodies”) and enumerates a “wish list” of properties for the perfect MIP that may guide future studies.
Steven C. Zimmerman was born in Chicago, Illinois. He obtained his Ph.D. with Prof. Ronald Breslow at Columbia University and carried out postdoctoral work with Prof. Sir Alan R. Battersby at the University of Cambridge. He joined the Department of Chemistry at the University of Illinois in 1985 where he currently holds the title William H. and Janet G. Lycan Professor of Chemistry. His research interests include synthetic organic, supramolecular, bioorganic, dendrimer, and polymer chemistry. |
N. Gabriel Lemcoff was born in Buenos Aires, Argentina in 1969. In 1991 he emigrated to Israel and started his chemical career at Tel-Aviv University, where he received his B.Sc. in Chemistry in 1995 and his Ph.D. in 2001 under the supervision of Prof. Benzion Fuchs in the area of macromolecular diacetal systems. He is currently a postdoctoral research associate in the group of Prof. Steven C. Zimmerman. His research interests are in the areas of synthetic organic chemistry, supramolecular chemistry, dendrimers, and molecular modeling. |
The scientific literature is filled with hypotheses that were subsequently proven wrong and rapidly forgotten. On rare occasion, however, incorrect hypotheses have value beyond explaining a particular experimental observation or phenomenon and they take on a life of their own. Such was the case with Pauling's 1940 proposal for how antibodies are produced in the human immune response.1 The hypothesis that antigens induce or imprint a binding site within the otherwise unfolded polypeptide of an antibody was extremely compelling (Fig. 1). Indeed, the idea provided the inspiration for Dickey who reported in 1949 that dye molecules could be imprinted into silica. This early study showed that in a very broad sense Pauling's concept could be put into practice experimentally.2
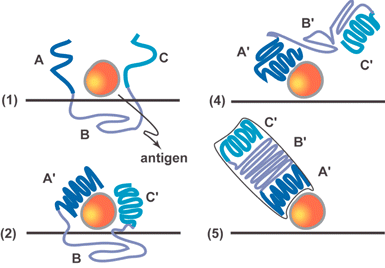 | ||
| Fig. 1 Schematic adapted from reference 1 showing four steps (1, 2, 4, 5) of Pauling's six step mechanism by which an antigen imprints structural information into an antibody molecule. | ||
The field lay more or less dormant until two landmark reports appeared, both of which are widely acknowledged as propelling polymer imprinting forward into the modern field it is today. The reports by Wulff3 and Mosbach4 showed that molecular templates, covalently and noncovalently, respectively, could imprint their own binding sites within polymers by the process shown schematically in Fig. 2. The noncovalent approach, wherein the functional monomer(s) assemble around the template, is most commonly used.5 Despite its less frequent use the covalent approach has certain advantages6,7 and to an extent the distinction between the two approaches is becoming blurred (vide infra). This review will focus on recent advances in the synthesis of MIPs and related synthetic hosts including our recently reported monomolecular imprinting approach.8 Particular emphasis is placed on current trends and especially limitations, the latter presented in the context of a “wish list” for the future properties.
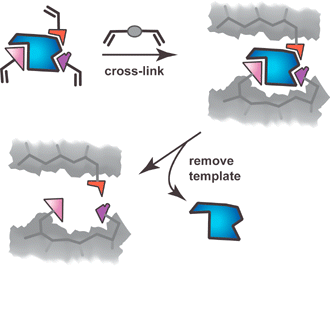 | ||
| Fig. 2 Schematic representation of the polymer imprinting process showing one binding site within the polymer matrix. Cross-linking functionality may be covalently or noncovalently linked to the template. | ||
Optimum properties of MIPs
An especially appealing feature of MIPs, from the perspective of applications, is their potential to replace antibodies in medical diagnostics and to be used as chemosensors in general.5,6,9–14 Thus, an ideal system might retain the affinity and selectivity of antibodies while adding several benefits including greater stability, ease of production, and lower cost. Progress in this area has accelerated to a point where one can start realistically to imagine what the perfect MIP will look like. This wish list, which is ennumerated in Table 1, is divided into three sections: synthesis, physical properties, and binding characteristics.| Method of synthesis |
| Preparable in one (or few) high yielding synthetic step(s) |
| Dynamic imprinting with error correction |
| Complete template removal |
| Able to be post-synthetically functionalized |
| Physical properties |
| Homogeneous imprinted sites of high stability |
| Ability to make either soluble or insoluble material |
| Readily processable |
| Ability to spectroscopically characterize binding sites |
| Binding characteristics |
| High affinity with possibility to tune |
| High selectivity with possibility to tune |
| Rapid binding kinetics |
| Transduction of binding into easy readout |
Recent advances in molecular imprinting
Making imprints more homogeneous
The method by which most MIPs are made all but guarantees the production of binding sites with different structures. Whether the differences in binding site structures produce measurable differences in the binding properties will depend on the specific system, but it is common to find a broad range of affinities. The classic study of diazepam (1) and theophylline MIPs reported by Mosbach illustrates the degree of hetereogeneity possible as well as its origin.15 As seen in Fig. 3, the hydrogen bonding between the diazepam template (1) and MAA (2) is not sufficiently strong to make assemblies such as 3 the predominant species in solution. Thus, an excess of 2 is added to push the equilibrium toward 3 and the resultant polymer contains many sites formed without templation or without the full complement of binding functionality (i.e., B sites in Fig. 3a). Some extraordinarily tight binding sites are present, but most sites have considerably lower affinity and presumably lower selectivity.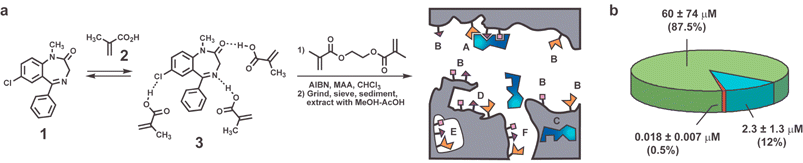 | ||
| Fig. 3 (a) Synthesis and schematic representation of a diazepam MIP (reference 15). The synthetic scheme emphasizes the need to noncovalently assemble methacrylic acid (MAA) molecules around the diazepam template (1) in a preequilibrium step preceding cross-linking with ethylene glycol methacrylate (EGDMA). Adapted with permission from reference 16, the schematic of the resulting gel emphasizes the heterogeneity of binding sites: high affinity site in macropore (A) and micropore (F), and lower affinity sites (B) in macropore, (C) trapped template, (E) embedded site, (D) highest affinity site with shape selectivity from polymer. (b) Chart showing dissociation constants and populations of three classes of binding sites needed to fit binding isotherm (data from reference 15). | ||
The heterogeneity found in MIPs is nontrivial to characterize in a quantitative manner. An advance in this area was reported by Shimizu who, beyond showing the limitation of the Scatchard plot analysis which treats MIPs as containing discrete classes of sites, demonstrated the first “affinity spectrum” (AS) for a noncovalently prepared MIP.17 The AS is a plot of association constant (Kassoc) vs. number of sites (N) and is based on a model that assumes a continuous range of binding constants. For a MIP reported by Shea and coworkers18 noncovalently prepared using a 9-ethyl adenine template the AS showed a rapid decrease in N with an increase in Kassoc (vide infra). This result is consistent with the observation that the few tight binding sites present are rapidly occupied and the overall properties of the MIP are then dominated by the large number of lower affinity sites.
Several strategies for reducing binding site diversity have been examined with the efforts achieving varying degrees of success. A particularly appealing approach takes advantage of the tight occupancy of high affinity sites with a selective chemical modification of the unbound, low affinity sites in the presence of an appropriate concentration of template (ligand). Thus, in 1997 Karube reported that methylation of a testosterone imprinted MAA-EGDMA polymer in the presence of ligand afforded a new polymer with small but significant enhancements in HPLC separation factors (i.e., testosterone vs. progesterone).19 Other investigators have examined similar strategies for reducing the heterogeneity in MIPs with somewhat mixed results. Shimizu found a modest increase in the fraction of high affinity sites after methylating the Shea adenine MIP described above, and using the AS method to characterize the changes in affinity.20 In a related study, Sellergren and Guiochon annealed a MIP prepared from L-phenylalanine anilide and found an increase in the saturation capacity with a slight increase in the mass transfer rate, but a decrease in enantiomeric resolution.21 Whitcombe and coworkers synthesized three MIPs designed to complex pyridine, quinoline, or acridine and treated each in separate experiments with three acid chlorides (acetyl chloride, naphthoyl chloride, and anthracene carbonyl chloride) whose sizes correspond to the ligand sizes.22 These studies, wherein the post-imprinting modification step was performed in the absence of template, showed a degree of matching of the imprinted binding site size and the size of the chemical capping agent. The results are similar to the molecular sorting reported by Fréchet in the derivatization of conventional polymers.23
Another strategy for increasing the homogeneity of MIPs involves increasing the preequilibrium constant shown in Fig. 3a. This can be done in a number of ways, for example by using binding contacts that are very strong (i.e., Kassoc ≥ 103 M−1). Such “stoichiometric noncovalent imprinting” uses a 1 : 1 ratio of functional monomer to the complementary group on the template. Tight binding favors sites A, D, and F at the expense of B sites in Fig. 3a.24 This approach usually involves strong ionic hydrogen bonding (e.g., carboxylate–guanidinium pair) or template–reactive monomer complexes held together by multiple, non-ionic hydrogen bonds.
In the extreme, the pre-equilibrium of Fig. 3a can be avoided altogether by using the covalent imprinting approach. However, the disadvantages of the covalent method are well documented. In an ingenious combination of the two methods, investigators have shown that cleavage of particular covalent bonds between the functional monomer and template may lead to ligand rebinding via noncovalent interactions.14,25,26 A sophisticated example of this approach, called the “sacrificial spacer” method, was reported by Whitcombe and coworkers.26 The tripeptide target, Lys-Trp-Asp (4), is covalently linked to reactive monomer 5 and then MIP synthesis is performed in the noncovalent manifold using 6 (Fig. 4). The sacrificial spacer (5) is ultimately removed by hydrolysis leaving behind a cavity with an appropriate fit and complementary functionality for noncovalently binding the template.
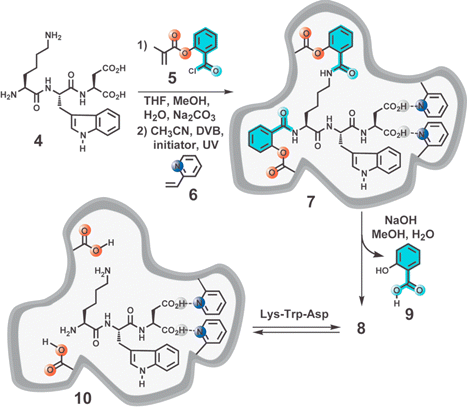 | ||
| Fig. 4 Schematic representation of the “sacrificial spacer” (highlighted in light blue) method of MIP synthesis. The amide groups of Lys-Trp-Asp are linked to sacrificial spacer 5 which following polymerization is hydrolysed away as 9. The cross-linking reaction is carried out in the presence of 2-vinylpyridine 6, which creates additional binding interactions to the Asp unit. Proposed complex formed between MIP and Lys-Trp-Asp: 10. | ||
Shimizu and coworkers used17 the AS method to analyze and compare the heterogeneity of the Shea 9-ethyl adenine MIP 11 (vide supra),18 a related ethyl adenine-9-acetate MIP 12 (not shown), and cholesterol MIP 13 prepared by Whitcombe25 using a carbonate group as the covalent, sacrificial spacer (Fig. 5). As seen in Fig. 5c there is a very pronounced difference between the two types of MIPs. The covalently prepared 13 has a narrow distribution of binding affinities showing a maximum at K = 1700 M−1. In contrast the noncovalently prepared 11 and 12 contain a much higher proportion of low affinity binding sites, but on the positive side the distribution decays more slowly toward the high log K side indicating the presence of a few very high affinity sites. The authors note that if the results are indicative of a general pattern of differences between the covalent and noncovalent approaches the following applications guide can be suggested: covalently prepared MIPs will be most useful for applications where a high percentage of “good” sites are required, such as chromatography, whereas noncovalently prepared MIPs might effectively be applied in cases where high affinities are needed and low concentrations can be tolerated (e.g., biosensors).
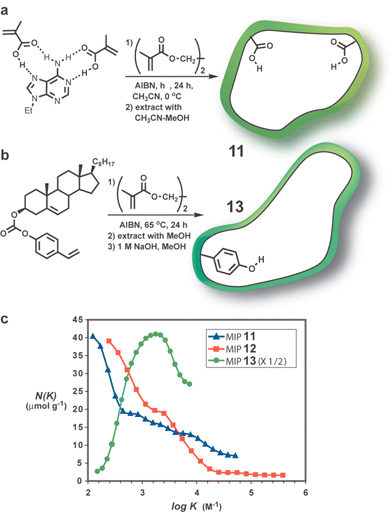 | ||
| Fig. 5 (a) Noncovalent synthesis of a 9-ethyladenine MIP as initially reported by Shea.18 MIP 12 was prepared similar to 11 but using ethyl adenine-9-acetate as template and chloroform as the porogen,17 (b) synthesis of 13 using sacrificial spacer approach,25 (c) AS distribution curves for MIP17 (data courtesy of Prof. K. Shimizu). | ||
The mixed approach developed by Whitcome (vide supra) clearly blurs the distinction between the covalent and noncovalent approaches. It further indicates that the template or imprint molecule need not be the ultimate target for complexation. This may prove a significant advantage when the MIP is targeted to a compound that is highly toxic or one that is unavailable in sufficient quantity.
Dynamic molding: an approach to error free, self-correcting molecular imprinting and a universal synthetic antibody
The most common approach to MIPs uses free radical polymerization reactions that are irreversible. For this reason, there is no mechanism for correcting errors in the synthesis, such as the linking of functional monomers in the absence of template. If reversibility could be built in, it would have the further advantage that any site, including templated sites with low affinity and non-templated sites, could equilibrate to high affinity ones. Such a process would be directly analogous to the dynamic combinatorial library approach for amplifying effective hosts and would help alleviate the heterogeneity problem (vide infra).To our knowledge there are no reported examples of MIPs synthesized dynamically (i.e., reversible cross-linking) with error correction, but there are examples where the selectivity of an existing MIP is altered through conformational and bond reorganization processes. For example, Hiratani and coworkers created a heteropolymeric gel capable of complexing Ca2+ ions and reversibly swelling in response to temperature changes.27 The gel was made from lead methylacrylate (PbMAA2), which served as both a reversible cross-linking agent and a covalent template for the ultimate complexation of Ca2+ ions by two carboxylate groups (Fig. 6). The polymerization of PbMAA2 was carried out with N-isopropylacrylamide (NIPA), an irreversable cross-linking agent (N,N′-methylenebis(acrylamide), BIS) and a reversible cross-linker (N,N′-bis(acryloyl)cystamide, BAC). Following removal of the Pb2+ ions the gel bound Ca2+ ions, presumably in the same sites occupied by the Pb2+ ions (Fig. 6a). Following reduction and reoxidation, the most favorable disulfide linkages were formed leading to a gel with reduced affinity for Ca2+ (Fig. 6b,c). Importantly, if the reoxidation was carried out in the presence of Ca2+ then the resulting “post-imprinted” gel exhibited an enhanced affinity.
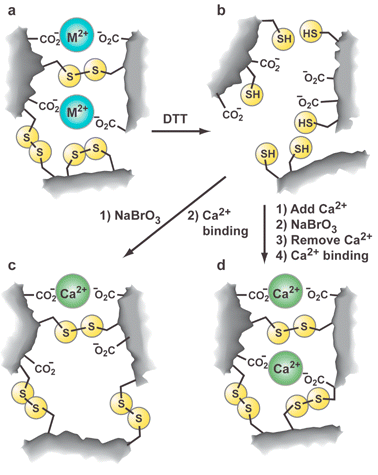 | ||
| Fig. 6 Schematic representation of thermally responsive gel imprinted for Ca2+ binding. (a) Initially imprinted gel (M2+ = Pb2+). After removal of Pb2+ ions gel binds Ca2+ ions (M2+ = Ca2+) giving initial gel. (b) Reduced gel obtained after reduction of initial gel with dithiothreitol (DTT) and removal of Ca2+. (c) Reoxidized gel formed by oxidation of reduced gel shows reduced Ca2+ affinity. (c) Post-imprinted gel formed by oxidation of reduced gel in the presence of Ca2+ ions shows enhanced Ca2+ affinity. | ||
The ultimate extension of the process outlined in Fig. 6 would be a polymer containing a range of binding functionality from hydrogen bond donor and acceptor groups to charged sites all connected by a rigid backbone that could reorganize to provide any constellation of binding sites. In the presence of an excess of the template and an external reorganization trigger the polymer would be imprinted. By acting as a kind of universal synthetic antibody such a system would come quite close to the process Pauling envisioned for the human immune response (Fig. 1). Progress in this area will be helped significantly by the recent, intense interest in developing dynamic polymers for their novel materials properties (e.g., self-healing ability).28–31
Transducing MIP binding into a readout
Beyond creating effective MIPs, considerable effort has focused on developing convenient methods to detect ligand uptake with the ultimate goal of creating chemosensors. The analytical techniques by which binding is transduced into a readout are varied and include measuring changes in the MIP's optical (e.g., fluorescence)32,33 and electrochemical properties,34,35 mass (QCM and SAW)36–38 and refractive index (SPR)39 as well as the recent use of biosensor-type approaches where binding of an analyte is coupled to an enzymatic reaction producing color or chemoluminescence.40,41 Most of these analytical detection methods have been reviewed recently.5,9An attractive approach to sensing involves integrating a reporter chromophore into the polymeric network so that analyte binding produces an optical readout. The analyte may bind in proximity to the chromophore changing its spectral properties through either a direct process such as fluorescence quenching or indirectly by a general change in the local environment. The latter approach is illustrated by the use of reporter monomer 14 containing the fluorescent dye, trans-4-[4-(N,N-dimethylamino)styryl]-N-vinylbenzylpyridinium chloride, in the synthesis of a MIP for adenosine-3′:5′-cyclic monophosphate (cAMP, Fig. 7a).42
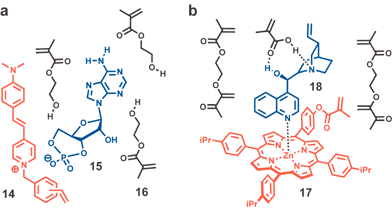 | ||
| Fig. 7 Representative prepolymerization assemblies of template (blue), reporter chromophore (red) and monomer/cross-linking agent (black). (a) In addition to 14–16, the mixture contains 2-ethyl-2-(hydroxymethyl)-1,3-propanediol trimethacrylate (TRIM) as the cross-linking agent. (b) Mono-functional Zn(II) porphyrin 17 complexed to (−)-cinchonidine. | ||
One strategy for increasing the detection selectivity uses reporter groups that signal binding only upon a direct bonding event between the chromophore and the target analyte. Commonly used in the development of molecular recognition based chemosensors, this strategy can increase the sensitivity and selectivity of analyte detection. As seen in Fig. 7b, Takeuchi and coworkers integrated a Zn(II)-porphyrin moiety into a MIP that complexed (−)-cinchonidine leading to quenching of its fluorescence.43 The high affinity sites of this MIP gave an association constant (Kassoc = 1.14 × 107 M−1) which was ten-fold higher than the Kassoc measured for two analogous MIPs, one prepared indentically but without 17 and the other without MAA. The authors previously reported application of the same strategy to a 9-ethyladenine selective MIP.44 Tong and coworkers used the same strategy to create a histamine selective MIP but using commercially available Zn(II) protoporphyrin as the fluorescent functional monomer.45 This particular porphyrin features two vinyl groups that may allow it to serve as a cross-linking agent.
Smaller and thinner: the top down approach
MIPs have traditionally been prepared by bulk polymerization followed by mechanical grinding into particles of the desired dimensions. This approach is simple and, unlike traditional emulsion polymerization, fully compatible with the noncovalent imprinting requirement of hydrogen bond-mediated assembly. However, substantial effort has focused on alternative synthetic manifolds because of the many limitations of the resulting MIP particles. In particular, slow mass transfer of analytes into and out of MIPs limits their use as chromatographic supports and as rapid chemosensors. Additionally, the template is often trapped deep within the polymer matrix but nonetheless leaches out at a rate sufficient to prevent trace analysis. Finally, the insoluble, heterogeneous polymer is difficult to characterize.Significant progress has been made in developing novel methods that produce smaller and thinner MIPs including by imprinting within films and microgels and on surfaces ranging in size from nanoparticles to micron-sized silicon wafers. This work has been reviewed recently by Mayes46 and thus a few selected examples are presented that illustrate the diversity of approaches under development.
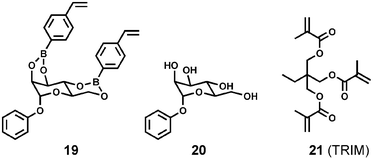
Wulff and coworkers explored the synthesis and recognition properties of microgels imprinted with template 19, a reactive monomer used previously in the preparation of traditional MIPs.47 Microgels with a high level of cross-linking (≥50% wt. cross-linker) were prepared in different solvents (porogen) using 19, MMA, and a cross-linking agent, such as EDMA or TRIM (21). Importantly, the microgels were fully soluble in a range of organic solvents. The yields of template removal were nearly quantitative for the lightly cross-linked gels but dropped as the extent of cross-linking increased. The imprinted microgels took up sugar 20 but showed only a small degree of enantioselective binding when presented with racemic 20. From membrane osmometry the number-averaged molecular weights were determined to be (2–7) × 105 Da, a value that is in the range of proteins. Based on these MW values and the extent of sugar uptake it was estimated that each microgel particle contained 10–100 binding sites. Thus, this report described a significant step toward imprinting a single binding site within a protein-sized synthetic macromolecule.
A remarkably clever approach to ensure that the imprinted sites reside at the polymer surface was described by Haupt and Mosbach (Fig. 8).48 These investigators immobilized 8-carboxypropyltheophylline onto aminopropyl silica (amide formation) with capping of residual amino groups. Using a previously optimized procedure for the synthesis of a classical theophylline MIP, the silica was treated with trifluoromethylacrylic acid (TFMAA) and divinylbenzene. In this new approach a solvent porogen is unnecessary because following dissolution of the silica the imprinted binding sites reside near the surface of the polymer particle. In comparison to the classical MIP, the surface imprinted polymer (sMIP) showed a similar high selectivity for theophylline over structurally similar guests caffeine and theobromine, but the sMIP's overall capacity was lower. Potential advantages of this approach include: (1) the ability to imprint guests that are insoluble in the imprinting solvent, (2) more homogeneous binding sites from controlling the orientation of the imprint molecule, and (3) more accessible binding sites due to their proximity to the MIP's surface.
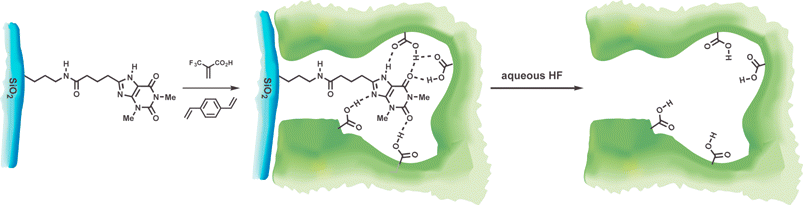 | ||
| Fig. 8 Aminopropyl derivatized silica gel is coupled to 8-carboxypropyltheophylline and a molecularly imprinted polymeric layer is grown on the silica surface.48 Treatment with HF dissolves the silica, removes the template, and leaves imprinted sites near the surface of the polymer. | ||
Monomolecular imprinting: the nanoscale or bottom up approach
One ultimate goal of the top down approach is to imprint a single binding site within a single macromolecule. The ability to isolate binding sites to separate molecules means that homogeneity might be achieved by separation or fractionation. As such, heterogeneity in the imprinting process sacrifices only the yield of the desired MIP, not its performance (e.g., selectivity or affinity). Is it possible to develop a bottom up strategy wherein the imprinting process leaves a single binding site in a single macromolecule? Very recently, several synthetic approaches to molecular hosts with single binding sites have emerged which share some conceptual and methodological similarities to the synthesis of MIPs.The most extensively studied of these approaches involves a dynamic combinatorial library (DCL) of receptors (hosts) wherein the template (guest) shifts the equilibrium toward the tightest binding receptor.49,50 An elegant example of this approach is the equilibrating library of macrocyclic disulfides reported by Otto and Sanders to which were added different templates to produce an enhancement in the population of different macrocyclic hosts (Fig. 9).51 Thus, a 1 : 1 : 1 mixture of dithiols 22–24 formed a small library of interconverting macrocyclic disulfides. In the absence of a template, the library contained 25 (22·23 mixed dimer) and 26 (22·23·24 mixed trimer) as the major components. In the presence of N-methylquinolinium iodide, the amount of several minor components increased significantly, especially 27 (22·242 mixed trimer) which becomes the second most prevalent species after 25. In the presence of a second template, the N-methylammonium iodide salt of morpholine, a different macrocyclic receptor, a 243 homo-trimer (structure not shown) was amplified. Thus, each guest imprints its structure within the equilibrating library of host molecules.
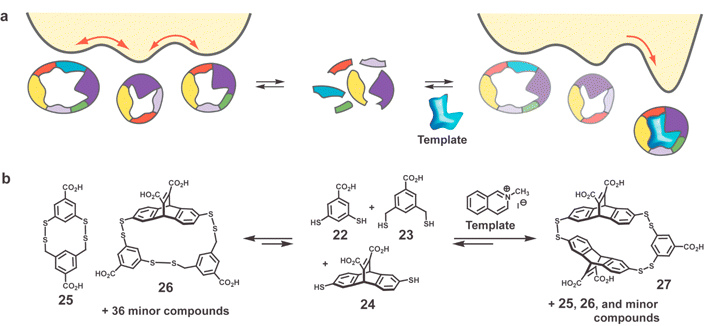 | ||
| Fig. 9 (a) Schematic representation of dynamic combinatorial library (DCL) approach. Energy profile in presence and absence of template is altered by energy of complexation leading to a type of amplification. Illustration adapted with permission from reference 51. (b) Dithiols 22–24 used as monomers for forming a library of macrocyclic disulfides.51 | ||
The DCL approach to synthetic receptors has drawbacks. The reaction chemistry, including kinetics and thermodynamics, required to make the scheme in Fig. 9a work is nontrivial. For example, the monomers must covalently assemble and interconvert in a facile manner on a suitable timescale, but there must be a mechanism for “trapping” the mixture after introduction of the template; otherwise upon isolation the amplified receptor will simply equilibrate back to the original mixture. Additionally, for small libraries, parallel synthesis may offer an attractive alternative, whereas for large libraries there are questions about whether the equilibirum can be shifted to a useful extent.52 Nonetheless, this is a very promising new strategy that warrants much more exploration.
How distinct are the DCL and MIP approaches? Beyond the number of binding sites per molecule, there are some obvious differences between the classical MIP and DCL approaches. For example, MIPs are polymeric hosts produced by irreversible chemical reactions whereas the DCL approach makes small “organic” binding sites reversibly. These differences, however, only reflect the current methods by which these approaches are implemented and there is every reason to believe that the distinction will become less apparent as more examples appear (see, for example, Fig. 6).
A novel molecular imprinting procedure that bears remarkable resemblance to Pauling's antibody imprinting hypothesis was reported by Shinkai, Reinhoudt, and coworkers (Fig. 10).53 These investigators took advantage of the tendency for poly(L-lysine) to interconvert between α-helical and β-sheet secondary structures as a result of subtle external influences. Thus, poly(L-lysine) was converted into peptide 28 bearing both alkanethiol side chains and arylboronic acid groups. The former allows the peptide to be anchored onto a gold surface, whereas the latter took advantage of the observation by Kobayashi that an analogous aryl boronic acid derivatized poly(L-lysine) bound D-glucose, the binding inducing a high degree of β-sheet structure in the peptide.54 The preference for this secondary structure was attributed to the formation of a 2 : 1 arylboronic acid·D-glucose complex. As shown in Fig. 10, the β-sheet secondary structure induced in 28 is partially locked by its fixation onto the gold surface of a QCM resonator. The D-glucose was washed off and the resulting imprinted layer showed a stronger signal upon rebinding of D-glucose than did a device produced with a D-fructose imprinted or non-imprinted peptide surface. Although this approach is not strictly monomolecular because the surface contains an array of peptides that are likely to be heterogeneous, the same general strategy could be extended to solution phase imprinting with a different “locking” chemistry.
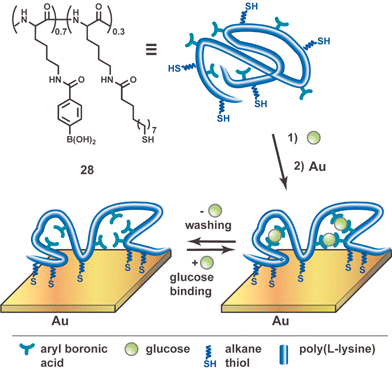 | ||
| Fig. 10 Schematic representation of conformational imprinting method. Addition of glucose induces secondary structure within poly(L-lysine) which is captured by anchoring the molecule to a gold substrate. Schematic adapted with permission from reference 53. | ||
Shinkai and coworkers described the first “homogeneous nanoscale imprinting system,” wherein a saccharide template imprinted two boronic acid groups onto the surface of [60]fullerene.55,56 The D-threitol-derived, saccharide template (29) is chiral and non-racemic (Fig. 11). The surface derivatization involves a chiroselective double Diels–Alder reaction, via an intermediate ortho-quinone methide, and subsequent hydrolysis gave 30 as a 72 : 28 ratio of enantiomers. In competitive complexation studies with D,L-threitol, 30, was shown to form a 70 : 30 diastereomeric ratio of complexes. The major isomer was shown to be (fA)-30·L-threitol + (fC)-30·D-threitol as expected given that D-threitol was used to prepare 30. The work reveals several advantages of this bottom-up approach, in particular, the ability to structurally identify individual imprinted binding sites.
![Nanoscale imprinting on [60]fullerene. Initial bis-arylboronic acid product, formed by acid hydrolysis of initial cycloadduct, was reacted with 2,2-dimethylpropane-1,3-diol to give the cyclic boronate whose absolute configuration was determined using the circular dichroism (CD) method of Harada and Diederich.57](/image/article/2004/CC/b304720b/b304720b-f11.gif) | ||
| Fig. 11 Nanoscale imprinting on [60]fullerene. Initial bis-arylboronic acid product, formed by acid hydrolysis of initial cycloadduct, was reacted with 2,2-dimethylpropane-1,3-diol to give the cyclic boronate whose absolute configuration was determined using the circular dichroism (CD) method of Harada and Diederich.57 | ||
Rebek and coworkers reported a dynamic, nanoscale imprint using a chiral “softball” capsule formed by the dimerization of 31 (Fig. 12).58 Containing a plane of symmetry, bis-glycouril 31 is achiral, but it dimerizes with the formation of a chiral capsule with eight hydrogen bonds along the seam. In the absence of external chiral influences, capsule 31·31 exists as a 1 : 1 mixture of enantiomers (racemate). To increase the stability of the capsule for imprinting, compound 32 was synthesized containing two hydroxy groups. The added functionality allowed the formation of four additional hydrogen bonds which slowed significantly the rate of interconversion of the enantiomeric 32·32 dimers.
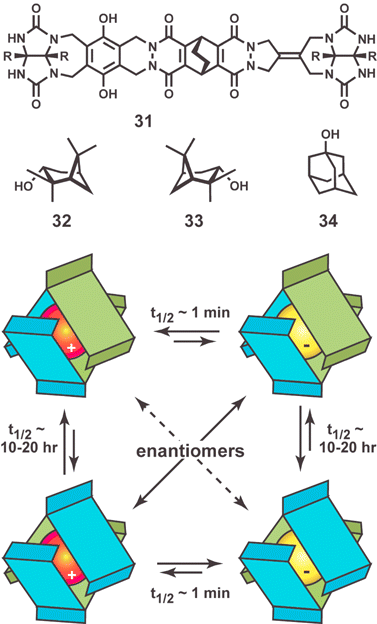 | ||
| Fig. 12 Structure of bis-glycoluril 31 and 32 and guests 33 and 34 which bind inside “softball” dimer 32·32. Schematic representation of chiral imprinting showing diastereomeric assembled complexes. Adapted with permission from reference 58. | ||
With the most stable capsule in hand, complexation studies were undertaken with 33. Within seconds of the addition of three equivalents of (+)-pinanediol (33) to a solution of dimer 32·32 two diastereomeric complexes formed in an approximately 1 : 1 ratio (Fig. 12). Over the course of a few days a thermodynamically controlled 2 : 1 ratio formed. After removal of solvent, and extraction of excess (+)-33, a solution of the assembled complex, (+)-33·32·32, was treated with an excess of 34 to fully displace the (+)-33. After removal of 33, the 34·32·32 complex was treated with (−)-33 and a 2 : 1 mixture formed which favored the less stable diastereomeric complex. This result indicates that the chiral imprint is retained following extraction of the chiral guest.
We recently described a monomolecular imprinting process wherein a single template (porphyrin) was dynamically imprinted into a single macromolecule (dendrimer).8 Thus, the overall process borrows elements of both the covalent approach to MIPs and the DCL strategy for producing synthetic hosts. As shown schematically in Fig. 13, the monomolecular imprinting method involves three key steps: (1) attaching dendrons to a template, which is either the target ligand or a structure designed to mimic its shape and functional group array, (2) cross-linking the end-groups, and (3) cleaving the focal point attachments to the template and removing it.
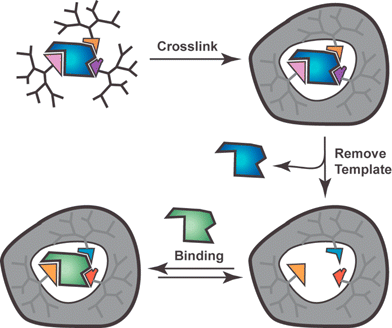 | ||
| Fig. 13 Schematic representation of monomolecular imprinting approach using dendrimers. Template may be covalently or noncovalently linked to dendrons. Removal of template may or may not alter functional groups at the core and template may be target ligand or an analog that produces appropriate constellation of functional groups. | ||
Although any macromolecule might be used for monomolecular imprinting, the use of dendrimers was considered advantageous for method development. In particular, the monodispersity of dendrimers meant that characterizing the steps in Fig. 13 would be easier than if a polydisperse polymer were used. Furthermore, with multiple end-groups a high number of cross-links could be obtained. Unfortunately these cross-links are structurally far from the core or template and the preparation of dendrimers usually requires multiple steps.
The actual chemistry is shown in Fig. 14. A key feature is the use of the Grubbs ring closing metathesis (RCM) reaction to cross-link the 64 homoallyl end-groups of 35.59 By virtue of its reversibility this process dynamically molds the dendritic framework around the core template. The beauty of the Grubbs process is evident in the fact that catalyst 36 tolerates the different functional groups present in 35 and produces a highly cross-linked 37 in good yield (88%). The RCM reaction produced products with differing numbers of cross-links. Here the advantage of the dendrimer approach was clear in that the individual cross-linked products were observed by MALDI-TOF-MS, with peaks corresponding to 28–32 out of a possible 32 cross-links being most prevalent. Basic hydrolysis quantitatively removed the template (porphyrin 39), with molecularly imprinted dendrimer (MID) 38 isolated in 43% yield. Similar size exclusion chromatographic (SEC) retention times were observed for 37 and 38 suggesting that the latter retains the same compact, highly cross-linked structure.
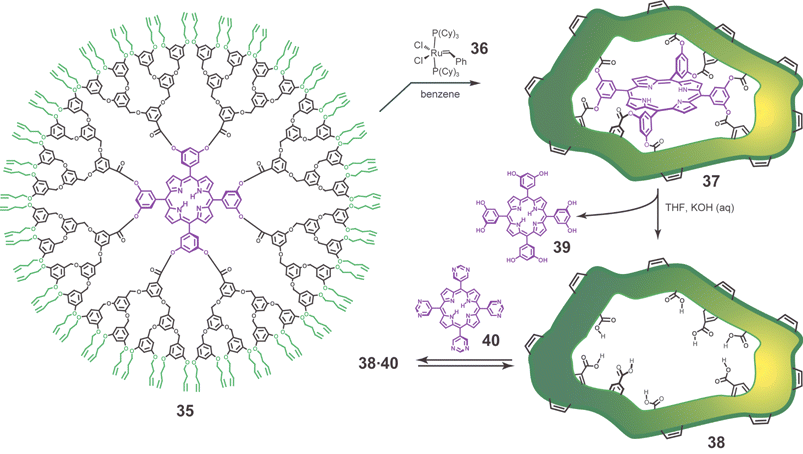 | ||
| Fig. 14 Scheme illustrating monomolecular imprinting method, the synthesis of molecularly imprinted dendrimers (MIDs).8 Dynamic cross-linking of 35 is performed with 4 mol% of 36 per alkene at a dendrimer concentration of 10−6 M. The imprinted dendrimer 38 does not bind template 39 because the addition of eight water molecules makes its binding cavity too small. However, porphyrins with complementary basic sites such as 40 form complexes in apolar organic solvents. | ||
Complexation studies with MID 38 and 11 different porphyrin guests, as well as a battery of control studies, support the following general conclusions: (1) >95% of the imprints are effective and their binding properties are homogeneous, (2) MID 38 is selective, binding porphyrins with at least four hydrogen bond donor/acceptor sites (e.g., tetrapyrimidinyl porphyrin 40) with Kassoc ≈ 104–105 M−1 in toluene, (3) the imprint is size selective, thus, template 39 is too large to bind, but the 2,6-dihydroxy isomer gives Kassoc ≈ 105 M−1 in 5% ethyl acetate–toluene, (4) the imprint is not highly shape selective with 5,10,15,20-tetrakis(2-pyridyl), tetrakis(3-pyridyl), and tetrakis(4-pyridyl)porphyrin all giving about the same Kassoc. In short, the monomolecular approach outlined in Fig. 13 works.
With this proof of principle example in hand, the major challenge is to improve and refine the monomolecular process. One serious obstacle to scale-up, the requirement for high dilution cross-linking (≈ 10−5–10−6 M) to prevent inter-dendrimer metathesis, has been solved by moving the alkene groups inside the dendrimer.60 It is very probable that dendrimers with alkene groups closer to the core, perhaps in each layer, will increase the rigidity of the imprint with increases in affinity and selectivity. In this regard, the cross-linked structure is infinitely tunable. An ideal monomolecular imprint might arise from dendrons that generate a structure so tightly cross-linked that the template/ligand cannot diffuse out. In this case one or more dendrons lacking alkene groups could be attached to the template so as to generate a channel for the template to escape (and the ligand to bind).
As indicated above, one advantage of the dendrimeric approach is the ability to characterize the cross-linked products. To be practical the monomolecular imprinting will ultimately have to be developed with more readily prepared macromolecular structures. Hyperbranched and star polymers are obvious choices. In this regard, we have recently shown that star polymers with porphyrin cores can be readily prepared, cross-linked, and cored.61
Another recent advance in the monomolecular imprinting approach involves attachment of an azodye to the focal point of the cross-linkable dendrons.62 As seen in Fig. 15, dendron 41 can be imprinted with a simple alkane diamine (butane-1,4-diamine) and the resulting cross-linked dendrimer, 42, rapidly signals binding of the template with a colour change. A Job plot analysis and 13C and 19F NMR studies show two point binding through formation of a bis-carbinolamine. However, the cross-linked dendrimer is small and flexible enough to allow a number of diamines to bind, albeit more weakly and slowly. The improvements discussed above for the porphyrin imprinting should be applicable here as well.
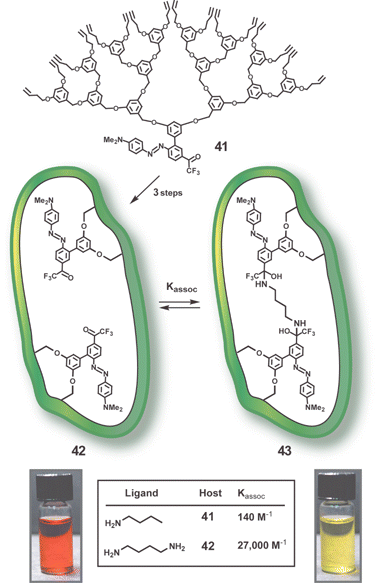 | ||
| Fig. 15 Schematic representation of monomolecular imprinting approach using dendron 41 with azodye at focal point.62 Conversion of 41 to bis-imine of butane-1,4-diamine, RCM reaction, and imine hydrolysis produces MID 42. MID 42 binds diamines by two point binding (see complex 43) which causes the visible colour change shown. | ||
Conclusions
To a large extent the enormous potential for MIPs to replace antibodies in bioanalytical assays and to be used in chemosensor applications has not been realized because of their inherent limitations. The heterogeneity in binding affinities, slow mass transfer in and out of the polymer matrix, overall low binding affinity, lack of a read-out for complexation, and trapped template slowly leaching out are the properties most often mentioned as being problematic. Described herein are several promising new strategies to address these problems, including some significant advances in the synthesis of MIPs. A clear trend, which we have termed the “top-down” approach, is toward nanoscale MIP synthesis. At the same time an entirely new, “bottom-up” strategy has appeared, wherein a single binding site is imprinted inside a single molecular scaffold. Several examples in this area feature dynamic “molding” processes that can both correct errors in the imprinting process and amplify the most productive imprints. It should be clear from the examples presented that the distinction between the top-down and bottom-up strategies is growing smaller and will ultimately disappear.In returning to the question posed in the title: are universal synthetic antibodies possible?, predictions along these lines are always risky. What can be said with certainty is that in the past several years the pace at which new and exciting developments in molecular imprinting have been reported has quickened. Furthermore, it is a surety that the continuing search for a universal approach to synthetic antibodies will lead to many additional discoveries that are both important and of fundamental significance.
Acknowledgements
The authors gratefully acknowledge the National Institutes of Health (GM61067) for support in writing this review and support of the work in the authors' laboratory described herein. Partial support of the Army Research Office is also acknowledged with gratitude.Notes and references
- L. Pauling, J. Am. Chem. Soc., 1940, 62, 2643 CrossRef CAS
.
- F. H. Dickey, Proc. Natl. Acad. Sci. USA, 1949, 35, 227 CAS
.
- G. Wulff and A. Sarhan, Angew. Chem., Int. Ed., 1972, 11, 341 CAS
.
- L. Andersson, B. Sellergren and K. Mosbach, Tetrahedron Lett., 1984, 25, 5211 CrossRef CAS
.
- K. Haupt and K. Mosbach, Chem. Rev., 2000, 100, 2495 CrossRef CAS
.
- G. Wulff, Angew. Chem., Int. Ed., 1995, 34, 1812 CrossRef CAS
.
- K. J. Shea, Trends Polym. Sci., 1994, 2, 166 Search PubMed
.
- S. C. Zimmerman, M. S. Wendland, N. A. Rakow, I. Zharov and K. S. Suslick, Nature, 2002, 418, 399 CrossRef CAS
.
- K. Haupt, Chem. Commun., 2003, 171 RSC
.
-
Molecularly Imprinted Polymers. Man-Made Mimics of Antibodies and their Application in Analytical Chemistry, ed. B. Sellergren, Elsevier, New York, 2001 Search PubMed
.
-
Drug Development Assay Approaches, Including Molecular Imprinting And Biomarkers, eds. E. Reid, H. M. Hill and I. D. Wilson, Royal Society of Chemistry, Cambridge, UK, 1998 Search PubMed
.
-
Molecularly Imprinted Materials – Sensors And Other Devices, eds. K. J. Shea, M. Yan and M. J. Roberts, Materials Research Society, Warrendale, PA, 2002 Search PubMed
.
-
Molecular Imprinting: From Fundamentals To Applications, eds. M. Komiyama, T. Takeuchi, T. Mukawa and H. Asanuma, Wiley, New York, 2003 Search PubMed
.
- M. J. Whitcombe and E. N. Vulfson, Adv. Mater., 2001, 13, 467 CrossRef
.
- G. Vlatakis, L. I. Andersson, R. Muller and K. Mosbach, Nature, 1993, 361, 645 CrossRef CAS
.
-
B. Sellergren, Molecular And Ionic Recognition With Imprinted Polymers, eds. R. A. Bartsch and M. Maeda, American Chemical Society, Washington, 1998, ch. 4 Search PubMed
.
- R. J. Umpleby II, M. Bode and K. D. Shimizu, Analyst, 2000, 125, 1261 RSC
. Shimizu reported recently that the binding behavior of MIPs can be accurately modeled by the heterogeneous Langmuir–Freundlich (LF) isotherm, a more straightforward analysis: R. J. Umpleby II, S. C. Baxter, Y. Chen, R. N. Shah and K. D. Shimizu, Anal. Chem., 2001, 73, 4584 Search PubMed
.
- K. J. Shea, D. A. Spivak and B. Sellergren, J. Am. Chem. Soc., 1993, 115, 3368 CrossRef CAS
.
- S. McNiven, Y. Yokobayashi, S. H. Cheong and I. Karube, Chem. Lett., 1997, 1297 CAS
.
- R. J. Umpleby II, G. T. Rushton, R. N. Shah, A. M. Rampey, J. C. Bradshaw, J. K. Berch Jr. and K. D. Shimizu, Macromolecules, 2001, 34, 8446 CrossRef
.
- Y. Chen, M. Kele, P. Sajonz, B. Sellergren and G. Guiochon, Anal. Chem., 1999, 71, 928 CrossRef CAS
.
- N. Kirsch, C. Alexander, M. Lubke, M. J. Whitcombe and E. N. Vulfson, Polymer, 2000, 41, 5583 CrossRef CAS
.
- V. Smigol, F. Svec and J. M. J. Fréchet, Anal. Chem., 1994, 66, 4308 CrossRef CAS
.
- G. Wulff and K. Knorr, Bioseparation, 2002, 10, 257 CrossRef CAS
.
- M. J. Whitcombe, M. E. Rodriguez, P. Villar and E. N. Vulfson, J. Am. Chem. Soc., 1995, 117, 7105 CrossRef CAS
.
- J. U. Klein, M. J. Whitcombe, F. Mulholland and E. N. Vulfson, Angew. Chem., Int. Ed., 1999, 38, 2057 CrossRef CAS
and references therein..
- H. Hiratani, C. Alvarez-Lorenzo, J. Chuang, O. Guney, A. Yu Grosberg and T. Tanaka, Langmuir, 2001, 17, 4431 CrossRef CAS
.
- X. Chen, M. A. Dam, K. Ono, A. Mal, H. Shen, S. R. Nutt, K. Sheran and F. Wudl, Science, 2002, 295, 1698 CrossRef CAS
.
- S. J. Rowan, S. J. Cantrill, G. R. L. Cousin, J. K. M. Sanders and J. F. Stoddart, Angew. Chem., Int. Ed., 2002, 41, 898 CrossRef
.
- T. Nishinaga, A. Tanatani, K. Oh and J. S. Moore, J. Am. Chem. Soc., 2002, 124, 5934 CrossRef CAS
.
- H. Otsuka, K. Aotani, Y. Higaki and A. Takahara, J. Am. Chem. Soc., 2003, 125, 4064 CrossRef CAS
.
- S. A. Piletsky, E. Terpetschnig, H. S. Andersson, I. A. Nicholls and O. S. Wolfbeis, Fresenius J. Anal. Chem., 1999, 364, 512 CrossRef CAS
.
- A. L. Jenkins, O. M. Uy and G. M. Murray, Anal. Commun., 1997, 34, 221 RSC
.
- S. A. Piletsky and A. P. F. Turner, Electroanalysis, 2002, 14, 317 CrossRef CAS
.
- A. Merkoçi and S. Alegret, Trends Anal. Chem., 2002, 21, 717 CrossRef CAS
.
- F. L. Dickert, O. Hayden and K. P. Halikias, Analyst, 2001, 126, 766 RSC
.
- M. Zayats, M. Lahav, A. B. Kharitonov and I. Willner, Tetrahedron, 2002, 58, 815 CrossRef CAS
.
-
K. Das, J. Penelle and V. M. Rotello, Langmuir, 2003, asap Search PubMed
.
- A. Kugimiya and T. Takeuchi, Biosens. Bioelectron., 2001, 16, 1059 CrossRef CAS
.
- I. Surugiu, L. Ye, E. Yilmaz, A. Dzgoev, B. Danielsson, K. Mosbach and K. Haupt, Analyst, 2000, 125, 13 RSC
.
- S. A. Piletsky, E. V. Piletska, B. Chen, K. Karim, D. Weston, G. Barrett, P. Lowe and A. P. F. Turner, Anal. Chem., 2000, 72, 4381 CrossRef CAS
.
- R. Turkewitsch, B. Wandelt, G. D. Darling and W. S. Powell, Anal. Chem., 1998, 70, 2025 CrossRef CAS
.
- T. Takeuchi, T. Mukawa, J. Matsui, M. Higashi and K. D. Shimizu, Anal. Chem., 2001, 73, 3869 CrossRef CAS
.
- J. Matsui, M. Higashi and T. Takeuchi, J. Am. Chem. Soc., 2000, 122, 5218 CrossRef CAS
.
- A. Tong, H. Dong and L. Li, Anal. Chim. Acta, 2002, 466, 31 CrossRef CAS
.
- N. Pérez-Moral and A. G. Mayes, Bioseparation, 2002, 10, 287 CrossRef CAS
.
- A. Biffis, N. B. Graham, G. Siedlaczek, S. Stalberg and G. Wulff, Macromol. Chem. Phys., 2001, 202, 163 CrossRef CAS
.
- E. Yilmaz, K. Haupt and K. Mosbach, Angew. Chem., Int. Ed., 2000, 39, 2115 CrossRef CAS
.
- P. A. Brady, R. P. Bonar-Law, S. J. Rowan, C. J. Suckling and J. K. M. Sanders, Chem. Commun., 1996, 319 RSC
.
- J.-M. Lehn and A. Eliseev, Science, 2002, 291, 2331 CrossRef CAS
.
- S. Otto, R. L. E. Furlan and J. K. M. Sanders, Science, 2002, 297, 590 CrossRef CAS
.
- J. S. Moore and N. W. Zimmerman, Org. Lett., 2000, 2, 915 CrossRef CAS
.
- A. Friggeri, H. Kobayashi, S. Shinkai and D. N. Reinhoudt, Angew. Chem., Int. Ed., 2001, 40, 4729 CrossRef CAS
.
- H. Kobayashi, K. Nakashima, Y. Ohshima, I. Hamachi and S. Shinkai, J. Chem. Soc., Perkin Trans. 2, 2000, 997 RSC
.
- T. Ishi-i, K. Nakashima and S. Shinkai, Chem. Commun., 1998, 1047 RSC
.
- T. Ishi-i, R. Iguchi and S. Shinkai, Tetrahedron, 1999, 55, 3883 CrossRef CAS
.
- H. Goto, N. Harada, J. Crassous and F. Diederich, J. Chem. Soc., Perkin Trans 2, 1998, 1719 RSC
.
- J. M. Rivera, S. L. Craig, T. Martín and J. Rebek Jr., Angew. Chem., Int. Ed., 2000, 39, 2130 CrossRef CAS
.
- T. M. Trnka and R. H. Grubbs, Acc. Chem. Res., 2001, 34, 18 CrossRef CAS
.
- L. G. Schultz, Y. Zhao and S. C. Zimmerman, Angew. Chem., Int. Ed., 2001, 40, 1962 CrossRef CAS
.
-
J. B. Beil and S. C. Zimmerman, Macromolecules, submitted Search PubMed
.
- E. Mertz and S. C. Zimmerman, J. Am. Chem. Soc., 2003, 125, 3424 CrossRef CAS
.
| This journal is © The Royal Society of Chemistry 2004 |