Materials that naturally assemble themselves
First published on 3rd December 2003
Abstract
One of key aims of modern chemistry is to explore and exploit the phenomenal complexity of matter that seems to arise spontaneously within ambient environments like that of our planet. Biology, of course, is a ultimate manifestation of a universal chemical canon encompassing interactions both at the molecular and supramolecular level. Inorganic building blocks play a significant role in the self-organised assembly of many biological structures at various scale-lengths but the details of their chemical behaviour and interaction with organic compounds are still not well-understood. Stephen Mann of the University of Bristol is a pioneer in the area; his team is probing the ground rules for biomineralisation in the hope of applying them to the self-assembly of practical materials with complex hierarchical structures.
 Professor Mann is Director of the Centre for Organised Matter Chemistry at the University of Bristol. He received his doctorate in 1982 from the University of Oxford for his work on biomineralisation and bioinorganic materials chemistry, later becoming lecturer and then reader at the University of Bath. His current research at Bristol is concerned with the biomimetic synthesis, characterisation and emergence of complex forms of organised matter across extended length scales. In his spare time, Professor Mann plays a Gibson Les Paul guitar, which he bought recently to mark being made a Fellow of the Royal Society.
Professor Mann is Director of the Centre for Organised Matter Chemistry at the University of Bristol. He received his doctorate in 1982 from the University of Oxford for his work on biomineralisation and bioinorganic materials chemistry, later becoming lecturer and then reader at the University of Bath. His current research at Bristol is concerned with the biomimetic synthesis, characterisation and emergence of complex forms of organised matter across extended length scales. In his spare time, Professor Mann plays a Gibson Les Paul guitar, which he bought recently to mark being made a Fellow of the Royal Society.
|
HOW DOES A COMPLEX SHAPE like a spiral shell or the spines of a sea urchin emerge from a simple crystalline substance—calcium carbonate? It seems truly extraordinary that Nature can assemble, with such exquisite control, a wide variety of functional mineral structures with highly specific morphologies—elegant skeletal frameworks, optical lenses (in trilobites), or gravity sensors in the inner ear—from just two kinds of mundane inorganic ions.
This process of biomineralisation has fascinated Stephen Mann, ever since his postgraduate days at Oxford in the early 1980s when he first started working in the newly fledged subject of bioinorganic chemistry under one of its leading exponents R. J. P. Williams. “It was the ‘wow’ factor that got us into it,” he says, “a limited number of solid-state inorganic materials such as calcium carbonate, silica and iron oxides could form new materials that bear no relation to the underlying crystallographic structure. We wanted to understand biomineralisation in a general sense from a chemical point of view, and develop overarching concepts that could be exploited to make new materials.”1
It was clear that biology offered a confined and controlled—and, of course, highly non-equilibrium—environment able to modulate the movement of ions and their destination, particularly in relation to crystal growth. Organic components such as proteins, lipid structures and larger macromolecular frameworks play a key role in directing the aggregation or nucleation of ions and their sequestration, either on a specific crystal face to create larger-scale morphologies with a lower symmetry than that of the basic unit cell, or to form part of a supramolecular organic–inorganic composite. Various dynamic combinations of interactions, often with feedback—chemical bonding, electrostatic attraction or repulsion, mechanical stress, spatial confinement—come into play to generate a specific reaction field at a defined length scale. In this way, macroscopic hierarchical structures can be autonomously built up from assemblies of smaller units formed at the nano and meso-scale—the process of morphogenesis.
Solid-state chemistry in a confined space
Mindful of these principles, Mann and his colleagues went on to investigate a series of biomimetic strategies ultimately aimed at synthesising unusual materials. The first experiments, carried out with Bob Williams, were to make model systems that followed the role of phospholipid membranes in modifying the formation of iron oxides and calcium carbonate. The compartmentalisation and templating afforded by these fundamental cell-membrane materials were thought to play a key role in biomineralisation. They discovered that spherical crystals of magnetite (found in magnetotactic bacteria) developed when iron oxide was confined inside lipid vesicles, whereas in their absence, another iron-oxide structure formed—needle-shaped goethite (as in limpet teeth).2 Similarly, calcium carbonate crystallised into flower-like crystals of vaterite on the surface of a soap film instead of the usual simple rhombohedral form.3 Vaterite (Fig. 1) occurs in certain marine creatures and is less thermodynamically stable than the other two calcium-carbonate polymorphs, calcite and aragonite. It is worth noting that it was in Williams’ group that the electron microscope, now a routine tool in materials science, first became a key analytical instrument for studying the evolution of biomineral structures. | ||
| Fig. 1 Vaterite occurs as elaborately-shaped spicules in marine animals called ascidans (scale bar 5 micrometres). | ||
This work inspired Mann, when he moved to the University of Bath and later to Bristol, to look at biological systems that could act as templates for materials synthesis. An intriguing organic–inorganic structure is the iron-storage protein ferritin which consists of a nanoparticle of ferrihydrite (5Fe2O3.9H2O) containing up to 4500 iron atoms encased in a stable polypeptide coat (apoferritin). One of Mann′s students, Fiona Meldrum, showed that the ferrihydrite cores can be removed from the protein cages and replaced with other nanoparticles of technological interest such as semiconductors or ferromagnets (Fig. 2).4 Indeed, another of Mann’s students, Eric Mayes, has since gone on to set up a company that makes cobalt–platinum nanoparticles, using apoferritin as a mould, for ultrahigh-density data-storage media.5
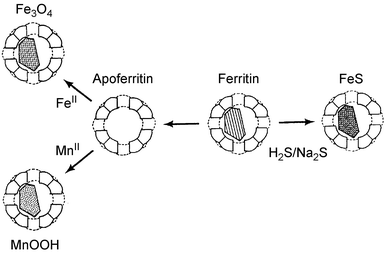 | ||
| Fig. 2 Artificial ferritins containing MnOOH, magnetic Fe3O4 or CdS can be prepared from apoferritin (demineralised ferritin). | ||
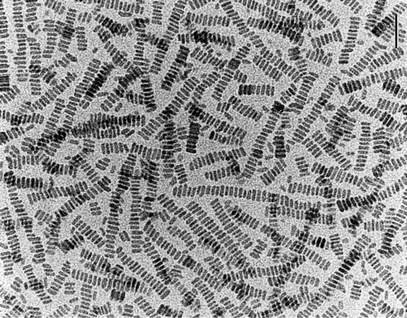
Even larger structures such as bacteria can offer unusual confined spaces for mimicking biomineralisation. Mann recalls how he developed one idea from a talk at the Materials Research Society given by Neil Mendelson from the University of Arizona who was studying Bacillus subtilis. This bacterium is used to make unfermented soybean curd, or ‘tofu’, and can be readily cultured. Remarkably, it can be drawn out as long threads made of filaments just one cell thick but close-packed into co-aligned hexagonal arrays. The individual bacterial filaments are half a micrometre wide, while the macroscopic fibre is a few millimetres across and several centimetres long (Fig. 3). The dry thread readily swells in water without damaging the underlying superstructure to offer a network of inter-filament channels through which nanoparticles such as those of silica can pass.
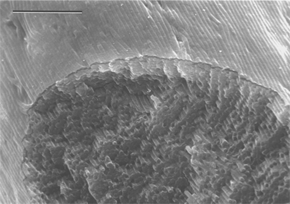 | ||
| Fig. 3 A cross-section of bacterial thread showing an internal hexagonal structure of co-aligned multicellular filaments (scale bar 10 micrometres). | ||
Mann and his team realised that the bacterial architecture furnished a suitable template for creating inorganic frameworks such as the mesoporous silica MCM-41 or zeolites. One of Mann′s students, Sean Davis, dipped the bacterial threads into an aqueous dispersion of the nanoparticles which diffuse into the channels in an orderly manner, creating a living composite.6 Calcination removes the bacterial template to give the solid mineral with a hierarchical porous structure at the nano and meso scale (Fig. 4). Mann notes that, of course, there is a simpler way to make minerals with these kinds of periodic structures using polymer networks and colloids. Indeed, such fabrication methods have almost become routine. However, the work illustrates nicely the basis of patterning mechanisms that Nature herself uses to introduce structural complexity into biological systems.
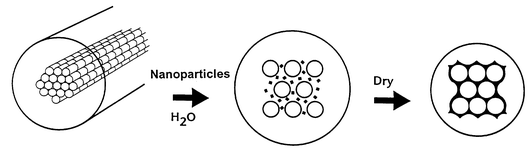 | ||
| Fig. 4 The mineralisation of the bacterial superstructure using inorganic nanoparticles and reversible swelling. | ||
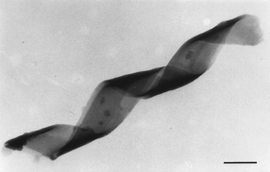
Some biological structures such as bone may be assembled as mutually interacting organic–inorganic composites in which each component modifies the behaviour of the other through interfacial recognition. A recent test-tube example designed by Mann’s group is the assembly of a silica–lipid nanocomposite from an unusual surfactant diacetylenic phosphatidyl choline (DC8,9PC) and tetraethoxysilane (Si(OCH2CH3)4), or TEOS. The phospholipid has a zwitterionic headgroup and two hydrocarbon tails, each containing diacetylenic units. Its overall chiral structure generates bending and twisting stresses which force the aligning lipid bilayers into strips that wind into a tube-shaped helical structure, rather like a drinking straw (Fig. 5). These pre-formed tubules can then be used as templates for making ultra-thin metal or inorganic cylinders by depositing the appropriate mineral and burning off the lipid.7,8 However, if DC8,9PC is added to water already containing TEOS, the two components co-assemble. The TEOS hydrolyses to produce silicate anions which interact with the lipid’s cationic headgroups and deposit along the lipid bilayers as they twist into the helical shape—in this case forming an open secondary architecture rather than a closed tube (Fig. 6).9
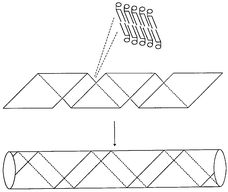 | ||
| Fig. 5 How DC8,9PC first forms a multilamellar helical ribbon and then a helical straw. | ||
Spontaneous generation of organisation
Understanding the design principles behind this kind of self-assembly has become a driving force behind Mann’s work. “Can you induce transitions in complex chemical systems that spontaneously generate higher levels of organisation?,” he says. Rather than rationally develop a complex material via sequential transformations that require intervention after each step, the idea is to understand how the initial conditions and the environment engender the scale-dependent emergent behaviour needed to create hierarchical structures. This is what Nature does and it is the general philosophy behind bottom-up nanotechnology. Chemical functionalisation may need to be built in from the start. For example, one of Mann’s students, Sandy Burkett, made a pre-functionalised MCM-41 by including a percentage of an organic silane in the tetraethoxysilane used to create the porous silica structure around a surfactant framework (which is then burnt off).10To investigate the fundamental rules, Mann has gone back to thinking about the processes that modulate the nucleation and growth of simple inorganic materials from their basic building blocks. Taking inorganic nanoparticles as his starting point, he has been investigating how information can be imparted to their crystal surfaces to alter their growth and patterns of aggregation. One ingenious approach is to functionalise particles by attaching an information-rich molecule with the potential for molecular recognition. Chad Mirkin at NorthWestern University stuck non-complementary single-stranded DNA (using thiol groups as the glue) onto two batches of gold particles and then added an oligonucleotide duplex which was complementary to the two grafted sequences. As the oligonucleotide strands interacted, the gold particles were assembled into aggregates which could be reversibly broken up by using heat to unwind the DNA.11
Mann notes, however, that such particles still have a simple spherical geometry which leads to the expected hexagonal close-packing; a much higher level of information could be introduced if the particles were anisotropic in shape, say, rod-shaped, and could be assembled into chain-like geometries leading to higher-order superstructures.12 His approach was to introduce surfactants that would preferentially coat one crystal face-type and so inhibit growth in that direction. At the same time, the hydrophobic surfactant tails would align in directions away from the polar inorganic–surfactant interface inducing a transition to a mesophase consisting of rows of nanoparticle rods anchored to interdigitated lipid bilayers.13
It turns out that water-in-oil microemulsions provide an idealised reaction environment for probing such nanoparticle–surfactant interactions. A series of experiments have been carried out by Mei Li, currently a postdoc in Mann’s team, in which nanocrystals of barium sulfate (or chromate) are prepared in isooctane from a mixture of barium ions as reverse micelles of barium bis(2-ethylhexyl)sulfosuccinate, Ba(AOT)2, and sulfate ions trapped in sodium bis(2-ethylhexyl)sulfosuccinate water-in-oil micelles. When mixed together in a ratio of 1 to 1, slow exchange between the micelles results in growth of barium sulfate/chromate crystals in confined water droplets only 3 to 4 nanometres across. Through further cooperative interactions with the surfactant, rod-shaped particles form and assemble into linear arrays as described above (Fig. 7).14
 | ||
| Fig. 7 Linear chains of BaCrO4 nanoparticles prepared in AOT reverse microemulsions (scale bar 50 nanometres). | ||
The process is highly sensitive to the molar ratios of the reactants. For example, only discrete particles, not assembled chains, form if the anion concentration is somewhat higher than that of the cations—up to 5 to 1 ratio. This is probably because the excess negative charge from the sulfate ions interferes with the electrostatic attraction between the anionic headgroups of the surfactant and the barium ions on the crystal surface.
When the molar ratio is reversed to give 5 to 1 excess barium, then something bizarre happens. The excess positive charge drives the surfactant to bind immediately to the surface of the nucleating crystal such that a periodic structure has no time to form. The result is a unique amorphous form of barium sulfate with particles, 5 nanometres in size, coated in surfactant. This intimate relation between surfactant and inorganic crystal leads to some extraordinary complex behaviour that seems truly biomimetic.15,16 The hydrophobic interactions between the surfactant tails cause the particles to aggregate and arrange themselves into linear arrays which fuse into bundles of surfactant-tethered 5-nanometre filaments (Fig. 8). Differential hydration along the filaments causes the bundles to distort, creating cone-shaped spiral shapes. These then amplify in scale like Russian dolls to produce self-similar millimetre-sized objects reminiscent of those created by natural biomineralisation processes (Fig. 9). Interestingly, similar structures are obtained from aqueous solutions of certain hydrophilic block copolymers and anionic polyelectrolytes.17
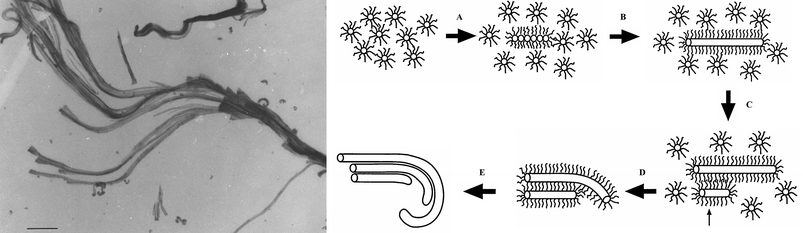 | ||
| Fig. 8 (LEFT) Twisted bundles of BaSO4 nanofilaments prepared in AOT reverse microemulsions from surfactant-stabilised amorphous nanoparticles (scale bar 500 nanometres); (RIGHT) the mechanism for their formation (the small arrow shows the nucleation of a secondary filament and surfactant interdigitation). | ||
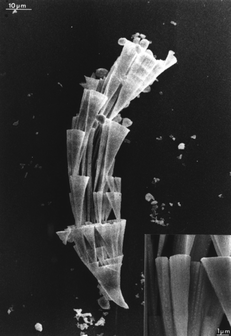 | ||
| Fig. 9 Cones of BaSO4 which form from the amorphous nanoparticles (scale bar 10 micrometres). They look distinctly ‘biological’. | ||
Surface charge or molar ratio, lattice energy, and degree of hydration at the surfactant-inorganic interface are clearly important in controlling the type of ordering that occurs. Mann is hoping to elucidate the rules leading to this kind of emergent behaviour. “At the moment we have only two or three examples; we need more to see what is generic between them,” he says. In this way, chemists can not only better understand the biomineralisation process but also start to programme ‘one-pot’ morphosynthetic strategies for advanced materials that are truly biomimetically-inspired.
Nina Hall was talking to Professor Mann.
Notes and references
- S. Mann, Biomineralisation Principles and Concepts in Bioinorganic Materials Chemistry, Oxford University Press, Oxford, 2001 Search PubMed.
- S. Mann, J. P. Hannington and R. J. P. Williams, Nature (London), 1986, 324, 565 CrossRef CAS.
- S. Mann, B. R. Heywood, S. Rajam and J. D. Birchall, Nature (London), 1988, 334, 692 CrossRef CAS.
- F. C. Meldrum, B. R. Heywood and S. Mann, Science (Washington D.C.), 1992, 257, 522 Search PubMed.
- Nanomagnetics web site: www.nanomagnetics.com.
- S. A. Davis, S. L. Burkett, N. H. Mendelson and S. Mann, Nature (London), 1997, 385, 420 CrossRef CAS.
- A. J. Patil Muthusamy, E. A. Seddon and S. Mann, Adv. Mater., 2003, 15, 1816 CrossRef.
- S. L. Burkett and S. Mann, Chem. Commun., 1996, 321 RSC.
- A. M. Seddon, H. M. Patel, S. L. Burkett and S. Mann, Angew. Chem. Int. Ed., 2002, 41, 2988 CrossRef CAS.
- S. L. Burkett, S. D. Sims and S. Mann, Chem. Commun., 1996, 1367 RSC.
- C. A. Mirkin, R. L. Letsinger, R. C. Mucic and J. J. Storhoff, Nature (London), 1996, 382, 607 CrossRef CAS.
- E. Dujardin, L.-B. Hsin, C. R. C. Wang and S. Mann, Chem. Commun., 2001, 1264 RSC.
- H. Cölfen and S. Mann, Angew. Chem. Int. Ed., 2003, 42, 2350 CrossRef.
- M. Li, H. Schnablegger and S. Mann, Nature (London), 1999, 402, 393 CrossRef CAS.
- M. Li and S. Mann, Langmuir, 2000, 16, 7088 CrossRef CAS.
- M. Li and S. Mann, Adv. Funct. Mater., 2002, 12, 773 CrossRef CAS.
- L. Qi, H. Cölfen, M. Antonietti, M. Li, J. D. Hopwood, A. J. Ashley and S. Mann, Chem. Eur. J., 2001, 7, 3526 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2004 |