Research Highlights
First published on 3rd December 2004
Free zone protein separations
Electrophoretic separations of large polyelectrolytes such as DNA and denatured proteins typically utilize physical gels to provide effective size discrimination of components. Separations performed in free solution are ineffective since large proteins (with molecular weights greater than 10 kDa) exhibit electrophoretic mobilities which are independent of protein size due to a uniform charge density along the peptide chain. Nevertheless, Harold Craighead and co-workers at Cornell University and Purdue University have recently described an approach which allows effective separation of denatured proteins in free solution without the use of sieving media. In these studies the authors show that if protein denaturation is performed using a mixture of alkyl sulfates (with different carbon chain lengths), differential binding of the alkyl sulfates with different carbon chain lengths on polypeptide surfaces leads to variation in free zone electrophoretic mobility.Specifically, separations were performed in Pyrex microfluidic chips fabricated using lithography, plasma etching, and thermal bonding techniques. Resulting microchannels had a depth of 3.8 µm and a half-depth width of 26 µm. In initial studies, four model proteins (with molecular weights between 14 and 116 kDa) were denatured with a commercial sulfate mixture with multiple chain lengths. As can be seen in Fig. 1 the four proteins are completely resolved in free-solution, with migration times that are not related to their molecular weights. Further experiments confirm that separations can also be achieved using a binary mixture of pure sodium dodecyl sulfate and sodium tetradecyl sulfate but not by a single component sulfate. These results indicate that differential binding of alkyl sulfates to different carbon chain lengths on the polypeptide surface generates variations in electrophoretic mobilities and thus allows denatured proteins to be separated in free-solution. Although the precise mechanism of differential binding is unclear, the authors believe that incorporation of alkyl sulfates with mixed chain lengths into the protein–sulfate complex generates surface heterogeneity, which is determined by the amino acid sequence. Overall, the approach provides an elegant, rapid and simple method for protein analysis and is ideally suited to integration with other on-chip processing methods.
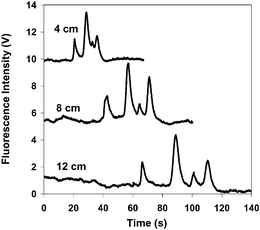 | ||
| Fig. 1 Free solution electrophoresis of four proteins denatured with an alkyl sulfate mixture. The separation was driven by 436 V cm−1 and the running buffer was 2.5 mM sodium borate + 0.14%(w/v) Pierce SDS. Fluorescence was detected at 4, 8 and 12 cm from the injection. The peaks were assigned as follows (12 cm): β-galactosidase (116 kDa, 67 s); conalbumin (78 kDa, 89 s); α-lactalbumin (14 kDa, 101 s); ovalbumin (45 kDa, 111 s). | ||
Chemical Communications, 2005, DOI: 10.1039/b411697f
Temperature gradient focussing for DNA hybridisation assays
Temperature gradient focusing (originally reported by Locascio and co-workers) within microfluidic systems involves the application of a temperature gradient across a microchannel. With an appropriate buffer (that exhibits a temperature dependent ionic strength) the temperature gradient creates a gradient in both the electric field and electrophoretic velocities. Charged species can then be immobilised and concentrated at a unique point where the sum of the bulk and electrophoretic velocities is equal to zero. This means that different species, with differing electrophoretic mobilities, will be focussed at discrete points along the gradient. The beauty of such an approach is twofold. Firstly, since the process inherently focuses and concentrates charged analytes, assays on low concentration samples are achievable. Furthermore, mixing efficiencies are both high and simple to implement.To demonstrate the efficacy of the approach, Michael Tarlov and colleagues at the National Institute of Standards and Technology, Gaithersburg and Loyola College have recently performed two kinds of DNA hybridisation assay using temperature gradient focusing within capillaries. In the first, single stranded DNA targets are focussed and concentrated at a specific location within a capillary (Fig. 2a). Fluorescently labelled PNA is then introduced and carried through this stationary band. If PNA/DNA hybridisation occurs the hybrid complex immobilises at a different spatial location, thus yielding a fluorescent band. If no binding occurs, the PNA remains neutral and is therefore unfocussed. In the second type of assay (Fig. 2b), fluorescently labelled PNA probes are hybridised with DNA target prior to analysis. Temperature gradient focussing then localises any hybrids at the low temperature end of the gradient. By adjusting the bulk flow rate, this band can be moved towards the high temperature end. At a given location (and therefore temperature) the hybrid will denature and this is accompanied by a decrease in observed fluorescence. The calculated melting point can then be used to detect single base pair mutations.
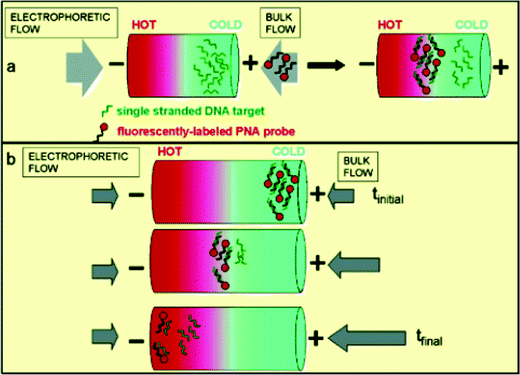 | ||
| Fig. 2 Schematic illustration of two TGF assays. The stationary assay (a) is performed by first focusing the ssDNA targets and then introducing the PNA probe into the bulk flow of the buffer. Scanning TGF (b) is performed by first focusing the DNA/PNA hybrid on the cold side of the temperature gradient and then changing the bulk flow to move analyte spatially through the temperature gradient so that its melting temperature can be measured. Adapted with permission. Copyright 2004 The American Chemical Society. | ||
In the first type of assay the authors use a temperature gradient of 20–65 °C across 2 mm of a 30 µm i.d. capillary. 18-mer single stranded DNA targets are focussed in the capillary at 204 V m−1, and then a fluorescently labelled PNA probe is introduced. After a short time lag, a new fluorescent band appears at 45 and 55 °C. Importantly, no such band is observed when the target is replaced with a noncomplimentary 18-mer target, thus confirming the identity of the original target. In the second type of assay the authors interrogate single stranded DNA targets containing sequences related to mutations in the transmembrane regulator gene. As described, DNA/PNA hybrids were moved through the temperature gradient to measure the duplex melting temperature. Sequence variations could be successfully detected through observed variations in the melt temperature of the wild-type and mutant sequence. Importantly, studies confirm that single base pair mutation analysis can be performed within a few minutes and at significantly lower concentrations than those required for conventional UV absorption analysis.
Journal of the American Chemical Society, 2004, 126, 13474
Size fractionation of microparticles
In biological systems molecules interact in a diversity of ways to create aggregated structures (e.g. genes, vesicles and cells). These aggregates normally exist and operate as individual ‘particles’, and thus characterisation of such structures and their properties at the single particle level is highly desirable. To this end, Hitoshi Watarai and colleagues at Osaka University have described the fabrication and operation of a simple microdevice that is able to sort microparticles in fluid media. The structure of this device is shown in Fig. 3a. Here separation of a glass coverslip and a plano-convex lens is precisely controlled through the use of piezo-electric element mounted to the underside of the lens. The gap separation can then be calculated by observing Newton's rings interference patterns formed between convex lens and flat glass surface. Such ring patterns are a result of optical interference and occur when the separation between surfaces is of the same order as the wavelength of light.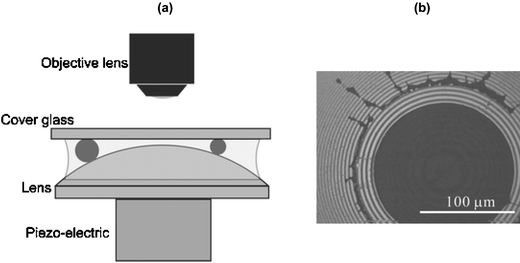 | ||
| Fig. 3 (a) Nano-gap produced between a flat glass coverslip and a plano-convex lens. Particles are trapped at a distance away from the centre of the lens that is related to their size; (b) photograph of trapped polystyrene particles with a diameter of 3 µm. Size measurement can be simply performed by counting the number of the stripes of the Newton rings. | ||
In initial experiments, an aqueous suspension of fluorescent microparticles (1.7 and 3.0 µm diameter) is injected into the gap between the lens and surface. As the water evaporates, particles are drawn towards the centre of the gap and trapped when the gap distance equals the particle diameter (Fig. 3b). Importantly, the gap distance (and thus particle size) can be directly determined from the ring pattern and is shown to provide for accurate size measurements of both particle populations. In addition, further studies indicate the potential of the microdevice in sizing DNA. Overall, this approach to particle sizing provides an interesting alternative to more established cell sorting and field-flow fractionation techniques due to minimal sample requirements, experimental simplicity and short analysis times should find many applications in the analysis of microparticles in biological media.
Chemical Communications, 2005, DOI: 10.1039/b411995a
Andrew J. de Mello
| This journal is © The Royal Society of Chemistry 2005 |
