Microtechnology: Meet neurobiology†
Thomas M.
Pearce‡
and
Justin C.
Williams
*
Department of Biomedical Engineering, University of Wisconsin, Madison, WI, USA. E-mail: jwilliams@wisc.edu; Fax: 1-608-265-3952; Tel: 1-608-265-3952
First published on 10th October 2006
Abstract
The field of neuroscience has always been attractive to engineers. Neurons and their connections, like tiny circuit elements, process and transmit information in a dramatic way that is intimately curious to researchers in the computer science and engineering fields. Of particular interest has been the recent push in applying microtechnology to the field of neuroscience. This review is meant to provide an overview of some of the subtle nuances of the nervous system and outline recent advances in lab on a chip applications in neurobiology. It also aims to highlight some of the challenges the field faces in the hopes of encouraging new engineering researchers to collaborate with neurobiologists to help advance our basic understanding of the nervous system and create novel applications based on neuroengineering principles.
Introduction
The field of neuroscience is an active and important area of investigation, ranging from elucidation of single-cell functions to neural network connectivity, mechanisms of learning and memory, and disease prevention, to name just a few. Even a simple neural network in a dish (or other in vitro environment) has the capacity to learn a task,1,2 and high order functions such as cognition and emotion are rooted in the interconnected circuit elements which are individual neurons.3,4 Neuroscience and engineering have always gone hand-in-hand, with bio-electronic interfaces and massive data processing power needed to probe the roots of these neurophysiological phenomena.5–8 Engineers have also taken advantage of insights gained from the nervous system, to create artificial neural networks in silicon as a computational tool.9,10 Despite this relationship between the fields, the recent explosion of engineered microdevices has not yet been widely applied for neuroscience purposes. Neurons fundamentally operate as mechanical, chemical, and electrical sensors and actuators on the microscale, making microdevices an ideal tool for interfacing with these cells at an unprecedented level. Conversely, neuroscience provides a nearly limitless number of useful, practical applications for the engineered microdevice. With the current state of microtechnological sophistication, neuroscience and engineering seem poised to embark upon another mutually beneficial, and enormously fruitful, relationship.The intent of this review is to explore the role of microtechnology, specifically lab-on-a-chip (LOC) devices, in relation to the field of neuroscience. As such, it is important to understand the difficulties and opportunities of working with the nervous system, as well as developing technology for interfacing with living neural tissue. Neurons inherently present challenges which are rather unique amongst tissue types. To effectively study these cells in a controlled manner, a number of conditions must be addressed. The location, growth, and connectivity of each individual cell within a larger culture must be controlled.11–15 Engineered surfaces are an excellent tool for overcoming this challenge, either through surface chemistry or microscale topography.16–18 The electrical activity of multiple, closely spaced, neurons must be sensed (or stimulated) simultaneously. Microelectrode arrays and microfabricated patch clamp electrodes provide experimental capabilities beyond the traditional macroscale methods currently in use.19 The fluid microenvironment of each cell must be tightly regulated in terms of chemical composition, temperature, and gas content, because neurons alter their behavior dramatically in response to their local surroundings.20,21 Unlike other cell types, neurons respond to environmental cues over a range of timescales, from hundreds of microseconds to days. Microfluidic technology provides the means to control these parameters in a manner impossible on a larger scale.22,23 Overall, LOC devices have an enormous potential for furthering the field of neuroscience.
Studying cells versus utilizing cellsLike other biotechnology applications, contrasting possibilities arise from exploiting the interface between neural tissue and technology—utilizing microtechnology to better understand basic physiology, versus using integrated living tissue as part of the technology to answer questions about other fields of interest. The distinction is not always clear. For example, a device to study the electrical response of a neuron to a certain chemical (studying the neuron) could also be used as a drug screening tool (using neurons to identify potential pharmaceutical compounds ).46 |
The distinction must be drawn early between LOC devices for generalized biological applications, and those for neuroscience purposes. The benefit of microtechnology to biological applications, a very active field of exploration, is reviewed elsewhere.16,18,24,25 To name just a few, microtechnology offers the following advantages to in vitro biological studies: low reagent/drug volumes, highly parallel experimentation, better mimicry of the natural tissue environment, precise experimental control of the cellular microenvironment, and low-cost disposable devices. These benefits hold true for neuroscience applications as well, but the focus of this review is on the challenges and advantages of microtechnology unique to the study of neurons. The scope of this review encompasses LOC devices only, and does not include implantable microdevices. Focus is placed on devices intended for use with living tissue, and not on devices for analysis of cellular contents or other similar applications.
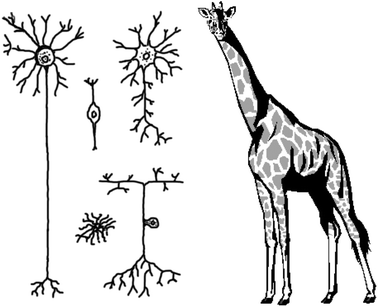
The scale of neuroscience: Neurons come in all shapes and sizesThe human brain contains approximately 100 billion neurons with 100 trillion interconnections, and many more supporting cells. Most organs in the body are composed of only a handful of cell types; by contrast the brain has hundreds, if not thousands, of distinct types of neurons, each defined in terms of its chemistry, shape, location and connections. This diversity stands in sharp contrast to all other tissue types found throughout the body, which have cells of a much more homogeneous structure and function. To illustrate, one careful, recent investigation of a class of interneuron that is found in a small local circuit in the retina, called the amacrine cell, found no less than 23 identifiable types.The illustration seen here is meant to illustrate some of the diverse shapes and sizes that neurons come in. The smallest neurons can be microns in diameter but other neurons have processes that extend over considerable distances. For example, the common giraffe has single primary afferent axons in the sciatic nerve that project several meters, and the squid giant axon is almost a millimeter in diameter.85 Due to the size of both individual cells and complex circuits, information transmission happens remarkably quickly, with action potentials traveling up to 100 metres per second and synaptic transmission occurring over an interval of hundreds of microseconds. A single neuron may have thousands of inputs and outputs, and integrates incoming information in a nonlinear fashion in both the spatial and temporal domains. |
Biology
To begin, a basic understanding of nervous tissue is required. Anatomically, neural tissue comprises all tissue found in the central (brain and spinal cord) and peripheral (everything else) nervous systems, including the conductive neurons and support cells such as neuroglia. The neuron is the electrically active cell of the nervous system, and is comprised of a cell body (soma) with neurites (axons and dendrites) extending outward. Neurons transmit electrical impulses via changing electrical potential across the cell membrane, arising from ion gradients and ion flux across the cell membrane. Various stimuli can trigger a change in membrane potential, including physical interactions, external voltages, and most commonly, chemical binding. In general, once a threshold potential has been reached within the body of a neuron, a wave of ion flux across the membrane (the action potential) is propagated down the length of an extending process (the axon), triggering the release of a chemical (neurotransmitter) onto the membrane of another cell (at the synapse). The post-synaptic cell binds the neurotransmitter and alters its own electrical properties accordingly. In this manner, external stimuli are sensed, muscles activated, and thoughts originated—and everything in between. Compared to other organ systems, the nervous system has an amazingly diverse and important set of functions. Determining the mechanisms of the nervous system is therefore of critical importance, but of immense complexity. A wide range of inherent challenges exists at all levels of neuroscience study; some of these difficulties transcend scale to persist throughout the field. An excellent resource for understanding principles of the nervous system is available from the Society for Neuroscience,26 and is available for free on their website (http://www.sfn.org). See Fig. 1 for an overview of the neuron.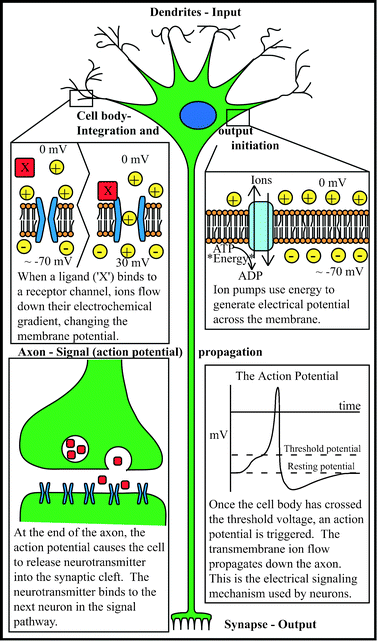 | ||
| Fig. 1 The inner workings of a neuron. | ||
History
In some ways, microtechnology and neuroscience have always been a good fit. Since the introduction of the patch-clamp electrode by Sakmann and Neher in 1976,27 electrophysiologists have used microscale implements to study the electrical activity of individual neurons. Beyond the glass pipette, other types of close-contact tissue-technology interfaces generally involve a conductive electrode placed in or near a neuron to detect electrical potential at the location of the exposed electrode tip.28 (Optical interfaces utilizing voltage- or ion-sensitive dyes exist as well, but fall outside the scope of this review.29) Neuroscience research (and by nature, the field of microtechnology) leaped forward as a result of the electronics miniaturization movement, as data recording and analysis capabilities underwent drastic improvements. Initially, intimate neural interface technology, outlined by Rutten,19 remained electrical in nature, with advances in electrode material properties and a move toward multichannel electrode arrays (MEAs) fabricated using the photolithographic techniques developed in the semiconductor industry.30 The pulled-glass patch clamp electrode has remained the tool of choice for single neuron recordings due to the quality of data and range of experiments that can be performed with this technique.The explosion of bio-oriented LOC microdevices in the early 2000's contained a relative lack of devices dedicated to solving neuroscience problems. One possible reason is that neurons are complex cells to work with and study, presenting challenges to LOC devices above and beyond other cell types (a common theme presented throughout this review). Another reason may be that many first-generation devices were not dedicated to solving any hypothesis-driven biological question in particular, but instead simply proving that cells could be grown and manipulated in a microenvironment. Some demonstrated basic fluid-handling operations such as valving, pumping, and mixing in the bio-device context.31 The technical achievements these devices demonstrate can now be put to use in solving the challenges facing neuroscience research; however, this is a largely undeveloped field thus far. We are, as a field, definitely reaching a level of expertise with microfluidics that we are able to create useful devices for biology. Applying these techniques to neurobiology is a logical progression of this development, given that we can start to appreciate the subtle challenges that the nervous system presents.
The attraction of neuroscience for engineers
Biologists and engineers often think in vastly different terms, from the basic jargon they use to the fundamental ways they approach problems. Subcellular mechanisms, based principally in protein biochemistry, are rarely organized into clear analytical diagrams, and their interactions are not often well characterized. The lack of a systematic approach has been lamented by some members of the biological science community32 but has not been widely addressed. For this reason, much of biology can seem incomprehensible to engineers (and likewise, engineering to biologists).
The solution in search of a problemThe article, ‘Can a Biologist Fix a Radio?—or, what I learned while studying apoptosis’ is a tongue-in-cheek look at biology from a biologist's point of view, and is an excellent and humorous take on a serious issue in biology.84 In it, the author outlines the strategies that would be taken by today's biologist to attempt to determine how a common radio functions, with the goal of fixing a malfunctioning one. The article calls for a more systematic approach to biology, similar to engineering, where the components of a cell or system are characterized so they can be related in a meaningful way, allowing predictions to be made regarding the function of the system as a whole.On the other side of this issue is the manner in which today's stereotypical engineer functions. In this world, the engineer designs and designs and designs, creating the most technically innovative and highly optimized solution possible. The difficulty arises when the solution must go searching for a problem, since the engineer often cares more about the technical achievement than the underlying purpose driving that advancement, sometimes becoming lost in the ‘technical beauty’ of the solution. This view of engineers may inspire biologists to forgo new technology for the safety and comfort of tried-and-true methods. It should be the goal of the engineer to change this perception, and remembering that when properly appreciated, biology can offer very interesting challenges. |
Unlike many other fields of cell and organ system biology, neuroscience has relatively well-characterized interactions. Neurons behave principally as input–output devices, a concept recognizable to most engineers. Whether the input is a molecular signal from another neuron or sensory stimulus (such as pressure or temperature) into a specialized sensory neuron, a stereotyped response is seen, which ends in signal propagation and output in the form of chemical release at the other end of the cell. This concept is innately familiar, more so than protein signaling cascades leading to gene transcription (although this also exists in the nervous system and can modulate input–output relationships). In addition, neural behavior is electrical in nature, so that stimulating and detecting activity uses a modality familiar to engineers. Neurons can be exquisitely sensitive transducers, and produce graded output signals which depend on the strength of the input. Finally, neurons organize into networks of electrical activity, which is attractive as a living computational tool.33
The LOC toolbox
The functional capabilities of lab-on-a-chip microdevices have expanded rapidly with novel fabrication techniques, new materials, and increasingly characterized size-dependent design considerations. The purpose of this review is not to exhaustively explain all of the available microscale tools, but rather to summarize the utility of LOC devices to neuroscience and to point to more in-depth studies and reviews regarding technical aspects of device design and fabrication.16,25Electrical interfaces—electrode arrays
Probably the earliest adoption of modern microtechnology in neuroscience was in developing sensors for detecting the extracellular electrical activity from multiple neurons in culture. This progressed largely due to advances in the microelectronics industry that made photolithographic electronic fabrication techniques widely available. This was a logical step in the adoption of microtechnology in neuroscience, as neurons are fundamentally electrical in nature, and engineers are inherently comfortable with electrical concepts. At the same time, advances in microelectronics and computing power made available the instrumentation to record from and analyze the electrical activity from hundreds of neurons simultaneously, and for lengthy durations, often for weeks to months.20Multielectrode arrays
The generation of action potentials from a neural cell gives rise to trans-membrane currents and subsequently extracellular voltage potentials. Placing a conducting sensor near the cell can sense these potentials. The extracellular potential from a neuron falls off quickly within a distance of tens of microns; therefore the sensor must be placed close to individual cells. Even within this distance the extracellular voltage is typically only 100's of µV. Additionally, to be able to isolate the extracellular potentials from individual neurons, the sensor has to be sufficiently small (on the order of the size of individual neuron cell bodies) to not pick up the conglomerate potential from dozens of nearby neurons. In general, as the size of the electrode gets smaller, it will pick up activity from a smaller surrounding area.As noted previously, while neurons are interesting to study in isolation, there is considerable interest in recording from multiple neurons with multiple sensors simultaneously, in an attempt to analyze the contributions of single neurons to network behavior.45 To accomplish this, many research groups have developed variations on what has become commonly referred to as the Multi-electrode Array (MEA). As the name implies, MEAs consist of a multitude of electrical sensors (electrodes) that are arranged in a regularly spaced arrangement. Typically these are fabricated using standard photolithography processes. They consist of lithographically defined insulated metal conductor lines that emanate from exposed metal sensor electrode pads. Although the detailed fabrication steps vary slightly from group to group, the underlying structure of most MEAs follows this basic formula. Starting with an underlying substrate (usually glass, to allow for viewing cells on inverted microscopes) a layer of metal is deposited, and subsequently patterned to define the electrical pads and traces. An insulating layer is then added to isolate the traces, and the layer then patterned to expose the electrode pads. The electrodes are usually spaced greater than 100 microns apart to avoid recording from the same neuron on multiple electrodes. Typically MEAs have electrodes arranged in square configurations (4 × 4, 6 × 6, 8 × 8), although the possible arrangements are limited only by the imagination and can be tailored to the needs of the particular experimental application. Typical applications involve experiments aimed at understanding neural development, neural network formation and high-throughput drug screening.46,47 For further information on MEA fabrication and use, see Rutten's detailed review of the field.19
Stimulation
Another practical use of MEAs is providing input to the neural cells in culture, in the form of electrical stimulation. Neural cells are intrinsically bimodal, in that they can both send and receive information, and can be excited by an external electrical field to produce action potentials that are identical to the ones produced by naturally occurring stimuli. MEAs, since they are fabricated out of conducting materials, can both sense and deliver electrical fields. In this way, a single electrode at one time can be used to stimulate a local neuron and at another time to record from it. In theory, at any given time, every electrode in the array can be used as either an input or output. This also offers a controlled way of assessing neural connectivity, as stimulating a single neuron can elicit time locked excitation of cells that it connects to. This can be a very powerful tool in looking at network function, as it provides the opportunity to selectively deliver a set of well defined inputs into the neural network, watch the output, and then change the inputs and compare the new outputs. Several groups have used this type of functionality to look at how artificially grown neural circuits can be ‘taught’ to do simple input–output tasks.48 There are certainly practical considerations to stimulating through the small sensors (current density , saturation, etc.) that can be found in prior reviews on the subject of electrical stimulation in neural cell cultures.49Electro-chemical detection
MEA devices can also be fabricated to have additional functionality beyond simple electrical recording or stimulation. There are several methods in which MEA electrodes can be also used as chemical sensors. The use of MEA electrodes as chemical sensors essentially involves detecting the current generated by a chemical reaction at the electrode surface. In general this is done by applying a potential across the electrodes and measuring current through the external circuit. The peaks in the CV curve correspond to characteristic oxidation or reduction potentials, which are specific for a given chemical species. In this way, the current generated is proportional to the concentration of the chemical species.50Chemical detection methods are of particular interest in neurobiology, as the fundamental electrical events in a neuron are modulated by chemical events and transmission of signals from cell to cell is propagated by neurotransmitters. Chemical detection is particularly challenging in neurons as most of the neurotransmitter is confined to the local area around the synaptic cleft. To detect the small changes in neurotransmitter release from individual synapses would require a small sensor to be placed in the vicinity of the synaptic cleft. To further complicate this scenario, the sensor needs to remain uncovered to allow diffusion of the neurotransmitter towards the sensor surface. For these reasons, most studies using chemical detection look for large changes in neurotransmitter levels that are triggered in response to stimuli applied across a large number of neurons. It is possible that there are many chemical species that are present at the electrode surface, which can have redox potentials that are similar to one another. This potentially limits the specificity of the measurement for a desired neurotransmitter. For example, dopamine has a similar redox potential as anionic ascorbic acid, so measurements of these species are difficult to differentiate.51 A common method for addressing this is to add a selective membrane over the electrode, which allows diffusion of the desired species towards the electrode surface, while preventing diffusion of competing species. An additional challenge to this technology is that the time course of neurotransmission is very short. Neurotransmitter release from the presynaptic cell, binding by the postsynaptic cell and subsequent unbinding and reuptake by the presynaptic cell, all happen within a matter of hundreds of microseconds.
Patch clamp electrodes
The patch clectrode has been in use for over thirty years and is one of the very early adoptions of microtechnology for biological applications.27 Patch electrodes consist of a glass pipette, with micron dimensions at the tip, which is filled with conductive solution and a small metal wire. The pipette tip is traditionally fabricated by heating a small glass tube, while pulling it under tension, in a controlled way that causes it to break at a small tip size. The tip is then reheated (flame polished) to produce a tip with precise dimensions and favorable surface properties. The pipette is then filled with an ionic solution that electrically connects with an Ag/AgCl electrode, positioned with precision manipulators onto the surface of a neural cell, and suction applied to seal a ‘patch’ of cell membrane onto the tip. In order for the technique to work, it is vitally important to achieve an electrical seal of a least a gigaohm between the membrane and the pipette tip to prevent leakage of current. Several variations are possible from here, which are discusssed more exhaustively in ref. 52 but in general they all involve isolating a patch of cell membrane and measuring the changes in electrical properties of the patch under various experimental conditions. This technique is very powerful for studying the function of single ion channels in neural cells (for its development, Neher and Sakmann were awarded the Nobel Prize for Medicine in 1991) and has become the gold standard for studying how biochemicals impact neural function. Although it has at its roots the basic building blocks of microtechnology (small size, great specificity), it does lack many of the hallmarks that have become associated with lab on a chip systems. It takes considerable experience to become proficient at the technique and it can only be done on one cell at a time. Features such as mass fabrication, parallel operations, integrated systems and microfluidics would certainly be of great benefit to patch clamp physiologists.To address these limitations, a number of groups have developed microfabricated patch clamp array systems. Most commonly, these are planar devices that have an array of small pores (typical diameter around 1–2 microns) that each essentially act on the same basic operating principle as a traditional patch clamp pipette.53–55 More recently, pores oriented along a vertical wall have been fabricated, allowing better visualization of the cells and microchannels.56 After fabrication of the holes and addition of electrodes to the back side of the hole, cells are added to the device and suction is applied to hold individual cells on each pore. The pores can be made through any number of fabrication processes, ranging from anisotropic silicon etching to laser ablation, to polymer molding. The primary advantage of this technology is in the automated fabrication and highly parallel architecture that is capable of collecting thousands of patch-clamp data points per day. This is several orders of magnitude more efficient than the traditional electrophysiologist who may spend an entire day staring through a microscope to patch a half-dozen cells. At present, however, microfabricated patch-clamp electrodes have lacked the same level of signal quality as the traditional pulled pipette electrode.57 Until this difficulty is repeatedly demonstrated to be overcome, resistance to this technology can be deservedly expected.
Chemical interface—microfluidics
Control of cells environmental surroundings is an important part of any in vitro biological application. The local environment, comprised of surfaces and fluid, is tightly regulated with high spatial and temporal control in the body. However, in standard cell culture conditions, all cells in a dish are subjected to bulk media conditions, with no local specification and slow regulation. In LOC devices, it is possible to provide fast, accurate control over the local fluid microenvironment surrounding the cell culture.43,58 In addition to more closely simulating the natural environment of cells, microfluidic control provides many distinct advantages to neuroscience applications as well.Perfusion control involves bathing the neural culture in a stream of moving media. Utilizing the properties of laminar flow, multiple streams may be brought together, with mixing occurring only by diffusion at the interface. The effect of this diffusive mixing is a chemical gradient across the culture, the properties of which depend on diffusion characteristics, the distance from the confluence of streams, the fluid velocity, and the initial concentrations.59 Gradients are extremely useful when studying neurons—cells polarize (send out axons and dendrites) according to gradients of molecular cues, and migrate toward or away from gradients of localization signals.42 Because of this, gradient-generating microdevices can be used to induce neural migration, development, and organization in vitro in a controlled manner (see Fig. 2).60,61 Microfluidically generated gradients can also be used to deposit surface-immobilized cues, to which cells can respond regardless of what is flowing in the solution phase.15
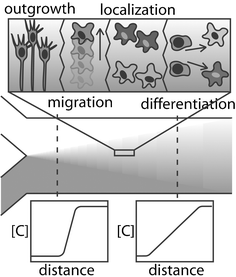 | ||
| Fig. 2 Chemical diffusion can elicit different neural behaviour depending on the type of chemical and relative spatial concentration gradient. | ||
Using perfusion control, it is also possible to target certain areas of a culture for chemical application.62 This can be used for multiple purposes—to drive cell proliferation or maturation, to stimulate or repress electrical activity, or to deliver or deprive nutrients, all in specific locations within the culture—or even specific parts of a single cell.43 This specifically benefits neural cultures since distinct elements within larger circuits can be chemically targeted with drugs or other environmental cues, giving information difficult to obtain in other experimental setups. Another way in which microfluidics mimics physiological conditions is in the speed with which the local environment can be altered. By moving a diffusion boundary across a cell, a chemical signal can be delivered on a millisecond timescale approaching that of synaptic signalling.63,64 This lies in direct contrast to the traditional approach, where the fluid in a macroscale perfusion chamber must be completely exchanged, with a minimum time on the order of seconds or longer.
Another microfluidic approach uses channels embedded in a substrate to deliver or sample fluid through a small opening, or a microaperture. This allows the overall conditions of the cell culture to be continuously perfused by bulk solution, while individual locations can be accessed using apertures. Similarly to perfusion control, this allows spatial and temporal chemical addressing to take place. In addition, microapertures can sample the chemicals released from a cell, giving additional information about the functional specification and activity of a cell or culture.65,66
Microfluidic strategies can also be used in cell patterning, often in conjunction with surface chemistry or topographical features.41,67 Since fluid streams flow in parallel without mixing, populations of neurons can be flowed into a culture chamber and deposited selectively along streamlines, which are controlled using channel geometry and flow rate. Due to the numbers of different neuronal cell types (see Box 2), and the different tissues with which neurons interact, such as muscle and connective tissue, the ability to generate patterned cultures of multiple cell types is an information-rich endeavour.
Surfaces and physical interfaces
In the developing brain, neurons follow both chemical gradients and mechanical guidance cues. In vitro, purely mechanical cues are capable of influencing neural growth, even in the absence of gradients of protein growth factors. Thus far, most attempts to elucidate the nature of these cues have primarily involved growing neural cells on surfaces with different bulk surface chemistries (proteins, growth factors) and or topographies (rough, smooth, hard, soft). The use of microfabrication techniques has allowed investigators to begin to exploit these properties on the microscale as a way to control the way that individual neurons grow and interact with other cells. Because the function of the nervous system fundamentally relies on how cells grow and contact each other, the ability to manipulate these interactions in vitro, through microtechnology, holds immense promise for a wide range of experiments.By restricting the physical area on which neurons can attach and grow, surface patterns can be used to cause selective growth and migration of different neural elements. For example, in Fig. 3, a pattern is shown that deposits adhesion molecules into ‘islands’ where cell bodies can attach and ‘bridges’ where neurites can extend. Both structures are created from the same molecules, using only the feature size to influence the behavior of the neurons. Cell bodies localize to the larger islands, because below a critical pattern width, the neurons would not have enough area to attach and spread out, and would become unhealthy and eventually die. A bridge pattern that is smaller than approximately 3 microns will only allow processes to grow on it, while preventing attachment and migration of the neuron cell bodies.68 This is a concept that has been explored in other areas of biology, where the physical size of patterned adhesion molecules has been shown to modulate cellular adhesion.69 In terms of neuroscience, the ability to segregate neurites from cell bodies allows directed connectivity between cells, and offers the possibility of locally controlling the environment of distinct network (or sub-cellular) components using microfluidics.70 Another use of surface patterning for studying neurons is the desire to localize cell bodies with electrode sites, on an MEA for example, to enhance signal detection. A pattern that restricts the cellular adhesion to within about 50 microns from the center of the electrode is generally a reasonable compromise between cell adhesion, isolation, and signal quality.
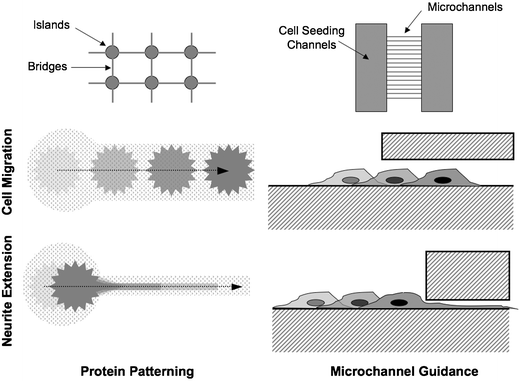 | ||
| Fig. 3 Examples of different physical confinement methods for restricting neuron migration and extension. | ||
Surface patterns can be formed in a number of different ways, each with distinct advantages and disadvantages. In general, all of these methods involve depositing a biomolecule (bio-ink), such as extracellular matrix proteins or growth factors, in a spatially defined manner with the intent of controlling the adhesion, growth or guidance of neurons on a surface. Lift-off fabrication involves the use of a sacrificial photoresist layer, with the substrate exposed in the desired pattern. Bio-ink is deposited on the entire surface and allowed to adhere, followed by chemical removal of the sacrificial layer. Lift-off is capable of depositing thick layers of protein (shown to be more effective for cell growth13) and high resolution, but requires harsh etchants which may be incompatible with the desired biomolecules.68 Microcontact printing uses a patterned PDMS stamp which is coated with bio-ink and pressed onto the substrate. In this manner, multiple substances can be sequentially deposited onto a surface, although alignment may be difficult.71–73 Stamps can be reused, but it is difficult to create thick protein layers with this techinque. Nozzle printing makes use of a modified ink-jet process to print bio-ink onto a translating substrate. In this method, many different molecules can be deposited with good alignment, but the minimum feature size is larger than with other methods.74–76 Microfluidics can also be used to deposit molecules onto a surface. In one method, a channel network in PDMS is temporarily adhered to a substrate and filled with a solution of biomolecules. After allowing time for adsorption or evaporation of the solution, the PDMS is peeled off. This is similar in principle to lift-off, but requires no etchant chemicals.37 Alternately, parallel laminar flow streams have been used to deposit adhesion molecules along defined streamlines.41 Because mixing occurs by diffusion, gradients naturally occur between streams and can potentially be used to better mimic natural in vivo growth cues for neural guidance. Self-assembled monolayers of photo-protected groups can also be used to create patterned surfaces. The substrate can be made reactive when exposed to light, then covalently linked to biomolecules in solution.77 Similar to microfluidics, surface concentration gradients can be achieved using this method, but without the restriction of following diffusion profiles along streamlines.
Interface integration
The benefits of individual microtechnologies in relation to the field of neuroscience, as summarized above, are noteworthy. For example, the Dynaflow system from Cellectricon (Gothenburg, Sweden) utilizes microscale apertures and fluidic channels to create laminar fluidic interfaces between streams of different concentrations. This device is used in conjunction with the conventional patch-clamp electrode, and makes use of the rapid fluid switching capabilities of laminar-flow microfluidics to investigate the dose response of neurons to various concentrations and substances.64 However, enormous advances surely will come with another strength of LOC devices, namely, integration of disparate components fabricated into a single device.
CoculturesIn their native state, neurons do not exist by themselves. In fact, neurons are the least abundant cell type in the brain, outnumbered ten to one by glial cells. While originally thought to be just structural support cells, glial cells have been shown to play many important roles in the neural environment, including facilitation of neural development, neuron differentiation, immunology and electrical conduction. This is an important consideration when developing future lab on a chip devices for neural applications.86 It has been shown that culturing with and without glial cells can result in dramatic differences in neuron behavior and function.87 Recently a number of groups have begun to devise elegant MEMS devices for co-culturing neurons and astrocytes to allow them to communicate chemically within the culture, but remain physically separate to facilitate study of individual neurons and networks.78,88 One interesting approach was to grow a co-culture of neurons and astrocytes on a bed of micro-pillars.78 It was found that for a given pillar density and size that astrocytes preferred to grow on top of the pillars while neurons tended to grow in the valleys in between. This is just one of many examples where lab on a chip technology has the potential to help study the interactions between neurons and glial cells. The ability to segregate different types of cells and control interactions will also become an important consideration for modelling the interactions between the disparate classes of interconnected neurons. |
While the devices utilizing one aspect of microtechnology can be considered a first step, microsystems with integrated electrical sensors, microfluidic perfusion control, and surfaces patterned both with chemicals and topography, are the direction the field is heading. Some progress has been made along this path already. Surface features and chemical patterns have been jointly used to better isolate cells and direct growth patterns.81 Microfluidic channels have been used to deliver chemicals to selected cells on a patterned surface.70 MEAs, which are often the starting point for further development, have been fitted with microfluidic channels, microcontacted printed with surface chemistry patterns, or fabricated amongst topographical features in order to better control and monitor cellular behavior.21,44,68 Microfabricated patch clamp electrodes are a highly pursued goal, precisely because the possibility of integrating the experimental prowess of patch-clamp with the benefits of other microtechnologies is a powerful framework for neuroscience research. As these mass-fabricated patch arrays become widely available, they could well supplant the MEA as the principal component of integrated neural microsystems.
The first microscale devices with multiple functionalities have already been fabricated and used. The majority of studies cited in this review have more than one microtechnology to offer, though not all of them are referenced in this section. It is important to remember that each device has a particular set of characteristics optimized for the biological study it is intended to serve. A result of science-driven device development is that there is no shortage of technical challenges and new technologies which remain to be created and utilized, despite the number of examples in each category presented here.
Getting started
As suggested by the title, one of the underlying objectives of this review is to generate interest in applying microtechnology to the field of neurobiology. Although it may be sufficient for the review to be merely informative in providing an overview of the field, as we suggest in the conclusions, there are numerous questions that remain in neurobiology. With this in mind, the review also hopes to provide some insight on finding intriguing neuroscience questions to investigate.As a thought exercise, consider browsing through a recent copy of Nature Neuroscience, a great resource in identifying high impact studies in the neuroscience field. For example, the May 2006 issue contains several articles that utilize in vitro cell culture models that might benefit from the appropriate use of microtechnology, in particular the article by Hartman et al.82 This study examines the ability of neurons and neural networks to maintain the appropriate level of activity despite varying inputs, which is important to the health and responsiveness of the tissue. For example, in the face of more than the optimal level of activity, inhibitory neurons may gain strength to bring the network back to within the proper range. In this study, the authors investigate this process by culturing a hippocampal network in vitro. Stimulation and recordings are carried out with patch-clamp electrodes, and network activity was globally controlled with drugs in the culture media. The results of the study show that the properties of individual inhibitory neurons respond to the global activity of the network, not the conditions in any single immediately connected cell.
By applying the types of microtechnology outlined in this review, a wealth of additional information could be gained from this experimental model. For example, instead of picking single cells to stimulate and record from, an array of electrodes, either patch-clamp or conductive pads, would allow more detailed analysis of the state of the network in relation to the strength of the inhibitory cells of interest. Adding a microfluidic culture chamber would give the ability to selectively target subsections of the network with drugs while recording from the entire culture simultaneously. Using physical barriers or patterned surfaces to place the cells in specific arrangements would enhance the power of the microfluidic perfusion chamber and the electrode array even further. Overall, the use of an engineered microsystem would lead to more information about how individual cells respond to their environment, including local and global network behavior.
Applications in neural developmentEach subspecialty of neuroscience, from developmental neurobiology to network physiology, has its own set of current problems. One particular area where microtechnology may have a significant impact is in neural development and guidance. There are many outstanding questions that remain in neurobiology that involve the interactions between developing neural cells and their local microenvironment. Some of these include; (1) interactions between surface bound guidance cues and soluble extracellular factors, (2) the interplay between surface mechanical geometries and surface chemistry, (3) the roles of competing factors (i.e. growth promoting vs. inhibiting) in determining neural guidance, (4) interactions between soluble factors and cell to cell contact, and (5) the dynamics of binding, transport and trafficking of neuro-active guidance molecules (i.e. growth factors). While many of these types of interactions have been posed, our understanding of the role of a cell's microenvironment could be greatly facilitated by the use of microtechnology.This is well illustrated by a number of recent reports where investigators have started to utilize microtechnology to elucidate basic mechanisms of interactions between a neuron and their microenvironment to study: (1) the integration of topographical and biochemical cues during growth of developing axons,89 (2) growth cone navigation in response to substrate bound gradients of chemical factors,90 (3) trafficking of growth factors during neurite growth.91 These studies very eloquently illustrate how microtechnology can be utilized to start to answer some of the more basic questions in neurobiology. They are also good references to illustrate how even relatively standard technology (simple channels, microstamps, and quantum dots) can be very effective when tailored to address biologically relevant questions. |
Conclusions
Using the technical achievements that have already been reported, an enormous number of experimental systems in neuroscience research can be enhanced. One of the most exciting prospects of lab-on-a-chip systems in neurobiology is the ability to generate data in ways unheard of only a few years ago. The benefit of this to the technologist is apparent—as long as there remain unanswered questions regarding the nervous system (which promises to hold secrets for a long, long time), the field is wide open for achievement (see, Box ‘Applications in neural development’ for examples). As outlined in this review, the study of neurons presents both challenges and opportunities unique amongst biological lab-on-a-chip applications. The hope is that by keeping these principles in mind while designing new systems to exploit microscale phenomena, an experimentally useful device for studying neurons can be generated. However, it is equally important to work hand-in-hand with the intended user so that devices are created which are not just technically innovative, but practical and useful to neurobiologists. As Whitesides' group illustrates (in a parallel review from the neuroscience point of view) a mutually beneficial relationship can, and should, be forged between the rapidly advancing field of neuroscience and the expanding field of microtechnology.83 A wealth of new tools, and the discoveries that come along with them, are awaiting these collaborations, and may well someday define a completely new field of ‘Cellular Scale Neuroengineering’.References
- T. B. DeMarse, D. A. Wagenaar, A. Blau and S. M. Potter, Autonomous Robots, 2001, 11, 305–310 Search PubMed.
- S. Marom and G. Shahaf, Q. Rev. Biophys., 2002, 35, 63–87 CrossRef.
- C. Koch and I. Segev, Nat. Neurosci., 2000, 3 Suppl, 1171–1177 CrossRef.
- E. E. Fetz, Science, 1969, 163, 955–958 CrossRef CAS.
- D. M. Taylor, S. I. Tillery and A. B. Schwartz, Science, 2002, 296, 1829–1832 CrossRef CAS.
- R. S. Clement, R. S. Witte, P. J. Rousche and D. R. Kipke, Neurocomputing, 1999, 26–27, 347–354 CrossRef.
- J. Csicsvari, D. A. Henze, B. Jamieson, K. D. Harris, A. Sirota, P. Bartho, K. D. Wise and G. Buzsaki, J. Neurophysiol., 2003, 90, 1314–1323 Search PubMed.
- P. M. Horton, L. Bonny, A. U. Nicol, K. M. Kendrick and J. F. Feng, J. Neurosci. Methods, 2005, 146, 22–41 CrossRef CAS.
- I. A. Basheer and M. Hajmeer, J. Microbiol. Methods, 2000, 43, 3–31 CrossRef CAS.
- A. K. Jain, J. Mao and K. M. Mohiuddin, Computer, 1996, 29, 31 CrossRef.
- N. M. Dowell-Mesfin, M. A. Abdul-Karim, A. M. Turner, S. Schanz, H. G. Craighead, B. Roysam, J. N. Turner and W. Shain, J. Neural. Eng., 2004, 1, 78–90 Search PubMed.
- D. W. Branch, B. C. Wheeler, G. J. Brewer and D. E. Leckband, IEEE Trans. Biomed. Eng., 2000, 47, 290–300 CrossRef CAS.
- C. D. James, R. Davis, M. Meyer, A. Turner, S. Turner, G. Withers, L. Kam, G. Banker, H. Craighead, M. Isaacson, J. Turner and W. Shain, IEEE Trans. Biomed. Eng., 2000, 47, 17–21 CrossRef CAS.
- A. M. Taylor, S. W. Rhee and N. L. Jeon, Methods Mol. Biol., 2006, 321, 167–177 Search PubMed.
- S. K. Dertinger, X. Jiang, Z. Li, V. N. Murthy and G. M. Whitesides, Proc. Natl. Acad. Sci. U. S. A., 2002, 99, 12542–12547 CrossRef CAS.
- N. Li, A. Tourovskaia and A. Folch, Crit. Rev. Biomed. Eng., 2003, 31, 423–488 Search PubMed.
- C. S. Chen, M. Mrksich, S. Huang, G. M. Whitesides and D. E. Ingber, Biotechnol. Prog., 1998, 14, 356–363 CrossRef CAS.
- G. M. Walker, H. C. Zeringue and D. J. Beebe, Lab Chip, 2004, 4, 91–97 RSC.
- W. L. Rutten, Annu. Rev. Biomed. Eng., 2002, 4, 407–452 Search PubMed.
- S. M. Potter and T. B. DeMarse, J. Neurosci. Methods, 2001, 110, 17–24 CrossRef CAS.
- T. M. Pearce, J. A. Wilson, S. G. Oakes, S. Y. Chiu and J. C. Williams, Lab Chip, 2005, 5, 97–101 RSC.
- J. Atencia and D. J. Beebe, Nature, 2005, 437, 648–655 CrossRef CAS.
- G. M. Walker and D. J. Beebe, Lab Chip, 2002, 2, 131–134 RSC.
- C. S. Chen, J. L. Alonso, E. Ostuni, G. M. Whitesides and D. E. Ingber, Biochem. Biophys. Res. Commun., 2003, 307, 355–361 CrossRef CAS.
- T. H. Park and M. L. Shuler, Biotechnol. Prog., 2003, 19, 243–253 CrossRef CAS.
- Brain Facts: A Primer on the Brain and Nervous System, Society for Neuroscience, 2005 Search PubMed.
- E. Neher and B. Sakmann, Nature, 1976, 260, 799–802 CAS.
- W. G. Regehr, J. Pine and D. B. Rutledge, IEEE Trans. Biomed. Eng., 1988, 35, 1023–1032 CrossRef CAS.
- M. Zochowski, M. Wachowiak, C. X. Falk, L. B. Cohen, Y. W. Lam, S. Antic and D. Zecevic, Biol. Bull., 2000, 198, 1–21 Search PubMed.
- J. Pine, J. Neurosci. Methods, 1980, 2, 19–31 CrossRef CAS.
- N. Sundararajan, D. Kim and A. A. Berlin, Lab Chip, 2005, 5, 350–354 RSC.
- Y. Lazebnik, Cancer Cell, 2002, 2, 179–182 Search PubMed.
- A. Harsch, C. Ziegler and W. Gopel, Biosens. Bioelectron., 1997, 12, 827–835 CrossRef CAS.
- M. P. Maher, H. Dvorak-Carbone, J. Pine, J. A. Wright and Y. C. Tai, Med. Biol. Eng. Comput., 1999, 37, 110–118 CAS.
- M. J. Mahoney, R. R. Chen, J. Tan and W. M. Saltzman, Biomaterials, 2005, 26, 771–778 CrossRef CAS.
- E. Claverol-Tinture, M. Ghirardi, F. Fiumara, X. Rosell and J. Cabestany, J. Neural. Eng., 2005, 2, L1–7 Search PubMed.
- G. M. Whitesides, E. Ostuni, S. Takayama, X. Jiang and D. E. Ingber, Annu. Rev. Biomed. Eng., 2001, 3, 335–373 Search PubMed.
- R. S. Kane, S. Takayama, E. Ostuni, D. E. Ingber and G. M. Whitesides, Biomaterials, 1999, 20, 2363–2376 CrossRef.
- Y. Nam, D. W. Branch and B. C. Wheeler, Biosens. Bioelectron., 2006 Search PubMed.
- N. E. Sanjana and S. B. Fuller, J. Neurosci. Methods, 2004, 136, 151–163 CrossRef.
- S. Takayama, J. C. McDonald, E. Ostuni, M. N. Liang, P. J. Kenis, R. F. Ismagilov and G. M. Whitesides, Proc. Natl. Acad. Sci. U. S. A., 1999, 96, 5545–5548 CrossRef CAS.
- J. H. Wittig, Jr., A. F. Ryan and P. M. Asbeck, J. Neurosci. Methods, 2005, 144, 79–89 CrossRef.
- A. A. Werdich, E. A. Lima, B. Ivanov, I. Ges, M. E. Anderson, J. P. Wikswo and F. J. Baudenbacher, Lab Chip, 2004, 4, 357–362 RSC.
- I. Suzuki, Y. Sugio, Y. Jimbo and K. Yasuda, Lab Chip, 2005, 5, 241–247 RSC.
- K. Shimono, M. Baudry, V. Panchenko and M. Taketani, J. Neurosci. Methods, 2002, 120, 193–202 CrossRef CAS.
- S. I. Morefield, E. W. Keefer, K. D. Chapman and G. W. Gross, Biosens. Bioelectron., 2000, 15, 383–396 CrossRef CAS.
- A. Stett, U. Egert, E. Guenther, F. Hofmann, T. Meyer, W. Nisch and H. Haemmerle, Anal. Bioanal. Chem., 2003, 377, 486–495 CrossRef CAS.
- D. A. Wagenaar, R. Madhavan, J. Pine and S. M. Potter, J. Neurosci., 2005, 25, 680–688 CrossRef CAS.
- D. A. Wagenaar, J. Pine and S. M. Potter, J. Neurosci. Methods, 2004, 138, 27–37 CrossRef.
- F. Crespi, J. Neurosci. Methods, 1990, 34, 53–65 CrossRef CAS.
- P. S. Cahill, Q. D. Walker, J. M. Finnegan, G. E. Mickelson, E. R. Travis and R. M. Wightman, Anal. Chem., 1996, 68, 3180–3186 CrossRef.
- B. Sakmann and E. Neher, Annu. Rev. Physiol., 1984, 46, 455–472 CrossRef CAS.
- K. G. Klemic, J. F. Klemic, M. A. Reed and F. J. Sigworth, Biosens. Bioelectron., 2002, 17, 597–604 CrossRef CAS.
- R. Pantoja, J. M. Nagarah, D. M. Starace, N. A. Melosh, R. Blunck, F. Bezanilla and J. R. Heath, Biosens. Bioelectron., 2004, 20, 509–517 CrossRef CAS.
- F. J. Sigworth and K. G. Klemic, Biophys. J., 2002, 82, 2831–2832 CrossRef CAS.
- C. Ionescu-Zanetti, R. M. Shaw, J. Seo, Y. N. Jan, L. Y. Jan and L. P. Lee, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 9112–9117 CrossRef CAS.
- F. J. Sigworth and K. G. Klemic, IEEE Trans. Nanobiosci., 2005, 4, 121–127 CrossRef.
- C. H. Hsu, C. Chen and A. Folch, Lab Chip, 2004, 4, 420–424 RSC.
- F. Lin, W. Saadi, S. W. Rhee, S. J. Wang, S. Mittal and N. L. Jeon, Lab Chip, 2004, 4, 164–167 RSC.
- B. G. Chung, L. A. Flanagan, S. W. Rhee, P. H. Schwartz, A. P. Lee, E. S. Monuki and N. L. Jeon, Lab Chip, 2005, 5, 401–406 RSC.
- A. Tourovskaia, X. Figueroa-Masot and A. Folch, Lab Chip, 2005, 5, 14–19 RSC.
- A. M. Taylor, M. Blurton-Jones, S. W. Rhee, D. H. Cribbs, C. W. Cotman and N. L. Jeon, Nat. Methods, 2005, 2, 599–605 CrossRef CAS.
- T. M. Pearce, J. J. Williams, S. P. Kruzel, M. J. Gidden and J. C. Williams, IEEE Trans. Neural Syst. Rehabil. Eng., 2005, 13, 207–212 CrossRef.
- J. Olofsson, H. Bridle, J. Sinclair, D. Granfeldt, E. Sahlin and O. Orwar, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 8097–8102 CrossRef CAS.
- M. C. Peterman, J. Noolandi, M. S. Blumenkranz and H. A. Fishman, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 9951–9954 CrossRef CAS.
- M. C. Peterman, J. Noolandi, M. S. Blumenkranz and H. A. Fishman, Anal. Chem., 2004, 76, 1850–1856 CrossRef CAS.
- S. Martinoia, M. Bove, M. Tedesco, B. Margesin and M. Grattarola, J. Neurosci. Methods, 1999, 87, 35–44 CrossRef CAS.
- C. D. James, A. J. Spence, N. M. Dowell-Mesfin, R. J. Hussain, K. L. Smith, H. G. Craighead, M. S. Isaacson, W. Shain and J. N. Turner, IEEE Trans. Biomed. Eng., 2004, 51, 1640–1648 CrossRef.
- C. S. Chen, M. Mrksich, S. Huang, G. M. Whitesides and D. E. Ingber, Science, 1997, 276, 1425–1428 CrossRef CAS.
- P. Thiebaud, L. Lauer, W. Knoll and A. Offenhausser, Biosens. Bioelectron., 2002, 17, 87–93 CrossRef CAS.
- L. Kam, W. Shain, J. N. Turner and R. Bizios, Biomaterials, 2001, 22, 1049–1054 CrossRef CAS.
- J. C. Chang, G. J. Brewer and B. C. Wheeler, Biomaterials, 2003, 24, 2863–2870 CrossRef CAS.
- J. M. Corey, B. C. Wheeler and G. J. Brewer, IEEE Trans. Biomed. Eng., 1996, 43, 944–955 CrossRef CAS.
- E. A. Roth, T. Xu, M. Das, C. Gregory, J. J. Hickman and T. Boland, Biomaterials, 2004, 25, 3707–3715 CrossRef CAS.
- T. Xu, J. Jin, C. Gregory, J. J. Hickman and T. Boland, Biomaterials, 2005, 26, 93–99 CrossRef CAS.
- T. Xu, C. A. Gregory, P. Molnar, X. Cui, S. Jalota, S. B. Bhaduri and T. Boland, Biomaterials, 2006, 27, 3580–3588 CAS.
- S. Costantino, K. G. Heinze, O. E. Martinez, P. De Koninck and P. W. Wiseman, Microsc. Res. Tech., 2005, 68, 272–276 CrossRef.
- A. M. Turner, N. Dowell, S. W. Turner, L. Kam, M. Isaacson, J. N. Turner, H. G. Craighead and W. Shain, J. Biomed. Mater. Res., 2000, 51, 430–441 CrossRef CAS.
- M. P. Maher, J. Pine, J. Wright and Y. C. Tai, J. Neurosci. Methods, 1999, 87, 45–56 CrossRef CAS.
- S. W. Rhee, A. M. Taylor, C. H. Tu, D. H. Cribbs, C. W. Cotman and N. L. Jeon, Lab Chip, 2005, 5, 102–107 RSC.
- P. Degenaar, B. L. Pioufle, L. Griscom, A. Tixier, Y. Akagi, Y. Morita, Y. Murakami, K. Yokoyama, H. Fujita and E. Tamiya, J. Biochem. (Tokyo), 2001, 130, 367–376 Search PubMed.
- K. N. Hartman, S. K. Pal, J. Burrone and V. N. Murthy, Nat. Neurosci., 2006, 9, 642–649 CrossRef CAS.
- D. B. Weibel, P. Garstecki and G. M. Whitesides, Curr. Opin. Neurobiol., 2005, 15, 560–567 CrossRef CAS.
- A. A. Butov, Adv. Gerontol., 2003, 12, 172–173 Search PubMed.
- A. C. Scott, Rev. Mod. Phys., 1975, 47, 487–533 CrossRef.
- J. C. Chang, G. J. Brewer and B. C. Wheeler, J. Neural Eng., 2006, 3, 217–226 Search PubMed.
- Y. Nam, J. Chang, D. Khatami, G. J. Brewer and B. C. Wheeler, IEE Proc. Nanobiotechnol., 2004, 151, 109–115 Search PubMed.
- L. Kam, W. Shain, J. N. Turner and R. Bizios, Biomaterials, 2002, 23, 511–515 CrossRef CAS.
- N. Li and A. Folch, Exp. Cell Res., 2005, 311, 307–316 CrossRef CAS.
- A. C. von Philipsborn, S. Lang, J. Loeschinger, A. Bernard, C. David, D. Lehnert, F. Bonhoeffer and M. Bastmeyer, Development, 2006, 133, 2487–2495 CrossRef CAS.
- T. Q. Vu, R. Maddipati, T. A. Blute, B. J. Nehilla, L. Nusblat and T. A. Desai, Nano Lett., 2005, 5, 603–607 CrossRef CAS.
Footnotes |
| † The HTML version of this article has been enhanced with a colour image. |
| ‡ Present address: Medical Scientist Training Program, Washington University in St. Louis, MO 63110, USA. |
| This journal is © The Royal Society of Chemistry 2007 |