A microfluidic method for removal of the zona pellucida from mammalian embryos
H. C.
Zeringue
a,
M. B.
Wheeler
b and
D. J.
Beebe
*a
aDepartment of Biomedical Engineering, University of Wisconsin-Madison, Madison, WI, USA. E-mail: djbeebe@wisc.edu
bDepartment of Animal Sciences, University of Illinois at Urbana-Champaign, Urbana, IL, USA
First published on 14th October 2004
Abstract
Many in vitro procedures involve manipulation of the zona pellucida (chimerics, transgenics, biopsy). We have demonstrated a microfluidic channel network to precisely control (spatially and temporally) the delivery of chemical treatments for the removal of the zona pellucida. Building devices in polydimethylsiloxane (PDMS) with channel dimensions on the same order as that of the embryo diameter (∼120 µm) allows precise control of the local fluid environment. The system uses pressure driven flows to control embryo positioning, embryo movement, and plug formation. Zona removal is achieved by briefly washing a plug of lysing agent (acid Tyrode's medium) over the embryo.
Introduction
The in vitro production (IVP) of mammalian embryos is central to several areas of biotechnology including treatment of human fertility, animal breeding, transgenic animal production and cloning. Recent procedural advances such as nuclear transfer, nuclear microinjection and intracytoplasmic sperm injection (ICSI) have been developed, but similar advances in the tools used for gamete manipulation have yet to make a similar impact on assisted reproduction. Microtechnologies are just beginning to engage IVP, male gamete manipulation and cellular biology.1,2 We present a microfluidic-based method for chemical manipulation of the mammalian embryo within a microfluidic device.The zona pellucida (ZP) is a passive glycoprotein membrane encasing the embryonic cells during the first 5–8 days of development. Manipulation of the ZP is common to many IVP procedures. ZP thinning, complete removal or partial removal are critical to chimera3 and transgenic procedures,4,5 fertilization, and 6 cell biopsy.7 The present report deals with complete ZP removal (ZPR) for chimera3 and transgenic4,5 production.
The method most commonly used for complete ZPR is chemical removal. Chemical removal involves placing a group of embryos into acidic lysing solution (typically acid Tyrode's medium) until the zona becomes visibly degraded, the embryo is then quickly pipetted back into holding medium. The low efficiency rates of chimera production are generally partially attributed to the imprecision and harshness of this procedure. We present a more precise method of ZPR, utilizing a shorter exposure to lysis solution.
In order to perform IVP of pre-implantation embryos in microfluidic systems, the following basic functions are required: (1) loading and unloading, (2) transport/holding, (3) culture, (4) chemical manipulation, and (5) mechanical manipulation. Individual embryo transport to predetermined locations in microfluidic systems, biocompatibility of microfabrication materials and mechanical removal of cumulus cells have previously been demonstrated.1 Here, we demonstrate the functionalities necessary for chimera, embryonic stem cell transgenic and viral-mediated transgenic formation.
Materials and methods
The channel network is formed from polydimethylsiloxane (PDMS) (Sylgard 184, Dow Corning, Midland, MI) using micromolding methods.8,9 The molded PDMS is sectioned and connection holes are bored through the PDMS. The PDMS is bonded to a microscope slide using an oxygen plasma treatment.8IVP procedure specifics are described elsewhere.10 Briefly, oocytes were aspirated from abattoir ovaries and incubated in maturation medium for 24 h at 39 °C, 5% CO2 in air. Oocytes were then co-incubated in fertilization medium with sperm for 24 h at 39 °C, 5% CO2 in air. After 24 h co-incubation, cumulus was removed from presumptive zygote-stage embryos by vortexing (3 min). ZPR was performed on denuded embryos.
Devices were autoclave sterilized for 30 min. Syringes were connected to the channels using tubing press-fit into the connection holes. The channel network was filled with HEPES wash solution (Whitaker Biosciences). Flow was adjusted using syringes to achieve embryo positioning at the parking region. A lysing plug (acidic Tyrode's medium11 with pH 2.5–2.8) is formed at the cross-junction by flowing lysis solution from the lysis inlet to the lysis outlet. The lysis plug is briefly washed over the zygotes by adjusting flow along the main channel.
Results and discussion
A microfluidic system was designed, fabricated and demonstrated to achieve chemical removal of the zona pellucida from mammalian embryos (Fig. 1). To perform ZPR in a microfluidic platform, the following basic functions were demonstrated: (1) loading and unloading, (2) transport/holding, (3) precise chemical and (4) mechanical manipulation. (1) The funnel well allows a large placement area where embryos are guided into the channel for easy embryo deposit/retrieval with standard pipetting techniques. (2) The channel network allows easy transportation of embryos (Fig. 1). (3) The cross-junction in the main channel permits formation of the fluid plug, used to chemically remove the ZP. The parking area (Fig. 1, P) is a constricted channel region to spatially hold embryos while maintaining continuous fluid flow. (4) The “v” design of the parking area allows embryonic cells to be brought into intimate contact and maintained during subsequent culture.12 Additionally, the micromolding fabrication process and techniques provide complete optical access for embryo analysis, rapid prototyping, and easy integration with other IVP functional components.1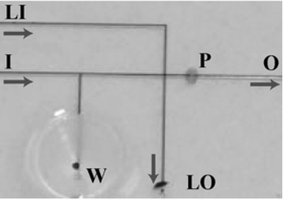 | ||
| Fig. 1 Close-up of the channel network of the zona pellucida removal device. Embryos are placed in the well (W). Fluid is drawn through the outlet (O), moving the embryos to the constricted parking (P) region. A plug of lysing agent is formed by flowing acid Tyrode's medium from the lysis inlet (LI) to the lysis outlet (LO). The embryos are then exposed to the acidic plug by flowing fluid from the main inlet (I) to outlet (O). Arrows show the direction of fluid movement. | ||
Fig. 2 shows the steps necessary for chimera formation demonstrated in a microfluidic device. Two embryos are brought to the parking area for ZPR (Fig. 2(a)). The lysis plug flows over the embryos (Fig. 2(b)) and is washed out of the local environment (Fig. 2(c)). Approximately 15 s later, the degraded ZP dislodges, leaving the embryonic cells in intimate contact (Fig. 2(d)). Gentle continuous fluid flow (approx. 1 mm s−1 average velocity) is used throughout the ZPR process. Recent preliminary data demonstrates bovine embryos develop to the blastocyst stage after microfluidic ZPR (unpublished data, A.L. Reeder and J.J. Rutledge13).
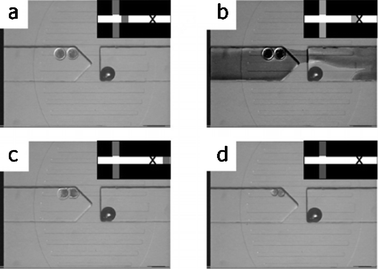 | ||
| Fig. 2 Series of photomicrographs showing chemical zona pellucida removal. Schematic of the channel network in the upper right corner of each photomicrograph shows the position of the lysis plug. (a) The embryos are first brought into the parking area. (b) Diffuse lysis plug (darkened† fluid) flows over the embryos. (c) Lysis plug is removed from parking region as ZP begins to degrade. (d) The denuded embryonic cells in intimate contact. A bubble is seen as a dark circle on the right side of the images and does not interfere with device operation. | ||
An unexpected result was the timing of ZP degradation and subsequent removal. In traditional ZPR protocols, the embryo is typically left in the lysis solution until visible degradation occurs. Our results show ZPR after an exposure time of a few seconds in the presence of gentle fluid flow. This result is significantly shorter than the typical 30 s to 2 min of acid exposure in traditional Petri-dish removal. A more brief exposure to acid could improve subsequent embryo development, increasing efficiencies for ZPR-related procedures.
This research also has potential implications for IVP procedures involving thinning and partial removal of the ZP, including zona hardening compensation, embryo biopsy, ICSI and transgenics. The problem of zona hardening is seen by lower rates of hatching from the ZP,14 inhibiting proper attachment and potentially playing a role in monozygotic twinning.15 Brief exposure to acidic solutions has been applied to help compensate for hardening. Acidic weakening of the zona matrix facilitates embryo hatching, attachment and continued development. For partial ZP removal procedures, one could deliver acidic solution to a distinct region of the ZP,16 removing a portion of the ZP (similar to present ZP drilling procedures17).
In this paper, we present a microfluidic platform for chimeric embryo formation. Precise control of embryo placement and flow rates permit controlled exposure to the lysing agent yielding predictable ZPR. The single fluid environment allows much of the shock and severe treatment from quickly changing environments to be removed from the ZPR process. The demonstration illustrates potential impacts micro technologies can have on the embryology industry. The functionalities presented here are important enablers of basic developmental biology studies.
Acknowledgements
The authors would like to thank Amy Reeder, Rick Monson and Jack Rutledge from the Department of Animal Sciences at UW for helpful discussions and access to preliminary data. Funding was provided by IEDR through UIR (UW-Madison).References
- D. J. Beebe, M. Wheeler, H. C. Zeringue, E. Walters and S. Raty, Microfluidic technology for assisted reproduction, Theriogenology, 2002, 57, 125–135 CrossRef CAS.
- R. S. Suh, N. Phadke, D. A. Ohl, S. Takayama and G. D. Smith, Rethinking gamete/embryo isolation and culture with microfluidics, Hum. Reprod. Update, 2004, 9, 451–561 Search PubMed.
- A. K. Tarkowski, Mouse chimeras developed from fused eggs, Nature (London), 1961, 190, 857–860 CAS.
- T. Tsukui, Y. Kanegae, I. Saito and Y. Toyoda, Transgenics by adenovirus-mediated gene transfer into mouse zona-free eggs, Nature Biotechnology, 1996, 14, 982–985 CrossRef CAS.
- A. Nagy, J. Rossant, R. Nagy, W. Abramow-Newerly and J. C. Roder, Derivation of completely cell culture-derived mice from early-passage embryonic stem cells, Proc. Natl. Acad. Sci. USA., 1993, 90, 8424–8428 CAS.
- G. Palermo, H. Joris, P. Devroey and A. C. V. Steirteghem, Pregnancies after intracytoplasmic sperm injection of single spermatozoon into an oocyte, Lancet, 1992, 340, 17–18 CrossRef CAS.
- H. Joris, A. De Vos, R. Janssens, P. Devroey, I. Liebaers and A. Van Steirteghem, Comparison of the results of human embryo biopsy and outcome of pgd after zona drilling using acid tyrode medium or a laser, Hum. Reprod., 2003, 18, 1896–1902 CrossRef CAS.
- B. Jo, L. M. V. Lerberghe, K. M. Motsegood and D. J. Beebe, Three-dimensional micro-channel fabrication in polydimethylsiloxane (pdms) elastomer, J. Microelectromech. Syst., 1999, 9, 76–81 CrossRef CAS.
- D. Duffy, J. C. McDonald, O. J. A. Schueller and G. M. Whitesides, Rapid prototyping of microfluidic systems in poly (dimethyl siloxane), Angew. Chem., 1998, 37, 550–575.
- A. Fisher, D. P. Bernal, C. Guiterrez-Robayo and J. J. Rutledge, Estimates of heterosis for in vitro embryo production using reciprocal crosses in cattle, Theriogenology, 2000, 54, 1433–1442 CrossRef.
- C. A. Ziomek, in The mammalian preimplantation embryo, ed. B.D. Bavister, Plenum Press, New York, 1971 Search PubMed.
- S. Raty, J. A. Davis, D. J. Beebe, S. L. Rodriguez-Zas and M. B. Wheeler, Culture in microchannels enhance in vitro embryonic development of preimplantation mouse embryos, Theriogenology, 2001, 55, 241.
- A. L. Reeder and J. J. Rutledge, personal communication, 2003 Search PubMed.
- E. Edi-Osagie, L. Hooper, P. McGinlay and M. Sief, Effect(s) of assisted hatching on assisted conception (ivf & icsi), Cochrane Database Syst. Rev., 2003, 4, CD001894 Search PubMed.
- M. Alikani, N. A. Cekleniak, E. Walters and J. Cohen, Monozygotic twinning following assisted conception: An analysis of 81 consecutive cases, Hum. Reprod., 2003, 18, 1937–1943 CrossRef.
- S. Takayama, J. C. McDonald, E. Ostuni, M. N. Liang, P. J. A. Kenis, R. F. Ismagilov and G. M. Whitesides, Patterning cells and their environments using multiple laminar fluid flows in capillary networks, Proc. Natl. Acad., 1999, 96, 5545–5548 Search PubMed.
- M. Moser, T. Ebner, M. Sommergruber, U. Gaisswinkler, K. Jesacher, M. Pucher, R. Wiesinger and G. Twes, Laser-assisted zona pellucida thinning prior to routine icsi, Hum. Reprod., 2004, 19, 573–578 CrossRef CAS.
Footnote |
| † Digital adjustment of photomicrograph contrast allows for better visualization of lysis solution. |
| This journal is © The Royal Society of Chemistry 2005 |
