Synthesis and characterization of water-soluble two-photon excited blue fluorescent chromophores for bioimaging†
Received
11th July 2005
, Accepted 7th October 2005
First published on 11th November 2005
Abstract
We report here the synthesis and characterization of a new type of non-ionic blue fluorescent water-soluble chromophores specifically designed for two-photon absorption microscopy. The water solubility is induced by introduction of short oligo(ethylene glycol) monomethyl ether moieties. This work has led to low molecular weight dyes with efficient two-photon absorption cross sections and high fluorescence quantum yield in organic solvents as well as in aqueous solutions.
Introduction
Materials with large two-photon absorption (TPA) cross section have attracted considerable attention in recent years owing to multiple applications in various fields, such as optical power limiting,1,2 three-dimensional micro-fabrication,3,4 photodynamic therapy by generation of singlet oxygen,5,6 and three-dimensional fluorescence microscopy.7,8 In this last case, due to the intensity dependence of TPA, the excitation is confined to a small volume in the focal plane of the imaging lens. As a consequence, photobleaching and photodamage are greatly reduced in comparison with one-photon imaging. Moreover, the infrared illumination used for TPA penetrates deeper into the sample than visible light excitation, allowing imaging in thick tissues. In addition, the absorption spectra are usually much broader in two-photon than in one-photon excitation, allowing simultaneous excitation of a wide variety of fluorophores emitting with different colors. As a consequence, two-photon microscopy is ideally suited for multi-color imaging. Unfortunately, most of the fluorophores commonly used in one-photon excitation are not optimized for TPA and are characterized by small two-photon cross sections σ2. This is especially true for fluorophores emitting in the blue, since for instance the widely used cascade blue and DAPI fluorophores are characterized by σ2 values lower than 1 Göppert–Mayer (1 GM = 10−50 cm4 s photon−1).9 Therefrom, there is a need for the design and synthesis of water-soluble organic chromophores having large σ2 values and blue emission with high quantum yield. It is now well established that a one-dimensional (1D) conjugated molecule of the general symmetrical structure D–π–aromatic core–π–D (D = donor, π = conjugated spacer) presents a high TPA cross-section.10 In our hands we have found that concentrated solutions of bis-stilbenic molecules consisting of a biphenyl aromatic core surrounded by two tris-alkoxy styrenic entities are efficient as optical limiters in the visible by multiphoton absorption.11 The alkoxy group has been chosen as a moderate donor to avoid undesirable absorption in the visible region. In order to improve the solubility of the chromophores in organic solvents we placed three dodecyloxy donor groups in 3,4,5-positions on the phenyl ring at each extremity of the 1D chromophore, denoted GMO-4 (Fig. 1).11,12 Furthermore, this chromophore presents an intense blue emission. In this paper, we present the design and synthesis of water-soluble derivatives of GMO-4, the tuning of their linear optical properties (absorption and emission) by modifying the rigidity of the aromatic core and the investigation of their two-photon excited fluorescence (TPEF) characteristics. These new water-soluble blue emitting TPEF materials are of potential interest in two-photon bioimaging.
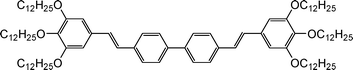 |
| | Fig. 1 Structure of GMO-4. | |
Experimental
General methods
All chemicals and reagents were purchased from Aldrich or Acros Organics and were used as received unless specified. THF was distilled over sodium under an argon atmosphere prior to use. 1H and 13C NMR spectra of the samples were recorded with 300 MHz Bruker Advance 300 instrument in CDCl3 solvent (Aldrich) with CHCl3 internal standard (7.24 ppm for 1H and 77 ppm, middle of the three peaks, for 13C spectra). High-resolution FAB mass spectra were recorded with a ZA-HF instrument with 4-nitrobenzyl alcohol as a matrix. TLC was run on Merck precoated aluminium plates (Si 60 F254). Column chromatography was run on Merck silica gel (60–120 mesh) or neutral alumina (Merck) 70–230 mesh.
Spectra
All UV-visible absorption spectra were recorded on a Hitachi U3000 Spectrophotometer in a dual beam mode using a matched pair of 1 × 1 cm quartz cells. Pure solvent was used as reference. Values of the molar extinction coefficients, εmax, of the compounds of interest were obtained as the average of at least five independent measurements with absorbance in the range 0.5–1. Fluorescence emission and excitation spectra were performed with optically dilute solutions (Abs. < 0.15) in 1 × 1 cm cells using a Photon Technology international, Inc. (PTI) spectrofluorometer.
Quantum yields
Fluorescence quantum yields were measured by the relative method, using recrystallized quinine sulfate in 0.5 M aqueous H2SO4 (ϕ = 0.54)13 as the reference. In these measurements, the slit widths were adjusted so that the spectral bandwidth of the absorption and emission instruments were identical at 1.0 nm, and the absorbances of the sample and the reference were chosen so they were in the 0.1–0.15 range and nearly identical at the same excitation wavelength. Emission quantum yields were then calculated according to the method described by Crosby and Demas, taking into account the differences between the refractive indices of the sample and reference solutions.13
Two-photon excitation experiments
The TPA cross-section spectra were obtained by up-conversion fluorescence measurements using a Nd:YAG pumped optical parametric oscillator that produces 2.6 ns [full width at half maximum (FWHM)] pulses in the 450–650 nm spectral range and using a Ti:sapphire femtosecond laser in the range 700–900 nm. The excitation beam is collimated over the cell length (10 mm). The fluorescence, collected at 90° of the excitation beam, was focused into an optical fiber connected to a spectrometer. The incident beam intensity was adjusted to ensure an intensity squared dependence of the fluorescence over the whole spectral range. Calibration of the spectra was performed by comparison with p-bis(o-methylstyryl)benzene, for which σ2 = 70 GM at 570 nm, and with the published 700–900 nm Rhodamine B two-photon absorption spectrum.9
Results and discussion
Molecular design and synthesis
To induce a good solubility of the GMO-4 derivative in water, we replaced the side alkyl chains by oligo(ethylene glycol) moieties. This methodology has been often used to obtain water-soluble polymers, therapeutic agents (such as texaphyrins for photodynamic therapy), and nanoparticles.14,15 Poly- and oligo-(ethylene glycols) (PEG and OEG) are also frequently used as spacers for bioconjugates.16–18 In order to limit the size (and thus the molecular weight) of the TPEF chromophores, we prepared a series of GMO-4 analogues bearing short side chains with one, two and three oxyethylene units in each donor chain (9, 8 and 7 respectively, Fig. 2). This permits also a tuning of the water solubility. The relatively low molecular weights so obtained (from 800 to 1500 g mol−1) constitute a major advantage of these compounds over other fluorescent constructs used in biology (such as quantum dots, dendrimers or fluorescent proteins).19 Furthermore, in order to block the free rotation around the two phenyl rings of the central core in 7 and 8, we have also prepared the analogues 10 and 11 (Fig. 2), centered on a dialkylfluorenyl core. Chromophores 10 and 11 correspond to 7 and 8 in which the whole conjugation is maintained. With these compounds, our aim was to tune the absorption and emission properties of the chromophores, for a better adjustment to the laser sources commonly used in two-photon microscopy. Moreover, the fluorenyl derivatives are well known for their high photostability under two-photon excitation.
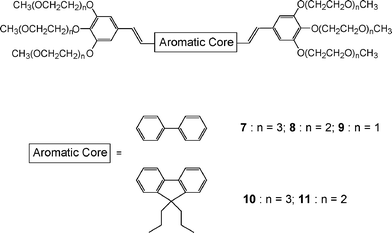 |
| | Fig. 2 Water-soluble chromophores synthesized in this study. | |
The key step for the synthesis of GMO-4 was a palladium catalytic double-Heck coupling reaction between 3,4,5-tridodecyloxystyrene and 4,4′-dibromobiphenyl (Scheme 1).12
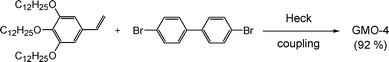 |
| | Scheme 1 Synthesis of GMO-4. | |
We prepared the styrene bearing three oligo(ethylene glycol) chains, and attempted the coupling with the 4,4′-dibromobiphenyl core. Unfortunately, this strategy was unsuccessful for the synthesis of our targeted compounds; due to retention of palladium by the reagents, as shown by high-resolution mass spectra. We opted then for a Wadsworth–Emmons coupling reaction between aldehydes 4a–4c and the aromatic cores 5 and 6 bearing two phosphonate moieties (Scheme 2).
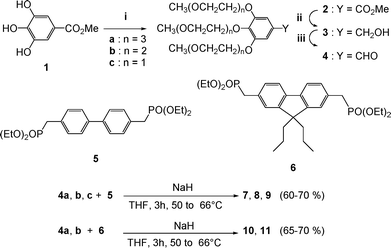 |
| | Scheme 2 Synthesis of chromophores 7–11. Reagents and conditions: (i) CH3(OCH2CH2)nOTs, K2CO3, acetone, 60%; (ii) LiAlH4, THF, 99%; (iii) MnO2, CH2Cl2, 96%. | |
First, commercially available methyl gallate 1 was reacted with oligo(ethylene glycols) monomethyl ether tosylates CH3(OCH2CH2)nOTs (n = 1, 2 and 3)14 to give the corresponding methyl esters 2a–2c.20 These were reduced into benzylic alcohols 3a–3c by lithium aluminium hydride then oxidized smoothly with MnO2 in CH2Cl2 to afford quantitatively the desired aldehydes 4a–4c.21 The bis-phosphonates cores 5 and 6 were obtained by Arbouzov reaction on the corresponding bis-bromomethyl derivatives as described in the literature.22–24 Compounds 7–11 were finally obtained by a Wadsworth–Emmons coupling reaction between the aldehydes 4a–4c with 5 and 4a and 4b with 6 in the presence of sodium hydride as depicted in Scheme 2.25 The structures of all these compounds have been confirmed by 1H, 13C NMR and HRMS. The stereochemistry of the created double bonds was difficult to analyze by 1H NMR, since we observed only a broad singlet for the vinylic protons. However, by refluxing 7 in toluene with a small amount of iodine, which is known to isomerize (Z)-stilbene into (E)-stilbene, no change was observed in the 1H NMR spectrum of the recovered product.26 This shows that the E products are obtained stereospecifically during the Wadsworth–Emmons reaction. In addition, the 1H NMR spectrum of 7 was recorded in water. In this solvent, the signal for the vinylic protons is no longer a broad singlet, but a typical AB system, with a coupling constant of 15.6 Hz, characteristic of a trans-substituted double bond. The procedures used for the synthesis of all compounds described in this study and the data obtained for their characterization are provided as ESI.†
Solubility
All compounds are soluble in common polar organic solvents (methylene chloride, ethanol, DMSO…). The water solubility of the five chromophores (7–11) has been estimated by the turbidimetric method.27 Results are summarized in Table 1.
| Compound |
7
|
8
|
9
|
10
|
11
|
| Water solubility/g L−1 |
190 |
20 |
<0.02 |
100 |
3 |
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P P |
1.9 |
1.7 |
— |
1.5 |
— |
In the biphenyl series, the water solubility is around 200 and 20 g L−1 for chromophores 7 and 8, respectively. However 9 is poorly water soluble, indicating that the hydrophilicity of the mono(ethylene glycol) unit is not sufficient to compensate the hydrophobicity of the hydrocarbon core. In the fluorenyl series, the introduction of propyl groups increases the hydrophobicity of the molecules. As a consequence, the solubility of the diethylene glycol substituted chromophore 11 in water is poor, but it remains in the 100 g L−1 range for the triethyleneglycol derivative 10. The solubility of 7 and 10 in physiological saline solution is similar to that in water. Indeed, one of the advantages of these molecules is their non-ionic character and thus, their low sensitivity to the ionic strength. The reference lipophilicity/hydrophobicity scale defined by the logarithm of the partition coefficient, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P, determined in the l-octanol/water partition system has been estimated by the shake-flask method.28 The various sizes of the OEG moieties and the structure of the aromatic cores induce log
P, determined in the l-octanol/water partition system has been estimated by the shake-flask method.28 The various sizes of the OEG moieties and the structure of the aromatic cores induce log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P values from 1.5 for the fluorenyl derivative 10 to 1.9 for the biphenyl derivative 7. Compound 8 has an intermediate value of 1.7. These values are interesting, as the optimum log
P values from 1.5 for the fluorenyl derivative 10 to 1.9 for the biphenyl derivative 7. Compound 8 has an intermediate value of 1.7. These values are interesting, as the optimum log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P values for central nervous system penetration and oral absorption are in this window (2 ± 0.7 and 1.8, respectively).29
P values for central nervous system penetration and oral absorption are in this window (2 ± 0.7 and 1.8, respectively).29
Photophysics
One-photon absorption and fluorescence emission spectra of the fluorophores with the longest solubilizing chains (7 for the biphenyl series and 10 for the fluorenyl series) in dichloromethane and water are shown in Fig. 3 and results are reported in Table 2. We observe for the biphenyl compound 7 a strong absorption in the near UV with a λmax = 362 nm (ε = 7.3 × 104 M−1 cm−1) in CH2Cl2 that is blue shifted by a few nm (λmax = 357 nm, ε = 6.2 × 104 M−1 cm−1) in water. These properties are only slightly affected by the OEG moiety length, as could be seen from the comparison with dyes 8 and 9. As expected, the enhanced conjugation due to the rigid fluorenyl core leads to a red shift of the absorption spectrum and an increase of the extinction coefficients for the fluorenyl series. Furthermore, the structure of the absorption spectra in this series indicates more resolved vibronic states. In dichloromethane, the λmax is shifted by about 20 nm for 10 and 11 (λmax = 382 nm for 10 and 383 nm for 11) in comparison with 7, 8 and 9 (λmax around 363 nm). For the fluorenyl series, solubilization in water does not significantly affect the absorption maximum (λmax = 383 nm) but reduces the absorption coefficient (ε = 5.8 × 104 M−1 cm−1 in water, 8.1 × 104 M−1 cm−1 in methylene chloride for 10).
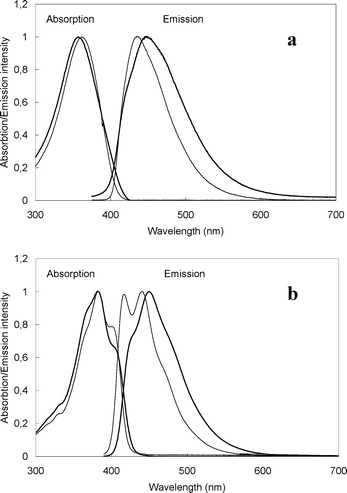 |
| | Fig. 3 Normalized absorption and emission for 7 (a) and 10 (b), in water (bold) and in methylene chloride (normal). | |
| |
Dichloromethane |
Water |
|
λ
abs/nm |
ε/M−1 cm−1 |
λ
em/nm |
Φ
a (%) |
λ
abs/nm |
ε/M−1 cm−1 |
λ
max/nm |
Φ
a (%) |
|
Errors in the absolute values of quantum yields are estimated to be lower than 10% when ϕ > 10%.
|
|
7
|
362 |
73![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
439 |
75 |
357 |
62![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
450 |
39 |
|
8
|
363 |
75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
437 |
79 |
357 |
59![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
454 |
46 |
|
9
|
363 |
71![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
437 |
70 |
— |
— |
— |
— |
|
10
|
382 |
81![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
450 |
81 |
383 |
58![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
453 |
72 |
|
11
|
383 |
86![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
450 |
85 |
— |
— |
— |
— |
Chromophores 7–9 show a fluorescence maximum around 438 nm in CH2Cl2 and 450 nm in water. The fluorescence quantum yield in methylene chloride is around 70–80% for these three dyes, and around 40–45% in water for compounds 7 and 8. For 10 and 11, two fluorescence maxima are apparent in methylene chloride (417 and 450 nm), while only one broad band, slightly red shifted (453 nm), is visible in water (for chromophore 10). It is interesting to note that the quantum yield decrease in water in respect with dichloromethane is less important for the fluorenyl derivative than for the biphenyl compounds (9% decrease for 10 compared to 35% decrease for 7 and 8). Hence, compound 10 appears to be a promising candidate for bioimaging in aqueous media. A complete one-photon photophysical study of these compounds will be reported later.
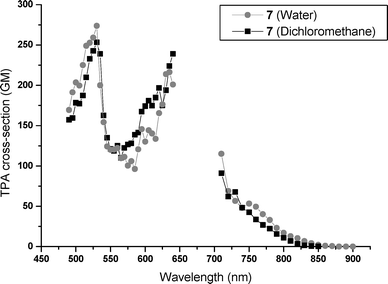
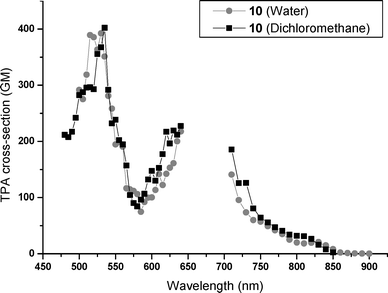
The TPA cross sections of these water-soluble chromophores were determined through their TPEF properties. The σ2 values have been determined in water and in methylene chloride. All chromophores present strong two-photon absorption in water and in dichloromethane. As for the one-photon properties, the length of the OEG chains does not affect significantly the two-photon properties. As a consequence, the two-photon properties are only reported for the most representative chromophores in both series, 7 and 10. Fig. 4 and 5 present the TPA spectra in water and methylene chloride from 450 to 900 nm, for chromophores 7 and 10, respectively. The two-photon absorption properties of 7 compare favorably with the two-photon properties of the GMO-4 parent molecule in dichloromethane solutions,11 indicating that the electronic structure is not highly affected by the replacement of the alkyl groups by the OEG moieties.
The TPA cross sections in water solutions are similar to the methylene chloride ones for the two series. An absorption maximum is depicted in the visible region at 520 nm (σ2 = 275 GM) for 7 and 530 nm (σ2 = 400 GM) for 10. Between 600 and 650 nm, we observe the rising part of an absorption band. The tail of this TPA band is observed in the near-IR region of the spectra. Due to the non-overlap of the emissions of the two different lasers used, there is a black window in our spectra. Consequently, the actual values of the σ2 maxima localized between 650 and 700 nm could not be measured. We note that the fluorenyl derivative 10 exhibits better TPA properties than its biphenyl analogue 7.
Conclusion
We reported here the design, synthesis and characterization of two series of efficient TPEF water-soluble dyes, with non-ionic character and intense blue fluorescence. These new dyes present two TP absorption bands in the visible range with σ2 maxima of a few hundred GM. The TP absorption is still intense in the near IR, typically about 100 GM at 720 nm in water. These values are much higher than the σ2 values of most classical dyes used in two-photon bioimaging (σ2 = 0.23 GM for fluorescein in water at pH = 11,30σ2 = 20.8 GM for rhodamine B in methanol,31σ2 = 50 GM for a perylene diimide derivative19). Since even lower σ2 values are reported for blue fluorescent dyes (0.16 GM for DAPI and 1 GM for cascade blue),9 our new dyes appear as valuable candidates for exploiting their blue TPEF properties in multiphoton microscopy. Preliminary TPEF imaging experiments have been made with mammalian living cells. Both chromophores passed easily through the membranes and spread homogeneously in the cytoplasm. Incubation over more than one hour was possible without sign of cell degeneration. These bioimaging results will be reported in a forthcoming paper.
Acknowledgements
We thanks CNRS for financial support. One of us (A. H.) thanks French ministry MENESR for a PhD fellowship.
References
- Y. Morel, A. Irimia, P. Najechalski, Y. Kervella, O. Stephan, P. L. Baldeck and C. Andraud, Two-photon absorption and optical power limiting of bifluorene molecule, J. Chem. Phys., 2001, 114, 5391–5396 CrossRef CAS.
- J. E. Ehrlich, X. L. Wu, I. Y. S. Lee, Z. Y. Hu, H. Rockel, S. R. Marder and J. W. Perry, Two-photon absorption and broadband optical limiting with bis-donor stilbenes, Opt. Lett., 1997, 22, 1843–1845 CAS.
- S. Kawata, H. B. Sun, T. Tanaka and K. Takada, Finer features for functional microdevices - Micromachines can be created with higher resolution using two-photon absorption, Nature, 2001, 412, 697–698 CrossRef CAS.
- K. D. Belfield, K. J. Schafer, Y. U. Liu, J. Liu, X. B. Ren and E. W. Van Stryland, Multiphoton-absorbing organic materials for microfabrication, emerging optical applications and non-destructive three-dimensional imaging, J. Phys. Org. Chem., 2000, 13, 837–849 CrossRef CAS.
- J. Liu, Y. W. Zhao, J. Q. Zhao, A. D. Xia, L. J. Jiang, S. Wu, L. Ma, Y. Q. Dong and Y. H. Gu, Two-photon excitation studies of hypocrellins for photodynamic therapy, J. Photochem. Photobiol. B, 2002, 68, 156–164 CrossRef CAS.
- F. Q. Meng, E. Nickel, M. Drobizhev, M. Kruk, A. Karotki, Y. Dzenis, A. Rebane and C. W. Spanger, Porphyrins with greatly enhanced two-photon absorption for photodynamic therapy, Abstr. Pap. Am. Chem. Soc., 2003, 226, U503–U504.
- J. D. Bhawalkar, A. Shih, S. J. Pan, W. S. Liou, J. Swiatkiewicz, B. A. Reinhardt, P. N. Prasad and P. C. Cheng, two-photon laser scanning fluorescence microscopy from a fluorophore and specimen perspective, Bioimaging, 1996, 4, 168–178 CrossRef CAS.
- W. R. Zipfel, R. M. Williams and W. W. Webb, Nonlinear magic: multiphoton microscopy in the biosciences, Nat. Biotechnol., 2003, 21, 1368–1376.
- C. Xu and W. W. Webb, Measurement of two-photon excitation cross sections of molecular fluorophores with data from 960 to 1050 nm, J. Opt. Soc. Am. B, 1996, 13, 481–491 Search PubMed.
- M. Rumi, J. E. Ehrich, A. A. Heikal, J. W. Perry, S. Barlow, Z. Hu, D. McCord-Maughon, T. C. Parker, H. Röckel, S. Thayumananvan, S. R. Marder, D. Beljonne and J.-L. Brédas, Structure-property relationships for two-photon absorbing chromophores: Bis-donor diphenylpolyene and bis(styryl)benzene derivatives, J. Am. Chem. Soc., 2000, 122, 9500–9510 CrossRef CAS.
-
Y. Morel, PhD Thesis, Grenoble, 2001.
- F. Lincker, P. Masson, J.-F. Nicoud, P. Didier, L. Guidoni and J.-Y. Bigot, Synthesis and characterization of efficient two-photon absorption chromophores with increased dimensionality, J. Nonlinear Opt. Phys. Mater., 2005, in press Search PubMed.
- G. A. Crosby and J. N. Demas, Measurement of photoluminescence quantum yields, J. Phys. Chem., 1971, 75, 991–1024 CrossRef.
- C. Lottner, K. C. Bart, G. Bernhardt and H. Brunner, Soluble tetraarylporphyrin-platinum conjugates as cytotoxic and phototoxic antitumor agents, J. Med. Chem., 2002, 45, 2079–2089 CrossRef CAS.
- P. Pengo, S. Polizzi, M. Battagliarin, L. Pasquato and P. Scrimin, Synthesis, characterization and properties of water-soluble gold nanoparticles with tunable core size, J. Mater. Chem., 2003, 13, 2471–2478 RSC.
- A. Simeonov, M. Matsushita, E. A. Juban, E. H. Thompson, T. Z. Hoffman, A. E. t. Beuscher, M. J. Taylor, P. Wirsching, W. Rettig, J. K. McCusker, R. C. Stevens, D. P. Millar;, P. G. Schultz, R. A. Lerner and K. D. Janda, Blue-fluorescent antibodies, Science, 2000, 290, 307–313 CrossRef CAS.
- S. Zalipsky, Functionalized poly(ethylene glycols) for preparation of biologically relevant conjugates, Bioconjugate Chem., 1995, 6, 150–165 CrossRef CAS.
- R. B. Greenwald, J. Yang;, H. Zhao, C. D. Conover, S. Lee and D. Filpula, Controlled release of proteins from their poly(ethylene glycol) conjugates: Drug delivery systems employing 1,6-elimination, Bioconjugate Chem., 2003, 14, 395–403 CrossRef CAS.
- A. Margineanu, J. Hofkens, M. Cotlet, S. Habuchi, A. Stefan, J. Q. Qu, C. Kohl, K. Mullen, J. Vercammen, Y. Engelborghs, T. Gensch and F. C. De Schryver, Photophysics of a water-soluble rylene dye: Comparison with other fluorescent molecules for biological applications, J. Phys. Chem. B, 2004, 108, 12242–12251 CrossRef CAS.
- M. W. P. L. Baars, R. Kleppinger, M. H. J. Koch, S. L. Yeu and E. W. Meijer, The localization of guests in water-soluble oligoethyleneoxy-modified poly(propylene imine) dendrimers, Angew. Chem., Int. Ed., 2000, 39, 1285–1288 CrossRef CAS.
- P. Jonkheijm, M. Fransen, A. P. H. J. Schenning and E. W. Meijer, Supramolecular organisation of oligo(p-phenylenevinylene) at the air-water interface and in water, J. Chem. Soc., Perkin Trans. 2, 2001, 1280–1286 RSC.
- J. Gruber and R. W. C. Li, Electrochemical synthesis of poly(4,4′-biphenylene ethylene)s, Eur. Polym. J., 2000, 36, 923–928 CrossRef CAS.
- M. J. Plater and T. Jackson, Polyaromatic amines. Part 3: Synthesis of poly(diarylamino)styrenes and related compounds, Tetrahedron, 2003, 59, 4673–4685 CrossRef CAS.
- J.-K. Lee, R. R. Schrock, D. R. Baignet and R. H. Friend, A new type of blue light emitting electroluminescent polymer, Macromolecules, 1995, 28, 1966–1971 CrossRef CAS.
- M. S. Wong, Z. H. Li, Y. Tao and M. D'Iorio, Synthesis and functional properties of donor–acceptor pi-conjugated oligomers, Chem. Mater., 2003, 15, 1198–1203 CrossRef CAS.
- J. F. Eckert, J.-F. Nicoud, J.-F. Nierengarten, S. G. Liu, L. Echegoyen, F. Barigelletti, N. Armaroli, L. Ouali, V. Krasnikov and G. Hadziioannou, Fullerene-oligophenylenevinylene hybrids: Synthesis, electronic properties, and incorporation in photovoltaic devices, J. Am. Chem. Soc., 2000, 122, 7467–7479 CrossRef CAS.
- A. Avdeef and B. Testa, Physicochemical profiling in drug research: a brief survey of the state-of-the-art of experimental techniques, Cell. Mol. Life Sci., 2002, 59, 1681–1689 CAS.
- European Chemical Bureau, Institute for health and consumer recommendations.
-
B. Testa, V. Pliskaand and H. Van de Waterbeend, Lipophilicity in Drug Design Action and Toxicology, VCH, 1995; vol. 4 Search PubMed.
- P. Kaatz and D. P. Shelton, Two-photon fluorescence cross-section measurements calibrated with hyper-Rayleigh scattering, J. Opt. Soc. Am. B, 1999, 16, 998–1006 Search PubMed.
- D. A. Oulianov, I. V. Tomov, A. S. Dvornikov and P. M. Rentzepis, Observations on the measurement of two-photon absorption cross-section, Opt. Commun., 2001, 191, 235–243 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis and characterization of compounds 1–11. See DOI: 10.1039/b509843b |
|
| This journal is © The Royal Society of Chemistry and Owner Societies 2006 |
Click here to see how this site uses Cookies. View our privacy policy here. 



![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P for chromophores 7–11
P for chromophores 7–11
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P
P![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P, determined in the l-octanol/water partition system has been estimated by the shake-flask method.28 The various sizes of the OEG moieties and the structure of the aromatic cores induce log
P, determined in the l-octanol/water partition system has been estimated by the shake-flask method.28 The various sizes of the OEG moieties and the structure of the aromatic cores induce log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P values from 1.5 for the fluorenyl derivative 10 to 1.9 for the biphenyl derivative 7. Compound 8 has an intermediate value of 1.7. These values are interesting, as the optimum log
P values from 1.5 for the fluorenyl derivative 10 to 1.9 for the biphenyl derivative 7. Compound 8 has an intermediate value of 1.7. These values are interesting, as the optimum log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P values for central nervous system penetration and oral absorption are in this window (2 ± 0.7 and 1.8, respectively).29
P values for central nervous system penetration and oral absorption are in this window (2 ± 0.7 and 1.8, respectively).29
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000