Lab-on-a-chip devices for global health: Past studies and future opportunities
Curtis D.
Chin
a,
Vincent
Linder
b and
Samuel K.
Sia
*a
aDepartment of Biomedical Engineering, Columbia University, 351 Engineering Terrace, 1210 Amsterdam Avenue, New York, NY 10027, USA. E-mail: ss2735@columbia.edu
bSAMLAB, Institute of Microtechnology, University of Neuchâtel, Rue Jaquet-Droz 1, P. O. Box 526, CH-2002 Neuchâtel, Switzerland
First published on 27th October 2006
Abstract
A rapidly emerging field in lab-on-a-chip (LOC) research is the development of devices to improve the health of people in developing countries. In this review, we identify diseases that are most in need of new health technologies, discuss special design criteria for LOC devices to be deployed in a variety of resource-poor settings, and review past research into LOC devices for global health. We focus mainly on diagnostics, the nearest-term application in this field.
Curtis Chin is a PhD student in the Department of Biomedical Engineering at Columbia University. He obtained his BS in Chemical Engineering from the Massachusetts Institute of Technology. |
Vincent Linder, PhD, is a research scientist at the University of Neuchatel and Claros Diagnostics. He holds a MSc in Chemistry and a PhD in Sciences from the University of Neuchâtel (Switzerland), where he worked on microfluidic technology for immunoassays. He completed his postdoctoral work in the Department of Chemistry at Harvard University. |
Samuel Sia, PhD, is an Assistant Professor of Biomedical Engineering at Columbia University. He holds a BS in Biochemistry from the University of Alberta (Canada), and a PhD in Biophysics from Harvard University. He completed his postdoctoral work in the Department of Chemistry at Harvard University. |
1. Introduction
Lab-on-a-chip (LOC) technologies have a tremendous but unproven potential to improve the health of people in developing countries. Ever since the modern inception of LOC and microfluidic technologies around 1990, use in remote settings has been perceived as potentially one of the most powerful applications of the technology by taking advantage of its small size, low volume requirement for samples, and rapid analysis. Indeed, portable LOC devices are now beginning to be used in remote settings, as a result of developments in integrating fluid actuation, sample pre-treatment, sample separation, signal amplification, and signal detection into a single device. As they stand, these devices are not yet appropriate for use in the extreme resource-poor settings of developing countries; nevertheless, these advances place the field of LOC research in a prime position to tackle the profound issue of global health, where the challenges in device designs are arguably the most demanding, and the need for new health technologies the greatest.There is an urgent need in developing countries for new health-related technologies, and specifically, new technologies for health diagnostics. For example, in one survey of international scientists familiar with the public health programs of developing countries, Singer and colleagues found that the top-ranking overall priority was “modified molecular technologies for affordable, simple diagnosis of infectious diseases”.1 Similarly, in a study by the Bill and Melinda Gates Foundation and the NIH to identify ‘Grand Challenges for Global Health’, two of the 14 priorities involved diagnosis and measurement of patients' health statuses (i.e. “develop technologies that allow assessment of individuals for multiple conditions or pathogens at point-of-care”, and “develop technologies that permit quantitative assessment of population health status”).2 LOC research holds substantial potential for fulfilling these priorities by automating complex diagnostic procedures that are normally performed in a centralized laboratory into a hand-held microfluidic chip; this capability could empower health-care workers and patients with important health-related information in even the most remote settings. To this effect, funding by philanthropic foundations (such as those from Doris Duke, Soros, and Gates) are leading the development of microfluidics technologies for diagnostics in developing countries. The broad aim of these scientific initiatives is to combine new diagnostic and prevention methods with treatment to improve public health,3 which is in turn linked closely to the macroeconomic health of a nation.4
In this review, we aim to aid interested scientists and engineers by systematically reviewing the fledgling field of LOC devices for global health. We will focus mainly on diagnostics, the nearest-term application of LOC devices, although we will also discuss other applications. We will first identify the most critical health conditions and diseases that are in need of new diagnostic methods, with an emphasis on those in need of new LOC diagnostic devices (Section 2). In subsequent sections, we will discuss the special design criteria that are needed for LOC devices to be deployed in developing countries (Section 3), and provide a review of past and current studies on LOC devices for global health, as well as examples of LOC devices that have not been—but could be—applied to these settings (Section 4). We will conclude with a summary and future directions (Section 5). Throughout the review, we will identify examples of past research, current promising technologies, and future challenges and opportunities.
2. Current need for diagnostic devices in developing countries
In developed and developing countries alike, early and accurate diagnosis is important for the health of individual patients as well as that of the general public: it permits prompt and proper treatment of patients, limits the spread of disease in the population, and minimizes the waste of public resources on ineffective treatments.1 In developing countries, the value of diagnosis for certain diseases is sometimes mitigated by the lack of available treatment (for example, in cases of certain neglected tropical diseases). On the whole, however, the value of diagnosis is very high in developing countries: early diagnosis, although not without logistical hurdles, can often lead to some kind of treatment (either directly against the condition or at worst palliative care), and investments in diagnostics and prevention can be more cost-effective than treatment.5 Moreover, point-of-care devices can improve the epidemiological surveillance of diseases,6 which is an especially challenging problem in developing countries.For scientists and engineers who aim to design new diagnostic technologies, a crucial question for achieving real-world impact is which health conditions in developing countries are most in need of diagnostic devices. In a study led by Murray and Lopez, the World Health Organization conducted an unprecedented and comprehensive initiative to compile statistics for comparing the relative burden of diseases, conditions, injuries, and risk factors on a global scale.7–9 In Table 1, we list the most common diseases by disability-adjusted life years (DALYs) in developing countries, a metric that accounts for years of life lost due to premature mortality as well as disability (in order to properly account for the impact of conditions that cause significant ill health but few direct deaths, such as neuropsychiatric conditions9,10).
| Diseasea | % DALYb | Type of assayc | Device | Disease | % DALY | Type of assay | Device |
|---|---|---|---|---|---|---|---|
| a Disease categories as grouped by Murray and Lopez,8 except maternal, perinatal, and nutritional conditions are shown as a separate category. b The burden of disease as measured by the percentage contribution to total disability-adjusted life years (DALYs) in middle- and low-income countries. Percentages were derived using data from WHO Global Burden of Disease 2002 revised estimates by World Bank income groups (high, upper middle, lower middle, and low income countries)9http://www3.who.int/whosis/menu.cfm?path=whosis,burden,burden_estimates,burden_estimates_2002N,burden_estimates_2002N_2002Rev&language=english c Data taken from ref. 5, 7–9, 18. Abbreviations used: immunochromatographic (IC), immunoassay (IA), enzyme-linked immunosorbent assay (ELISA), microscopy (mic.), venereal disease research laboratory (VDRL), rapid plasma regin (RPR), fluorescent treponemal antibody-adsorption (FTA-ABS), T. pallidum hemagglutination assay (TPHA), agglutination (agg.), culture (cul.), iodine (iod.), vitamin (vit.), gram stain (GS), cerebrospinal (CSF), hypertension (hypertens.), iron (Fe), infections (infect.), Down syndrome (DS), disorder (dis.), ischaemic cerebrovascular disease (isc.), rheumatory heart disease (rheum.), inflammatory heart disease (inflamm.), depression (deprs.), hepatitis B surface antigen (HBsAg), antibody to hepatitis B surface antigen (anti-HBs), antibody to hepatitis B core antigen (anti-HBc), alpha-fetoprotein (AFP), hepatitis C virus (HCV), C- reactive protein (CRP), prostate-specific antigen (PSA), brain naturietic peptide (BNP), hypertensive heart disease (hyp.)., nephritis and nephrosis (neph.), benign prostatic hypertrophy (bphyp.), low weight (LW.), birth asphyxia (BA), hemorrhage (hem.), sepsis (seps.), conditions (cond.), road accidents (RA), drownings (dr.), self-inflected injuries (SII), white blood cell (WBC), Sx/Hx/PE (symptoms/medical history/physical exam), red blood cell (RBC). | |||||||
| Communicable diseases | 32.1 | Non-communicable diseases | 43.5 | ||||
| Respiratory infect. (lower, upper, ottis media) | 6.8 | IA | Sec. 4.2 | Neuropsychiatric conditions (unipol & bipol deprs, others) | 11.7 | Sx/Hx/PE | N/A |
| Slide agg. w/antisera | Sec. 4.2 | Hormone levels | Sec. 4.5 | ||||
| GS and cul. of CSF | Sec. 4.4 | ||||||
| HIV/AIDS | 6.1 | IA for α-HIV Ab (± vs quant) | Sec. 4.2 | Cardiovascular diseases (isc., hyp., rheum., inflamm.) | 9.5 | ELISA of CRP, BNP | Sec. 4.2 |
| RT-PCR for HIV RNA | Sec. 4.3 | Cholesterol test | Sec. 4.5 | ||||
| CD4+ counts | Sec. 4.4 | ||||||
| Diarrheal diseases (rotavirus, cholera) | 4.5 | EIA | Sec. 4.2 | Sense order diseases (cataracts, hearing loss, glaucoma) | 4.6 | Sx/Hx/PE | N/A |
| Latex agg. assay of stool | Sec. 4.2 | ||||||
| RT-PCR | Sec. 4.3 | ||||||
| Malaria | 3.4 | Mic. of blood smears | Sec. 4.4 | Cancer | 4.2 | IA of biomarkers (e.g. PSA) | Sec. 4.2 |
| IC test for HRP-2, LDH, PS | Sec. 4.2 | Gene expression | Sec. 4.3 | ||||
| PCR for plasmodium | Sec. 4.3 | ||||||
| Tuberculosis | 2.5 | Tuberculin skin test | N/A | Respiratory diseases (COPD, asthma, others) | 3.5 | Spirometry | N/A |
| Mic. and sputum culture | Sec. 4.4 | Sx/Hx/PE | N/A | ||||
| Release of IFN-γ from blood | Sec. 4.2 | ||||||
| Measles | 1.6 | Virus isolation | Sec. 4.3 | Digestive diseases (liver cirrhosis, peptic ulcer disease) | 3.0 | Complete blood count | Sec. 4.4 |
| Monitor specific IgG titers | Sec. 4.2 | Electrolytes, creatinine | Sec. 4.5 | ||||
| IA for virus (MV) IgM | Sec. 4.2 | Elevated liver enzymes | Sec. 4.2 | ||||
| Pertussis | 0.9 | PCR of nasal secretions | Sec. 4.3 | Congenital abnormalities (heart dis., DS) | 1.9 | Karyotype analysis trisomy 21 | N/A |
| Culture | Sec. 4.4 | RBC count | Sec. 4.5 | ||||
| Sx/Hx/PE | N/A | ||||||
| Tetanus | 0.5 | Culture | Sec. 4.4 | Musculoskeletal dis. (osteoarthritis, rheumatoid arthritis) | 1.8 | Differential WBC | Sec. 4.4 |
| IA | Sec. 4.2 | ELISA rheumatoid factor | Sec. 4.2 | ||||
| Meningitis | 0.4 | CSF glucose | Sec. 4.5 | Genitourinary dis. (neph., bphyp.) | 1.0 | Sx/Hx/PE (physician) | N/A |
| CSF cell count | Sec. 4.4 | ||||||
| GS & cul. of CS | Sec. 4.4 | ||||||
| Lymphatic filariasis | 0.4 | ELISA | Sec. 4.2 | Diabetes mellitus | 1.0 | Plasma/glucose test | Sec. 4.5 |
| IC test of W. bancrofti | Sec. 4.2 | Insulin | Sec. 4.2 | ||||
| Mic. blood samples midnight | Sec. 4.4 | ||||||
| Hepatitis B & hepatitis C | 0.3 | IA HBsAg, anti-HBs, antiHBc | Sec. 4.2 | Endocrine disorders | 0.5 | Hormone levels | Sec. 4.5 |
| IA liver enzyme, AFP | Sec. 4.2 | X-rays, radiological exams | N/A | ||||
| HCV detection & genotying | Sec. 4.3 | ||||||
| Syphilis | 0.3 | VDRL | Sec. 4.2 | Oral conditions (dental caries, edentulism, others) | 0.5 | Sx/Hx/PE (dentist) | N/A |
| RPR | Sec. 4.2 | X-rays, radiological exams | N/A | ||||
| FTA-ABS of TPHA | Sec. 4.2 | ||||||
| Chlamydia | 0.3 | PCR from urine dipstick | Sec. 4.3 | Skin diseases | 0.3 | Sx/Hx/PE (physician) | N/A |
| ELISA of C. trachomatis antigens | Sec. 4.2 | ||||||
| NAAT, hybridization test | Sec. 4.3 | ||||||
| Gonorrhea | 0.2 | Mic & cul. urethral cervical | Sec. 4.4 | ||||
| Trachoma | 0.2 | Culture | Sec. 4.4 | Maternal, perinatal, and nutritional conditions | 11.8 | ||
| Antigen detection | Sec. 4.2 | Perinatal cond. (LW., BA., trauma) | 7.0 | Sx/Hx/PE (physician) | N/A | ||
| Leishmaniasis | 0.2 | IC test | Sec. 4.2 | ||||
| Mic. & cul. spleen, bone marrow | Sec. 4.4 | Nutritional deficiencies (protein-energy, Fe-anaemia) | 2.4 | IA albumin | Sec. 4.2 | ||
| Trypanosomiasis & schistosomiasis | 0.2 | Agg. test for IgM, PCR parasite | Sec. 4.2–3 | Cell count anemia | Sec. 4.4 | ||
| Mic. & cul. spleen, bone marrow | Sec. 4.4 | Maternal cond. (hem., seps., hypertens.) | 2.4 | Haemotology | Sec. 4.4–5 | ||
| Intestinal nematode infect. | 0.2 | Mic. & cul. anal swab | Sec. 4.4 | ||||
| Japanese encephalitis | 0.1 | IA of blood and spinal fluid | Sec. 4.2 | ||||
| Injuries | 12.5 | ||||||
| Unintentional injuries (RA, falls, fires, dr.) | 9.2 | Analytical toxicology | Sec. 4.2,5 | ||||
| Intentional injuries (violence, SII, war) | 3.3 | Culture (eg. C. tetani) | Sec. 4.4 | ||||
| IA | Sec. 4.2 | ||||||
As expected,10 infectious diseases constitute a large burden of disease in developing countries (32.1%; by comparison, they represent only 3.7% of total DALYs in developed countries). The trifecta of HIV/AIDS, malaria, and tuberculosis (TB), which has merited a dedicated focus from the international community (most notably the Global Fund, which has thus far committed $5.5 billion, http://www.theglobalfund.org), constitutes an important 12% of DALYs in developing countries. The social impact of these diseases stretches beyond the DALY statistics, however, since HIV/AIDS (along with common coinfections of TB) targets healthy adults, thereby leaving behind villages of orphans which destroy the underlying fabric of entire communities. Other infectious diseases are also important. Most significantly, lower respiratory infections and diarrheal diseases (such as rotavirus and cholera) impose large burdens; these diseases are also the biggest killers of children,10 even more so than vaccine-treatable childhood-cluster diseases (such as diphtheria, measles, pertussis, and tetanus). Another important category of infectious diseases is neglected tropical diseases (which includes lymphatic filiariasis, dengue, Chagas disease, leishmaniasis, onchocerciasis, schistosomiasis, trypanosomiasis, trachoma, and guinea worm), which cause 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 deaths annually. Although they do not contribute as significantly as some other infectious diseases by the measure of DALYs in the Global Burden of Disease report, the real burden of disease is likely to be higher than these estimates, with up to 90% of the burden concentrated in sub-Saharan Africa (for example, there are 200 million cases of hookworm infections in Africa alone).11 Current methods for diagnosing neglected diseases are cumbersome, invasive, and largely inadequate (e.g. for human African typanosomiasis and visceral leishmaniasis, see ref. 12), a consequence of the low priority given to neglected diseases for research funding (as pointed out poignantly by studies such as the 10/90 Report on Health Research, http://www.globalforumhealth.org, and ref. 13). In one analysis to define priorities for diagnostics development, Mabey and colleagues charted the need versus feasibility for selected diseases (including many neglected diseases), with the conclusion that African trypanosomiasis, visceral leishmaniasis, and TB are three of the tests most in need of development.14 Other important diseases include sexually-transmitted infections other than HIV/AIDS (such as hepatitis B and C, chlamydia, gonorrhea, and syphilis), some of which (most notably, hepatitis B and C, and HIV) are bloodborne pathogens that can also be transmitted by contaminated needles as well as contaminated blood supply for transfusions.15
000 deaths annually. Although they do not contribute as significantly as some other infectious diseases by the measure of DALYs in the Global Burden of Disease report, the real burden of disease is likely to be higher than these estimates, with up to 90% of the burden concentrated in sub-Saharan Africa (for example, there are 200 million cases of hookworm infections in Africa alone).11 Current methods for diagnosing neglected diseases are cumbersome, invasive, and largely inadequate (e.g. for human African typanosomiasis and visceral leishmaniasis, see ref. 12), a consequence of the low priority given to neglected diseases for research funding (as pointed out poignantly by studies such as the 10/90 Report on Health Research, http://www.globalforumhealth.org, and ref. 13). In one analysis to define priorities for diagnostics development, Mabey and colleagues charted the need versus feasibility for selected diseases (including many neglected diseases), with the conclusion that African trypanosomiasis, visceral leishmaniasis, and TB are three of the tests most in need of development.14 Other important diseases include sexually-transmitted infections other than HIV/AIDS (such as hepatitis B and C, chlamydia, gonorrhea, and syphilis), some of which (most notably, hepatitis B and C, and HIV) are bloodborne pathogens that can also be transmitted by contaminated needles as well as contaminated blood supply for transfusions.15
Like infectious diseases, the burden of non-communicable diseases is significant (at 43.5% DALY, it even exceeds that of infectious diseases by a large margin) (Table 1); unlike infectious diseases, the burden of non-communicable diseases in developing countries is often underappreciated.16 The specific list of important non-communicable diseases is familiar to readers from Western countries: cardiovascular disease (such as ischaemic heart disease and stroke), cancer, neuropsychiatric conditions (such as unipolar depressive disorder), and respiratory diseases (such as chronic obstructive pulmonary disorder and asthma). As the standard of living in developing countries improves and average life span increases, the burden of disease will gradually shift to the non-communicable diseases; this shift is exacerbated by changes in diet (towards saturated fats and sugars) and high tobacco use.16 Already, obesity and diabetes are increasingly prevalent in developing countries.17 Even for children in developing countries, asthma, epilepsy, dental caries, diabetes, rheumatic heart disease, and injuries are becoming increasingly prominent contributors to morbidity.18 As these trends develop, accessibility of the corresponding diagnostic technologies in developing countries cannot be assumed from their likely availability in Western countries, due to the special constraints of resource-poor settings (see Section 3).
Maternal, perinatal and nutritional diseases contribute a significant fraction of DALYs (11.8%) in developing countries (Table 1). Two important risk factors for material diseases include anemia and vitamin A deficiency;10 although treatment is the most important consideration for these two micronutrient deficiencies, diagnosis can lead to improved epidemiological surveillance. Overall, malnutrition is the single most important cause of loss in global health, with the greatest effect felt in sub-Saharan Africa.10 To combat malnutrition in children under five years of age, a simple bracelet made by Medicins Sans Frontieres can be used to measure the mid upper-arm circumference in order to diagnose the stage of malnourishment; biochemical measurements may be useful for more specific diagnoses (e.g. serum albumin levels for protein-energy malnutrition).
Finally, intentional and unintentional injuries (including war) constitute a significant DALY fraction (12.5%) that rivals that of other categories, and which is higher than that in Western countries (9.1%) (Table 1). A number of behavioral changes can be undertaken for preventing injuries in developing countries.19 From the perspective of diagnostic devices, this burden may call for devices for detecting poisons, diagnosing neuropsychiatric conditions (such as epilepsy) and substance of abuse to ensure prompt treatment, and diagnosing tetanus infections to strengthen epidemiological surveillance.
To diagnose this wide array of diseases and conditions, assays with a variety of methodologies will be needed. The types of assays that are currently used to diagnose them are listed in Table 1; some assays are in great need of new diagnostic methods, and some are not. For each diagnostic assay, potential corresponding LOC devices are also listed. Analysis of this table, which cross-lists diseases and technologies, reveals a couple of points. (1) Similar classes of analytes (e.g. proteins, nucleic acids) serve as useful markers for very different diseases and conditions; hence, similar designs of diagnostic technologies will be applicable for disparate classes of diseases. (For example, yes/no protein markers are useful for diagnosis of HIV/AIDS as well as indicators of coronary heart disease). In Section 4, we will review potential LOC technologies as grouped by analytes. (2) Multiple classes of assay technologies are needed to produce complete diagnostic information for groups of related diseases, and often even for a single disease (for confirmatory testing, identification of resistant subtypes, and/or staging of a disease). (For example, yes/no testing for antibodies, analysis of RNA levels, and counting of CD4+ lymphocytes are all crucial information for diagnosing and staging HIV/AIDS.) This observation calls for carefully considering the integration of multiple modular technologies at the earliest design stages of LOC diagnostic devices for developing countries.
3. Third-world design constraints
Like no other setting, the use of LOC devices in developing countries poses a set of extremely challenging design criteria. For maximum range of use, a LOC device would have to perform reliably under the well-documented constraints of low cost, absence of trained workers, lack of electricity, poorly equipped laboratories, and transportation and storage in unrefrigerated conditions with rough handling.14 In practice, however, not all of these constraints apply to all settings in developing countries. For example, in developing countries, different design criteria apply to centralized testing in a national laboratory, in a rural health clinic, and in a remote setting with no infrastructure (Table 2). Similarly, there exist subtle but important distinctions in the constraints. For ‘low cost’, the economics of centralized testing may allow for the purchase of a moderately priced or even expensive fixed instrument (tens of thousands of dollars), if the cost of disposables is kept sufficiently low. By contrast, remote point-of-care testing requires low cost in both the fixed instrument and the disposable (pennies). Since these considerations hold direct pertinence to the design of the diagnostic technology, it is beneficial to be aware of the final targeted use of the device at the earliest design stages. For example, in the extreme points of the landscape of resource availability in developing countries, devices targeted for national centralized laboratories may include currently available technologies for Western countries (e.g. 96 well plate assays using an expensive and bulky fluid-handling machine and detector), and devices for point-of-care testing in rural settings will need to be designed with all of the constraints in mind.| Constraint on design criteria |
|
|||||||
|---|---|---|---|---|---|---|---|---|
|
High-
income countries national testing centerb |
Low- &
middle- income countries centralized lab (private)c |
Military |
Extra-
terrestrial sensors |
High-
income countries point-of- care (POC) |
Low- &
middle- income countries national/ regional testing centerd |
Low- &
middle- income countries rural health clinice |
Low- &
middle- income countries remote POCf |
|
a I = important, S = somewhat important, U = unimportant.
b In a typical in-house hospital laboratory in Western countries, for example, costs of reagents are $5 for immunoassays, and $5–$30 for nucleic acid tests.138 Reimbursement rates are typically $20–$50 per test for immunoassays, and $50–$300 for nucleic acid tests. Fixed instruments (such as liquid handling robots and detectors) can cost hundreds of thousands of dollars, although they can be leased or borrowed using reagent-rental agreements. Typically, turnaround times for most tests of between 0.5 and 2 days (from the time it is ordered to the time the result is provided to the physician). Excluding sample transportation to the lab, turnaround time for emergency testing is about 1 h. Most reagents are stored in the fridge until time of use.
c This heading describes centralized testing centers that are funded by private organizations (e.g. philanthropies, NGO). These testing centers are typically located in urban locations, and can be as well-equipped as testing centers in Western countries. An example is the MTCT-Plus Initiative that funded two clinical laboratories in Maputo and Nampula to provide diagnostics services for supporting HIV programs in Mozambique.
d Centralized testing centers in national or regional health centers are typically funded by the government, and are responsible for many of the tests in the country. The quality of these health centers vary substantially, depending on the investment of the government into public health infrastructure.4 For example, a cell-counting instrument (about $20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) would be among the most expensive instruments, with typical equipment including a spectrophotometer, a microscope and a centrifuge costing $1000 to $2000 each.
e Rural towns often have a local health clinic or a hospital, with a wide range of conditions. Often, their clinical laboratories are run by poorly trained workers, are poorly equipped, and have intermittent or no ground electricity. One such laboratory would be equipped, for instance with a centrifuge (manual or electric), a microscope (for counts of cells like platelets or total WBC, the diagnosis of TB using colorations and the direct observation of stool), semi-quantitative strip tests for clinical chemistry applications (i.e., pH and sugar in urines), agglutination tests for HIV, and the reagents for basic tests like blood type compatibility on glass slides.
f These tests can be administered anywhere in developing countries. Currently used non-LOC tests in this category include yes/no immunochromatographic tests for malaria (i.e., a Paracheck Pf, which at a price of $1 can be limited to an emergency case for ruling out infection of Plasmodium falciparum) or semi-quantitative strip tests for clinical chemistry. An external instrument must be light, portable, rugged, and cheap (tens of dollars or cheaper, for widespread use), with very cheap disposable microfluidic chips (pennies). For example, optical microscopes in such settings can be only equipped with a mirror as a light source to facilitate maintenance. 000) would be among the most expensive instruments, with typical equipment including a spectrophotometer, a microscope and a centrifuge costing $1000 to $2000 each.
e Rural towns often have a local health clinic or a hospital, with a wide range of conditions. Often, their clinical laboratories are run by poorly trained workers, are poorly equipped, and have intermittent or no ground electricity. One such laboratory would be equipped, for instance with a centrifuge (manual or electric), a microscope (for counts of cells like platelets or total WBC, the diagnosis of TB using colorations and the direct observation of stool), semi-quantitative strip tests for clinical chemistry applications (i.e., pH and sugar in urines), agglutination tests for HIV, and the reagents for basic tests like blood type compatibility on glass slides.
f These tests can be administered anywhere in developing countries. Currently used non-LOC tests in this category include yes/no immunochromatographic tests for malaria (i.e., a Paracheck Pf, which at a price of $1 can be limited to an emergency case for ruling out infection of Plasmodium falciparum) or semi-quantitative strip tests for clinical chemistry. An external instrument must be light, portable, rugged, and cheap (tens of dollars or cheaper, for widespread use), with very cheap disposable microfluidic chips (pennies). For example, optical microscopes in such settings can be only equipped with a mirror as a light source to facilitate maintenance.
|
||||||||
| Absence of ground electricity | U | U | I | I | U | U | S | I |
| Low cost of fixed instrument | U | U | U | U | U | S | I | I |
| Low cost of disposables | U | U | U | U | S | I | I | I |
| Absence of trained end-users | U | U | S | N/A | I | S | S | I |
| Absence of equipped end-use facilities | U | U | I | I | I | S | I | I |
| Rapid Analysis | U | U | I | S | I | U | I | I |
| High sensitivity & specificity | I | I | I | I | I | I | I | I |
| Large fluctuations in temperature | U | S | I | I | I | S | I | I |
| Portability | U | U | I | I | I | S | I | I |
| Rough handling | U | S | I | I | S | S | I | I |
In the centralized laboratories of Western countries (run by companies such as Quest Diagnostics as well as in-house centers in hospitals) with skilled personnel, established infrastructure, and high financial resources, sophisticated tests such as microscopy, ELISAs, and nucleic acid amplification tests are routinely performed (Table 2).14 Although it would be desirable for diagnostic tests in this setting to meet criteria such as rapid analysis to improve efficiency of operation, there exist few constraints (compared to other settings for diagnostics) that result from low capital or poor infrastructure.
In developing countries, there exist a small number of privately funded centralized testing centers that have essentially the same infrastructure and resources as the testing centers in Western countries (Table 2). By contrast, in most centralized testing centers of developing countries, cost (due to limited budgets via public financing) and availability of skilled workers (due to attrition of the best workers) are still limited compared to the centers of Western countries. Thus, the cost of the microfluidic device (which includes both the material and the manufacturing process) must be kept low in most settings in developing countries. Moreover, for remote point-of-care testing in developing countries, the fixed instrument must be portable and cheap, and the disposable must be extremely cheap. All components of the device (including the instrument and disposable) must be robust and rugged under a variety of environmental conditions. Overall, remote point-of-care testing in developing countries imposes perhaps the severest constraints on the design of LOC devices, with extremely low cost a distinguishing feature from most applications in the Western world.
Although interest in designing LOC devices for developing countries is only beginning, it will be beneficial to exploit the commonalities in the settings of developing countries with other resource-poor settings in Western countries (Table 2). For example, one can leverage the large body of existing research on LOC designs for point-of-care testing for physicians' and home use,20,21 devices for military applications22 and first responders, and extraterrestrial sensors23–25 in designing LOC devices for developing countries (Fig. 1). In all these settings, integration, portability, low power consumption, automation, and ruggedness are important qualities.
 | ||
| Fig. 1 Pictures of current LOC devices for use in remote settings. (A) A handheld device, iSTAT, for measuring electrolyte levels. Taken from http://www.istat.com with permission of i-STAT Corporation. (B) A portable device and a fixed instrument for analyzing DNA from the company Cepheid. Taken from http://www.cepheid.com with permission of Cepheid Inc. (C) Mars Organic Analyzer, an extraterrestrial LOC device. Reprinted from ref. 24. Copyright 2005 by the National Academy of Sciences of the United States of America, all rights reserved. | ||
These general design constraints suggest some LOC components and procedures to be more appropriate than others in resource-poor settings. In Fig. 2, we outline a sample of appropriate LOC technologies for use in developing countries. In general, the differences in appropriate LOC technologies are pronounced between centralized testing versus point of care, with cost being the distinguishing feature between technologies for use in high-income versus low-income countries.
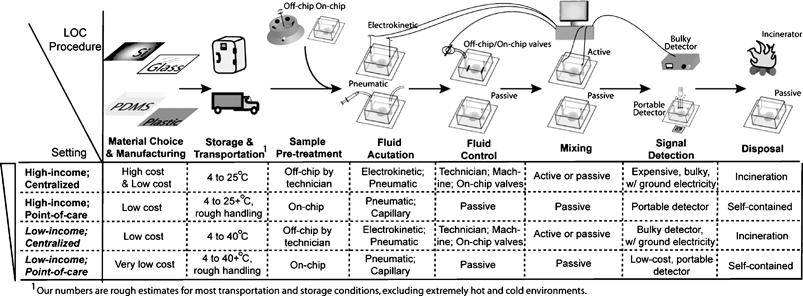 | ||
| Fig. 2 A range of appropriate LOC procedures for different settings. | ||
Material and manufacturing
To minimize the cost of the microfluidic device, it is important to reduce the footprint of expensive components such as glass ($500–4000 m−2),26 quartz, and silicon (a number of challenging issues exist in the scale-up of silicon micromachining for biological devices27). An alternative is to use plastics, which are inexpensive (the manufacturing cost of an injection-moulded device is generally less than $0.3028), available in an abundant choice of materials, and appropriate for single-use disposal to avoid cross-contamination. Challenges in plastic include minimization of batch-to-batch variation, improvement in chemical resistance, improvement in control over surface chemistry, and compatibility with fluorescence.26,29,30 Additional steps for processing the microfluidic chip (such as pre-treatment of surfaces with capture reagents) should be simple and scalable to minimize the cost of the manufacturing process.Storage and transportation
Unlike controlled research environments, the LOC device will be subjected to a variety of environmental conditions. Reagents, including those stored inside the microfluidic chip, must be stable to fluctuations in temperature as well as physical shocks. Methods for stabilizing dry reagents (such as trehalose,31 a chemical that has been used to stabilizing dried proteins in conventional 96 well ELISA assays) and wet reagents32,33 will be needed.Sample pre-treatment
A number of sources of physiological fluids are available. Although whole blood (from venipuncture or finger prick) and its derivatives (plasma and serum) are most common, the use of non-invasive samples such as saliva34 and urine35 are gaining prominence. For real-world samples, sample pre-treatment before the analysis step is needed. Pre-treatment steps include sampling, extraction, filtration, pre-concentration, and dilution;36 a centrifugation or filtration step is necessary, for example, to obtain plasma or serum from whole blood. In a centralized laboratory, these steps can be performed by a technician or a liquid handling robot before injection into the microfluidic chip. In remote settings, however, automation and integration of these steps in the LOC device is ideal.37–43 To minimize cost and power consumption, passive methods may be most appropriate.Fluid actuation
An ideal LOC device for developing countries should be capable of actuating the flow of fluids with reliable flow rates using inexpensive and compact instrumentation. Electrokinetic actuation of fluids is popular in research laboratories, but it requires a charged surface for electroosmotic flow (which limits the type of material that can be used) and a high voltage supply. Pneumatic actuation may be most practical for portable applications, with battery- or hand-powered vacuum sources.44 Capillary force is a simple method for pumping fluids in portable LOC devices.45Fluid control
For complex assays, a series of different reagents need to be delivered into the microfluidic chip. In centralized testing facilities, these procedures can be performed manually by a technician, an external liquid handling robot, or on-chip valves that are controlled by an external instrument. For portable automated devices, passive delivery of a series of reagents is an attractive option.32,45–47Mixing
Some assays will require mixing of samples with different reagents. In such cases, active micromixers can be used if a power supply is available.48 Passive mixers, which rely on the geometry and topography of the microchannels, can also be used to mix and dilute samples.49 In general, however, heterogeneous assays (which include many immunoassays) do not require mixing since the analyte is captured on the surface.Signal detection
An intrinsic challenge in microfluidics is detection of a signal emanating from a small physical region; in developing countries, this detection must be inexpensive, and ideally, use compact instrumentation that consumes little power. Fluorescence is a sensitive and popular detection method, but typically requires expensive and complicated optics and consumes significant power, whereas absorbance can be low-cost.44,50 With simple electronic modulation, the background in optical detection can be shielded from ambient light.44 Electrical measurements such as conductance are also potentially appropriate low-cost and portable detection methods for use in developing countries.51Disposal
In point-of-care settings in both Western and developing countries, it is ideal to contain the chemical reagents and blood samples in the LOC device for disposal. Also, because incineration is often not accessible, environmentally friendly chemicals are preferred.Overall strategy for development of LOC devices
More broadly, in developing a LOC device for a new setting, scientists and engineers can take two different approaches: adapt existing methods or design new technologies. Both approaches have been undertaken in the past for developing countries. Adaptation of Western-world technologies for use in developing countries has a number of precedents in technology-transfer projects; to be successful, these projects must carefully take into account the local needs, culture, and constraints.52 It is arguably more challenging (but perhaps more scientifically rewarding) to develop new technologies with consideration of local factors from the earliest stages of design. Collaboration with local partners could produce a device that best suits the health needs of the local people (rather than a device driven by the convenience of available technology); with the right initial testing group, this route can succeed extremely well as a disruptive technology.534. Review of current work on LOC devices for global health
4.1 Overview
Examination of diagnostic tests that are currently used in the field in developing countries can bring insights for designing LOC devices. Most of the current diagnostic tests used in developing countries are simple to use and provide rapid results.54 The most prevalent example is immunochromatographic tests (also known as dipstick or lateral-flow tests), which provide yes/no results in minutes in the form of a visible band (these tests typically use gold colloids or latex beads conjugated to antibodies).55–57 Moreover, immunochromatographic strips are cheap to produce. These strip tests, however, are not quantitative, and are not sufficiently sensitive for the detection of all important markers. As such, development of strip tests for diseases in developing countries (such as chlamydia and trachoma from Lee's group)58 is ongoing. LOC devices bring exciting capabilities of high analytical performance, but they must be made cost-effective, among other important requirements (Table 2). Also, LOC devices can also potentially be used for multiplexed and parallel analysis of many relevant markers at once; this capability, however, is challenging since different analytes typically call for the designs of different LOC methods. Below, we review LOC studies that have been applied (or have the potential to be applied) for use in developing countries, as grouped by the class of analytes.4.2 Proteins
A wide range of diseases is characterized by changes in protein concentrations in a patient's physiological fluids. These diseases span viral infections (e.g. anti-HIV antibodies as a marker for HIV/AIDS), bacterial infections (e.g. enterotoxin B as a marker for Staphylococcus aureus), parasitic infections (e.g. histidine-rich protein 2 as a marker for malaria), and non-communicable diseases (e.g. PSA as a marker for prostate cancer) (Table 1). Immunoassays are routinely used, with high sensitivity and specificity, to detect and quantitate protein markers. The most commonly used samples from the patient are whole blood, serum, and plasma, with less common samples being saliva, urine, feces, sperm, tears and sweat. In developing countries, the use of fluids that can be sampled non-invasively can encourage adoption of tests and decrease the incidence of infection due to contaminated needles. For example, saliva and urine offer a simple and safe alternative to blood sampling, but they typically contain lower concentrations of protein makers than in blood; successful detection will necessitate an improved sensitivity in the LOC device. As an example, a LOC immunoassay developed by McDevitt and colleagues to measure levels of C-reactive protein in saliva required a 1000-fold improvement in sensitivity over ELISA in microtiter plates (by using porous beads to increase the surface density of capture probe on the solid phase).59 Microfluidic chips to detect enteric antigens from human stool, a complex sample matrix, are being developed.60Enzyme immunoassays, which comprise most protein tests, typically require the established infrastructure of centralized testing facilities to accomplish complex reagent handling and optical detection. LOC devices have the potential to transpose antigen–antibody assays into assay formats that are much less demanding in infrastructure. At least two important hurdles exist in the processes of miniaturization and automation: storage of multiple reagents and fluid handling capability to carry out the complex protein assay, and detection of the signal in the microfluidic system. Below, we review approaches that have addressed these challenges using inexpensive and portable solutions that are, or have the potential to be, compatible with use in resource-poor settings.
We and Whitesides reported a simple and reliable technique for storing and delivering a sequence of reagents to a microfluidic device to carry out automated immunoassays32 (Fig. 3A). In this method, cartridges made of commercially available tubing were filled by sequentially injecting plugs of reagents separated by air spacers (which prevented the reagents from mixing with each other); in this form, the reagents were stable to shocks, and antibody solutions could be stored for months without loss in activity. By applying negative pressure at the outlet, all reagents were dispensed sequentially, and a solid-phase immunoassay could be completed in 2 minutes with low nM sensitivity. Another attractive method for passive fluid control and delivery is the autonomous capillary system,45,47 although this method currently requires a multi-step fabrication process. In contrast to passive delivery, active valves can also be used to deliver a complex series of pre-stored reagents. The groups of Beebe33 and Whitesides66 have developed inexpensive, hand-operated valving systems for point-of-care immunoassays. For long-term storage at high temperatures, storage of the reagents in a dry form may be more stable than as wet liquids. Yager and colleagues demonstrated that enzymes could be stored dry in LOC devices in mixtures of dextran and trehalose.67
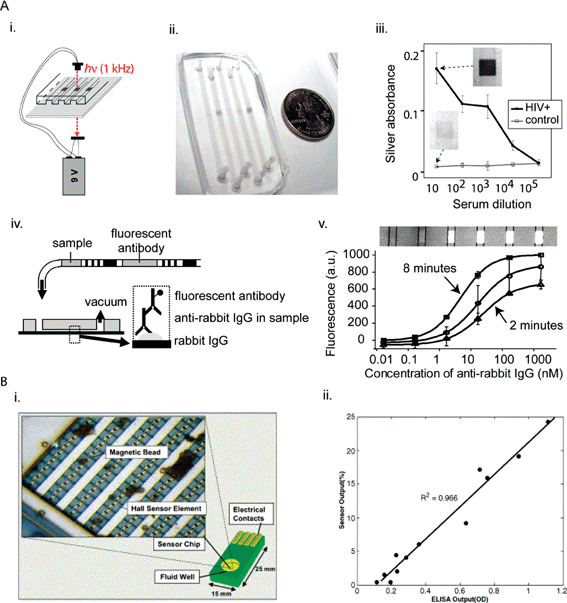 | ||
| Fig. 3 Simple and low-cost LOC methods for detecting proteins in developing countries. (A) Optical detection of proteins and reagent storage and delivery. (i) Schematic representation of the POCKET immunoassay powered by a 9 V battery. (ii) Actual device. (iii) Apparent silver absorbance values of anti-HIV-1 antibodies from HIV-positive patients and control patients. (iv) Schematic representation of reagent-loaded cartridges. (v) Overlay of fluorescence and brightfield images of the immunoreaction area, with fluorescent signal corresponding to presence of labeled detection antibodies on antigen stripes. The concentrations indicated above the picture refer to the concentration of sample tested in each microchannel. Reprinted from ref. 32 with permission from ACS Publications. (B) Immunomagnetic separation and detection of proteins with CMOS Hall sensors. (i) Schematic representation with inset showing actual chip. (ii) Comparison of the outputs of CMOS chip and ELISA. Reprinted from ref. 78 with permission from Elsevier. | ||
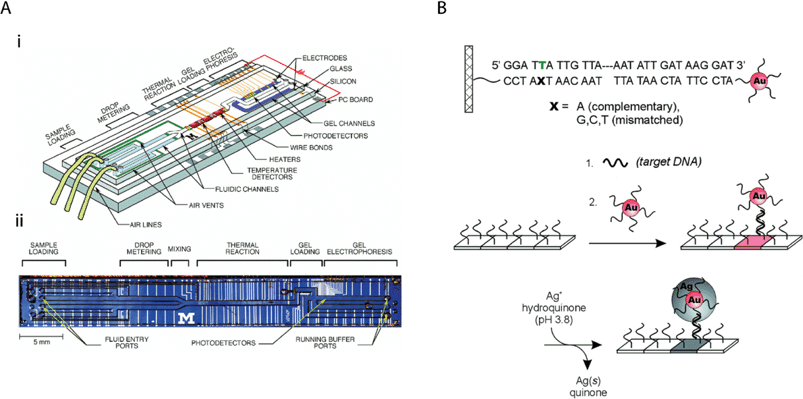 | ||
| Fig. 4 LOC methods for detecting nucleic acids that can be adapted for use in developing countries. (A) Integrated nanolitre DNA analysis device. (i) Schematic representation with two liquid samples and electrophoresis gel present. (ii) Optical micrograph of device. Reprinted with permission from ref. 90. Copyright 1998 AAAS. (B) Schematic representation of oligonucleotide-conjugated nanoparticles for probing DNA sequence arrays. Reprinted with permission from ref. 50. Copyright 2000 AAAS. | ||
All common fluid handling steps required for protein assays, including actuation and control of fluid flow, have been demonstrated on centrifugal microfluidic devices.68,69 For example, the Bioaffy chip from the company Gyros can quantify clinical markers such as α-fetoprotein, interleukin-6 and carcinoembryonic antigen down to low pM concentrations.70 Also, Bernard and colleagues used a common compact-disc player as a digital detector of silver-based signals of C-reactive protein in immunoassays;71 the simplicity of this overall format makes it attractive for use in resource-poor settings.72
For high-sensitivity assays, Mirkin and co-workers have reported an assay based on DNA-coated nanoparticles, magnetic separation, and PCR to detect protein markers at up to 6 orders of magnitude in improvement in the limit of detection compared to conventional assays.73 This format can in principle be adapted to a low-cost LOC device. High-sensitivity and quantitative assays can greatly benefit certain applications, including the measurement of levels of HIV-1 p24, a viral protein antigen which has been advocated as a potential simpler alternative to viral load or CD4 counting for monitoring AIDS progression in HIV patients and diagnosing HIV/AIDS in newborns.74
With appropriate amplification schemes, surface plasmon resonance (SPR), which detects at the surface minute changes in the index of refraction induced by the binding of molecule, can approach the sensitivity of ELISA.75 The company Texas Instruments Sensors & Controls (now renamed Sensata Technologies) has developed a portable SPR sensor (named Spreeta) for heterogeneous antibody-antigen binding and solid-phase DNA hybridization; this disposable device is designed to be manufacturable in very large quantities.76 The device can be integrated with a temperature-controlled instrument that runs on a 12 V battery to detect enterotoxin B in urine, milk, and sea water, with a sensitivity in the fM range.77 Currently, a Spreeta evaluation module is available at $200 per sensor (http://aigproducts.com/surface_plasmon_resonance/spr_evaluation_module.htm). Since the gold-coated chip could be regenerated up to 80 times before disposal, each assay can potentially be priced below $1 in the future.77
Boser, Harris, and colleagues developed on a 2.5 × 2.5 mm2 CMOS chip an array of Hall sensors to quantify the number of magnetic beads associated to immunocomplexes at the surface of the sensor (Fig. 3B).78 The use of magnetic beads facilitated removal of unbound antibodies conjugated to magnetic beads, and produced signals at the surface that were recorded by the Hall sensors. The average reading from 120 sensors was sufficient to quantify dengue fever antibodies in clinical serum samples with a good correlation compared to ELISA assays. When combined with proper fluidic control, this method can potentially be developed into an integrated simple and low-cost LOC device for immunoassays.
4.3 Nucleic acids
Analysis of nucleic acids offers powerful diagnostic information that complement protein analysis of antigens and antibodies. For example, by analyzing conserved DNA or viral RNA sequences, PCR and RT-PCR can be used to specifically detect infectious diseases important in developing countries (such as HIV/AIDS, hepatitis B and C, and TB).79,80 For HIV/AIDS, quantitative measurements of RNA levels (based on amplification of the 5′-long terminal repeat) provide information on the stage of diseases; as such, low-cost methods for PCR have been studied for use in developing countries.81–83As a technology, nucleic acid detection can be very sensitive due to amplification, and specific due to the intrinsic complementarity of the base-pairing interactions. Nevertheless, the building of an integrated LOC device for detecting nucleic acids is typically more challenging than for proteins. Overall, there are at least three LOC design issues for nucleic acid detection.
A number of groups have successfully integrated sample pre-treatment with analysis.84,87 For example, Quake's group used valves to automate cell isolation, cell lysis, nucleic acid purification, and analysis on the same microchip.86 Integration of sample pre-treatment with analysis is important to achieve ease of use, as well as to improve sensitivity by reducing sample losses in between steps. For example, integration of cell capture, cell lysis, mRNA purification, cDNA synthesis, and cDNA purification has been demonstrated for a RT-PCR microfluidic chip.88 Grodzinski and co-workers have developed a self-contained device in plastic that integrates sample preparation, cell capture, cell preconcentration and purification, cell lysis, PCR, DNA hybridization, and electrochemical detection to analyze DNA from pathogenic bacteria.89 In addition, metering samples is important to automate sample preparation,90 an important consideration in remote settings.
PCR/RT-PCR. Miniaturizing PCR on LOC devices has the potential to reduce the cost of reagents, speed up analysis, and automate the procedure for use in remote settings by integrating multiple functionalities such as cell concentration and lysis, DNA extraction, removal of PCR inhibitors, amplification of DNA, and separation and detection of the amplified products of interest.84 In the most straightforward adaptation of conventional PCR into LOC devices, a microchamber or microwell can be created in which the sample and PCR reaction mixture are thermally cycled (Fig. 4A). In one of the first studies by Burns and colleagues (which featured microfluidic channels, mixers, heaters, temperature sensors, and fluorescence detectors90), the low voltages and power suggested that hand-held battery operation is feasible; this technology is now being commercialized by the company HandyLab. Although directed more for biodefense than global health, the company Cepheid has developed a miniature analytical thermal cycling instrument (MATCI) that consists of silicon-micromachined reaction chambers with integrated heaters, optical windows, and diode-based fluorescence detection.22,91 Although the current size of these instruments may be too large for use in remote testing, they may be appropriate for centralized testing centers in developing countries. Similarly, since most LOC devices that use well-based PCR require bulky instruments as well as expensive and complex manufacturing, they may be most appropriate for use in centralized testing centers.92–94 The design of disposable micro-PCR devices on polycarbonate plastic95 may ultimately be suitable for use in resource-poor settings, although it currently lacks extensive integration.
In contrast to well-based LOC PCR, continuous-flow PCR systems operate by passing a sample continuously over regions of different temperatures. Continuous-flow systems offer flexible design geometry (for changing the number of amplification cycles), and fast transition times for heating and cooling (which depend on the flow rate and kinetics for reaching thermal equilibrium).84,96 Another design that is potentially simple to manufacture and simple to use include passive reactors in closed-loop designs that operate without valves.97 An interesting novel scheme for PCR amplification takes advantage of convective flow inside a Rayleigh–Bénard cavity to yield comparable performance to conventional PCR;98 because this system requires only a single heating element held at a fixed temperature, it can potentially be made low cost, although design challenges exist in adapting the system to a LOC device.84
Isothermal. The need for temperature cycling in PCR has made it challenging to build low-cost and simple devices suitable for point-of-care testing. An exciting development that bypasses thermocycling is isothermal DNA amplification, which includes techniques such as single-strand displacement amplification, rolling circle amplification, and ligase chain reaction.84 Harrison and co-workers have integrated isothermal amplification in an electrokinetic LOC device that used cycling probe technology to amplify a DNA sequence from S. aureus.99 More recently, Zhang and co-workers demonstrated loop-mediated isothermal amplification (LAMP) in a cross-shaped microfluidic system in PMMA.100 Also, Gulliksen and colleagues used an isothermal amplification method in a microfluidic device made of cyclic olefin copolymer for multiplexed detection of human papilloma virus at-the-point-of-care application.101 In the future, other schemes (such as helicase-dependent DNA amplification102) may be integrated into LOC devices.
A potentially low-cost and sensitive detection system for nucleic acids that may be appropriate for resource-poor settings is oligonucleotide-conjugated nanoparticle probes, as demonstrated by Mirkin and others (and now commercialized by the company Nanosphere) (Fig. 4B). Coupled with silver reduction amplification, they can be quantitated by an inexpensive scanometric reader,50 and potentially by a low-cost and portable reader.44 These nanoparticles, when reduced to form a micron-sized metallic bridge across an electrode gap, can also result in quantifiable changes in conductivity,73 which can in principle be measured by a low-cost and portable conductivity meter. Other electrochemistry-based methods for detecting DNA have been reported.107 Simple detection of amplified nucleic acid products with a dipstick method for resource-poor settings has been demonstrated.60,108
4.4 Cells
Analysis and counting of cells are important for diseases such as anaemia and hematology (via erythrocyte and complete blood counts), as well as for monitoring the progression of AIDS. Flow cytometry, the current standard for cell analysis and counting, can measure up to 10 or more cell properties and separate and isolate cells at rates up to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells per second without loss of viability.109 Since conventional flow cytometers are bulky, expensive, and mechanically complex, they are currently limited to well-financed centralized testing centers.
000 cells per second without loss of viability.109 Since conventional flow cytometers are bulky, expensive, and mechanically complex, they are currently limited to well-financed centralized testing centers.
Due mainly to the importance of counting CD4+ lymphocytes for monitoring the progression of AIDS, a number of initiatives have started to support the development of an inexpensive and compact device for cell counting for global health. In one non-LOC method, Mwaba and colleagues used filter papers to store dried blood samples, which were transported to a centralized facility for ELISA testing using anti-CD4 antibodies.110 The results of this simple technology were encouraging but exhibited limitations in accuracy. With support from the Gates Foundation, Imperial College London is supporting the development of a simple, low-cost, and semi-quantitative CD4+ lymphocyte-counting device (http://www1.imperial.ac.uk/medicine/about/divisions/medicine/infectious_diseases/cd4_initiative/) that exhibits cut-offs at 200, 350, 500 cells mm−3 with 10% coefficient of variation. Perhaps more so than simple membrane-based tests, LOC devices have the potential to meet these targets due to their increased versatility in design and enhanced analytical performance.
A different approach to count cells takes advantage of the relatively large size of cells (compared to the scale of microfabrication techniques) by capturing them for analysis. For example, in an initial study based on the capture of microbeads, McDevitt and colleagues micromachined arrays of pyramidal cavities on silicon wafers; these cavities housed microspheres that produced optical changes in the presence of analytes.112 Subsequently, Rodriguez, Walker, and colleagues adapted this device (by adding a polycarbonate, track-etch filter that selectively trapped lymphocytes and not red blood cells) to capture and measure the levels of CD4+ lymphocytes from blood samples in Botswana (Fig. 5).113 The results were in good agreement with those obtained with a conventional flow cytometer. This device (now being commercialized by the company LabNow) is potentially cheaper than other available systems for single-purpose flow cytometry114 and microbead separation; because of the cost, bulkiness, and power requirements of an epifluorescence microscope and a camera, however, the current system is likely most suitable for centralized testing centers rather than remote point-of-care testing. Other inexpensive flow cytometric methods have been developed, including capabilities for multiplexing.115
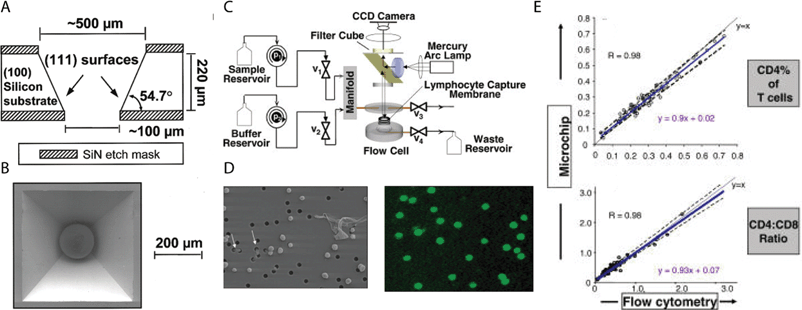 | ||
| Fig. 5 A low-cost LOC device for counting CD4+ lymphocytes. (A) Schematic representation of pyramidal wells in Si. (B) Scanning electron micrograph of single well with microbead. Reprinted from ref. 112 with permission from ACS publications. (C) Schematic representation of the device system. (D) (Left) Transmission image of membrane flow cell showing selective capture of lymphocytes. Holes are 3 µm in diameter. (Right) Fluorescent antibody staining of lymphocytes. (E) Results of cell counting from microchip versus flow cytometry. Reprinted from ref. 113. | ||
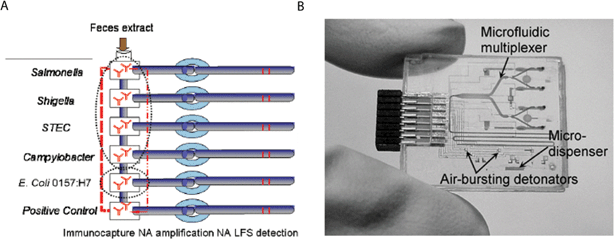 | ||
| Fig. 6 Integrated LOC devices with potential for use in developing countries. (A) Schematic representation of LOC for detecting enteric diseases. Reprinted from ref. 60 with permission from the International Society for Optical Engineering. (B) Picture of a plastic LOC device for point-of-care clinical diagnostics. Reprinted from ref. 139 with permission from the Proceedings of the IEEE. | ||
A third approach for capturing and counting cells is immunomagnetic separation, which typically uses antibody-conjugated superparamagnetic beads to isolate the cells of interest. Tibbe and co-workers used antibody-labeled ferromagnetic nanoparticles to perform a differential white blood cell count (with analysis of neutrophils, lymphocytes, monocytes, and eosinophils).116 In a magnetic field, immunomagnetic cells are aligned in a capillary along the magnetic field lines for fluorescent analysis. This design functions with potentially simple fluidic control, but still requires a conventional fluorescence imaging system.
Cell counting has important applications beyond the monitoring of HIV/AIDS progression. For example, McDevitt and colleagues adapted their microbead system to build a multifunctional LOC device for performing leukocyte counts and measuring C-reactive protein levels, two important indicators of coronary heart disease.117 Like the CD4+-lymphocyte counting device, this device may be useful in centralized testing centers in developing countries (in this case, to address the increasing incidences of cardiovascular disease, which constitutes 9.5% of total DALYs in developing countries). In a different application, Chiu and colleagues used a microfluidic device to show decreased deformability in erythrocytes when infected with P. falciparum.118 Although this study did not focus on a diagnostic application, it suggests a potential route for diagnosing and monitoring malaria infection using a LOC device. For example, in a study by Gascoyne and colleagues in Thailand using dielectrophoresis and field-flow fractionation, parasitized erythrocytes eluted more quickly than normal erythrocytes.119
Another promising cell-based application of LOC devices for global health is the miniaturization of microbiological culture assays. Currently, conventional microbiological techniques are used to identify drug-resistant bacterial strains; this identification is critical for administering efficacious therapy for TB patients. Techniques in culturing bacteria in microfluidic chips120–122 can potentially automate this laborious process (and other microbiological assays involving differential cultures) even in remote settings. Devices that culture and analyze pathogens and cells in microfluidic devices could moreover be adapted to perform diagnostic procedures that are conventionally microscope-based (such as blood smears for diagnosis of malaria). Low-cost microscopy using optofluidics, if it can be made robust, may be an attractive technique in resource-poor settings.123 The power of LOC technologies can potentially be augmented with genetic and molecular biological approaches, such as Jacobs' clever luminometric technology for low-cost TB diagnosis using luciferase-reporter phage124 (now commercialized by the company Sequella).
4.5 Clinical chemistry
Currently, the most popular LOC technologies for analyzing electrolytes are based on electrochemical detection. An active area of research in this field is potentiometric sensing using ion-selective field-effect transistors (ISFET); ISFETs, however often require a large reference electrode.27 Nevertheless, integration of electrochemical detection and semiconductor technologies have resulted in commercial products, such as the iSTAT from Abbott Diagnostics.20 This device is a portable blood analyzer that uses microfabricated thin-film electrodes to measure levels of electrolytes (Na+, K+, Cl−, Ca2+), general chemistries (pH, urea, glucose), blood gases (pCO2, pO2), and hematology (hematocrit). The electrochemical detection system includes amperometry, voltammetry, and conductance, depending on the analyte.Despite this important achievement, there are limitations of MEMS devices that feature electrochemical detection. The lack of suitable manufacturing facilities makes it expensive and, for some devices, impractical to scale up the manufacturing of the sensor. Although it is in principle possible to leverage existing microelectronics fabrication facilities for the manufacturing of biological and chemical sensors, there exist a number of challenging issues, such as differences in dimensions (below 1 µm for microelectronics and above 5 µm for sensors), in thickness and type of gate insulator, in materials (e.g. for conductors, Al, polysilicon, and Cu for electronics, versus Au and Pt in sensors), and in the passivation layer (e.g. high-density silicon nitride for sensors that are exposed to solutions).27,125,126 Although their current design still relied on a bulky instrument for fluid actuation and detection, Madou, Bachas and colleagues developed a LOC device that measured electrolyte levels optically using optodes;127 in the future, it may be possible to design a low-cost, integrated, optical device for electrolyte measurements.128
4.6 Non-diagnostics applications of LOC devices in developing countries
Although health diagnostics is the nearest-term application of LOC devices in developing countries, other important areas of global health can benefit in the future from this technology. For example, LOC diagnostic devices can be used for environmental sensing and monitoring.60 Sandia Labs, for example, is developing a portable device to monitor water quality by detecting pathogenic bacteria and toxins.129In addition to diagnostics and sensors, LOC technologies can be used to promote the development of therapeutic compounds. The use of LOC technologies (through high-throughput drug screening, genomics, and proteomics) for drug discovery has been reviewed in detail elsewhere;130 as research into drug discovery for neglected diseases increases (in universities and industrial companies such as the Novartis Institute for Tropical Diseases), LOC technologies can play an increasingly important role. More broadly, Singer and colleagues have investigated the potential contribution of biotechnology, genomics, and nanotechnology to address issues in global health and development;1,131,132 LOC technology has a clear potential to address these issues through its close association with all these research fields.
Even when drugs (and vaccines) are available, simple needle-free delivery into the patient is an important challenge in developing countries.2 Current approaches for improving drug delivery in developing countries include inhalation and oral delivery, and are a focus of a non-profit organization (Medicine in Need) based on the work of Edwards et al.133 Langer and colleagues have developed LOC devices for programmed time-release of drugs.134,135 In the long term, these technologies can conceivably be combined with other miniaturized medical devices (such as wireless capsule endoscope) for use in health centers without large infrastructure.
5 Conclusion
There is a pressing need for new health technologies for diagnosing and treating communicable and non-communicable diseases in developing countries. The LOC field is well-positioned to contribute to this challenge by leveraging recent advances in integrated devices for use in settings with low or moderate resources (such as point-of-care health testing, military sensors, and extraterrestrial devices), and through advances in the young but growing field of LOC devices for developing countries.What will be the roadmap in the near future for designing and deploying LOC devices in developing countries? First and foremost, new devices will be needed (Fig. 6); the design criteria of these devices are vast, demanding, and context-dependent, and they will need to be considered carefully from the early stages of development. Beyond the scientific challenges, successful deployment of the device in developing countries will involve a complex interplay of political and socioeconomic considerations.136 Scientists will therefore need to work closely with members from NGOs and local governments as early as possible (and well before a field-testable device is ready) to ensure proper distribution channels (Table 4 and Table 5). Since much of the work in engineering and development will best take place in the industrial rather than academic setting, the private sector has a significant role to play as well (Table 3). As an example of the importance of such multidisciplinary efforts, the Bill and Melinda Gates Foundation is supporting a consortium of academic researchers (Yager and co-workers), industry (Micronics, Nanogen, and Invetech), and an NGO (PATH) to develop a multifunctional LOC device for infectious diseases137 (Fig. 6A), and another consortium of academic researchers (Kelso and co-workers) and industry (Abbott and Inverness Medical Innovations) to develop a low-cost diagnostic device. Finally, potential obstacles can be anticipated from studies on the introduction and dissemination of LOC devices in developed countries; these obstacles include cost per test and reliability (see the market study FlowMap, available at http://www.microfluidics-roadmap.com/).
| Company | Website | Focus | Funding source |
|---|---|---|---|
| Cepheid | http://www.cepheid.com/ | DNA (TB) | FIND, US gov. |
| Claros Diagnostics | http://www.clarosdx.com/ | Proteins | Private |
| HandyLab | http://www.handylab.com/ | DNA, proteins | NIST, private |
| iStat | http://www.istat.com/ | Clinical chemistry markers | Private |
| LabNow | http://www.labnow.com/ | CD4 for HIV/AIDS | George Soros, private |
| Micronics | http://www.micronics.net/ | Enteric disease pathogens | PATH, University of Washington, NIH, Gates Foundation |
| Nanogen | http://www.nanogen.com/ | Cardiac biomarkers, DNA/RNA | Private |
| Nanosphere | http://www.nanosphere-inc.com/ | DNA, proteins | NIAID, NIH, private |
| Sensata | http://www.sensata.com/ | Proteins, viruses, bacteria | Private |
| Sequella | http://www.sequella.com/ | Proteins (TB) | Private |
| NGOs | Website | Focus | Year Founded |
|---|---|---|---|
| a Abbreviation: sexually transmitted infections (STIs). | |||
| FIND | http://www.finddiagnostics.org/ | TB | 2003 |
| PATH | http://www.path.org/ | Diarrheal diseases, malaria, STIs, AIDS, and cervical cancer | 1977 |
| Tuberculosis Diagnostics Initiative | http://www.who.int/tdr/diseases/tb/tbdi.htm | TB | 1996 |
| WHO Malaria Rapid Diagnostics Tests | http://www.wpro.who.int/sites/rdt | Malaria | 2005 |
| WHO Sexually Transmitted Diseases Diagnostic Initiative | http://www.who.int/std_diagnostics/ | Chlamydia, gonorrhea, syphilis | 2001 |
| WHO/TDR | http://www.who.int/tdr/ | TB, AIDS, malaria, STIsa | 1975 |
| Organization | Website | Focus |
|---|---|---|
| a Abbreviation: immunochromatographic tests (ICTs). | ||
| Binax, Inc. | http://www.binax.com/ | ICTsa for malaria, influenza, filariasis, pneumonia, strep A |
| Gede Foundation | http://www.gedefoundation.org/ | AIDS-related diagnostics (flow cytometry, real-time PCR, hematology analyzer, genotyping services) |
| Human GmbH | http://www.human.de/ | Various (clinical chemistry and hematology analyzer, microscopy, ELISA) |
| Partec | http://www.partec.de/ | Flow cytometry |
| Sustainable Sciences Initiative | http://www.ssilink.org/ | Various (low-cost PCR, antibody-detection) |
In the long term, miniaturization of medical technologies has the potential to improve public health, and perhaps even change the basic methods by which patients are diagnosed and treated, in developing countries. This pathway has a well-known precedent in information and communication technologies. Over the last 10 years in developing countries, adoption of cell phones and wireless internet has resulted in ‘leapfrogging’ over conventional communication technologies (such as landlines) that require significant infrastructure.52 In the next 10 years, will LOC methods follow a similar path, by promoting in developing countries a leapfrog over conventional medical technologies (such as radiology, microbiological culture, and centralized testing centers)?
Acknowledgements
We acknowledge Roberto Delatour from Medecins Sans Frontieres and George Whitesides for helpful discussions, Benjamin Wang for help on the figures, and Andreas Martinez for help in compiling Table 1. This work was supported by an Early Career Award from the Wallace H. Coulter Foundation.References
- A. S. Daar, H. Thorsteinsdottir, D. K. Martin, A. C. Smith, S. Nast and P. A. Singer, Nat. Genet., 2002, 32, 229–232 CrossRef CAS.
- H. Varmus, R. Klausner, E. Zerhouni, T. Acharya, A. S. Daar and P. A. Singer, Science, 2003, 302, 398–399 CrossRef CAS.
- A. Alwan and B. Modell, Nat. Rev. Genet., 2003, 4, 61–68 CrossRef CAS.
- J. Sachs, Macroeconomics and Health: Investing in Health for Economic Development, World Health Organization, Geneva, Switzerland, 2001 Search PubMed.
- R. Laxminarayan, A. J. Mills, J. G. Breman, A. R. Measham, G. Alleyne, M. Claeson, P. Jha, P. Musgrove, J. Chow, S. Shahid-Salles and D. T. Jamison, Lancet, 2006, 367, 1193–1208 CrossRef.
- B. H. Robertson and J. K. A. Nicholson, Annu. Rev. Public Health, 2005, 26, 281–302 CrossRef.
- C. J. L. Murray and A. D. Lopez, Lancet, 1997, 349, 1498–1504 CrossRef CAS.
- C. J. L. Murray and A. D. Lopez, Lancet, 1997, 349, 1436–1442 CrossRef CAS.
- World Bank World Development Report 1993: Investing in Health, Oxford University Press, New York, NY, 1993 Search PubMed.
- A. Lopez, C. Mathers, M. Ezzati, D. Jamison and C. Murray, Global Burden of Disease and Risk Factors, Oxford University Press and the World Bank, New York, NY, 2006 Search PubMed.
- D. H. Molyneux, P. J. Hotez and A. Fenwick, PLoS Med., 2005, 2, 1064–1070 Search PubMed.
- E. Torreele, C. Royce, R. Don, A. M. Sevcsik and S. Croft, PLoS Med., 2006, 3, e282 Search PubMed.
- P. J. Hotez, D. H. Molyneux, A. Fenwick, E. Ottesen, S. Ehrlich Sachs and J. D. Sachs, PLoS Med., 2006, 3, e102 Search PubMed.
- D. Mabey, R. W. Peeling, A. Ustianowski and M. D. Perkins, Nat. Rev. Microbiol., 2004, 2, 231–240 Search PubMed.
- H. H. Lee and J. P. Allain, Vox Sang., 2004, 87, 176–179 CrossRef.
- R. Beaglehole and D. Yach, Lancet, 2003, 362, 903–908 CrossRef CAS.
- J. C. Seidell, Br. J. Nutr., 2000, 83, S5–S8 CAS.
- J. L. Deen, T. Vos, S. R. Huttly and J. Tulloch, Bull. W. H. O., 1999, 77, 518–524 CAS.
- S. N. Forjuoh and G. Li, Soc. Sci. Med, 1996, 43, 1551–1560 CrossRef CAS.
- I. R. Lauks, Acc. Chem. Res., 1998, 31, 317–324 CrossRef CAS.
- A. J. Tudos, G. A. J. Besselink and R. B. M. Schasfoort, Lab Chip, 2001, 1, 83–95 RSC.
- P. Belgrader, S. Young, B. Yuan, M. Primeau, L. A. Christel, F. Pourahmadi and M. A. Northrup, Anal. Chem., 2001, 73, 391–391 CrossRef CAS.
- C. T. Culbertson, Y. Tugnawat, A. R. Meyer, G. T. Roman, J. M. Ramsey and S. R. Gonda, Anal. Chem., 2005, 77, 7933–7940 CrossRef CAS.
- A. M. Skelley, J. R. Scherer, A. D. Aubrey, W. H. Grover, R. H. C. Ivester, P. Ehrenfreund, F. J. Grunthaner, J. L. Bada and R. A. Mathies, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 1041–1046 CrossRef CAS.
- T. Akiyama, S. Gautsch, N. F. de Rooij, U. Staufer, P. Niedermann, L. Howald, D. Muller, A. Tonin, H. R. Hidber, W. T. Pike and M. H. Hecht, Sens. Actuators, A, 2001, 91, 321–325 CrossRef.
- H. Becker and C. Gartner, Electrophoresis, 2000, 21, 12–26 CrossRef CAS.
- J. Janata, Proc. IEEE, 2003, 91, 864–869 CrossRef CAS.
- A. J. Ricco, T. D. Boone, Z. H. Fan, I. Gibbons, T. Matray, S. Singh, H. Tan, T. Tian and S. J. Williams, Biochem. Soc. Trans., 2002, 30, 73–78 CAS.
- A. de Mello, Lab Chip, 2002, 2, 31N–36N RSC.
- K. R. Hawkins and P. Yager, Lab Chip, 2003, 3, 248–252 RSC.
- E. Garcia, J. R. Kirkham, A. V. Hatch, K. R. Hawkins and P. Yager, Lab Chip, 2004, 4, 78–82 RSC.
- V. Linder, S. K. Sia and G. M. Whitesides, Anal. Chem., 2005, 77, 64–71 CrossRef CAS.
- J. Moorthy, G. A. Mensing, D. Kim, S Mohanty, D. T. Eddington, W. H. Tepp, E. A. Johnson and D. J. Beebe, Electrophoresis, 2004, 25, 1705–1713 CrossRef CAS.
- Y. Li, P. Denny, C. M. Ho, C. Montemagno, W. Shi, F. Qi, B. Wu, L. Wolinsky and D. T. Wong, Adv. Dent. Res., 2005, 18, 3–5 Search PubMed.
- V. Srinivasan, V. K. Pamula and R. B. Fair, Lab Chip, 2004, 4, 310–315 RSC.
- A. J. de Mello and N. Beard, Lab Chip, 2003, 3, 11N–19N RSC.
- S. S. Shevkoplyas, T. Yoshida, L. L. Munn and M. W. Bitensky, Anal. Chem., 2005, 77, 933–937 CrossRef CAS.
- J. P. Brody and P. Yager, Sens. Actuators, A, 1997, 58, 13–18 CrossRef.
- S. Song, A. K. Singh, T. J. Shepodd and B. J. Kirby, Anal. Chem., 2004, 76, 2367–2373 CrossRef CAS.
- S. Song and A. K. Singh, Anal. Bioanal. Chem., 2006, 384, 41–43 CAS.
- R. H. Liu, M. A. Stremler, K. V. Sharp, M. G. Olsen, J. G. Santiago, R. J. Adrian, H. Aref and D. J. Beebe, J. Microelectromech. Syst., 2000, 9, 190–197 CrossRef.
- T. Rohr, C. Yu, M. H. Davey, F. Svec and J. M. J. Frechet, Electrophoresis, 2001, 22, 3959–3967 CrossRef CAS.
- P. Sethu, L. L. Moldawer, M. N. Mindrinos, P. O. Scumpia, C. L. Tannahill, J. Wilhelmy, P. A. Efron, B. H. Brownstein, R. G. Tompkins and M. Toner, Anal. Chem., 2006, 78, 5453–5461 CrossRef CAS.
- S. K. Sia, V. Linder, B. A. Parviz, A. Siegel and G. M. Whitesides, Angew. Chem., Int. Ed., 2004, 43, 498–502 CrossRef CAS.
- D. Juncker, H. Schmid, U. Drechsler, H. Wolf, M. Wolf, B. Michel, N. de Rooij and E. Delamarche, Anal. Chem., 2002, 74, 6139–6144 CrossRef CAS.
- D. L. L. Chen and R. F. Ismagilov, Curr. Opin. Chem. Biol., 2006, 10, 226–231 CrossRef CAS.
- S. Cesaro-Tadic, G. Dernick, D. Juncker, G. Buurman, H. Kropshofer, B. Michel, C. Fattinger and E. Delamarche, Lab Chip, 2004, 4, 563–569 RSC.
- S. K. Sia and G. M. Whitesides, Electrophoresis, 2003, 24, 3563–3576 CrossRef CAS.
- X. Jiang, J. M. Ng, A. D. Stroock, S. K. Dertinger and G. M. Whitesides, J. Am. Chem. Soc., 2003, 125, 5294–5295 CrossRef CAS.
- T. A. Taton, C. A. Mirkin and R. L. Letsinger, Science, 2000, 289, 1757–1760 CrossRef CAS.
- S. J. Park, T. A. Taton and C. A. Mirkin, Science, 2002, 295, 1503–1506 CrossRef CAS.
- United Nations Development Programme, Human Development Report 2001: Making New Technologies Work for Human Development, Oxford University Press, New York, NY, 2001 Search PubMed.
- S. L. Hart and C. M. Christensen, MIT Sloan Manage. Rev., 2002, 44, 51–56 Search PubMed.
- M. Usdin, M. Guillerm and P. Chirac, Nature, 2006, 441, 283–284 CrossRef CAS.
- M. Cheesbrough, District Laboratory Practice in Tropical Countries, Part 1, Cambridge University Press, Cambridge, UK, 2000 Search PubMed.
- M. Cheesbrough, District Laboratory Practice in Tropical Countries, Part 2, Cambridge University Press, Cambridge, UK, 2000 Search PubMed.
- D. D. Cunningham, Anal. Chim. Acta, 2001, 429, 1–18 CrossRef CAS.
- C. E. Michel, A. W. Solomon, J. P. Magbanua, P. A. Massae, L. Huang, J. Mosha, S. K. West, E. C. Nadala, R. Bailey, C. Wisniewski, D. C. Mabey and H. H. Lee, Lancet, 2006, 367, 1585–1590 CrossRef.
- N. Christodoulides, S. Mohanty, C. S. Miller, M. C. Langub, P. N. Floriano, P. Dharshan, M. F. Ali, B. Bernard, D. Romanovicz, E. Anslyn, P. C. Fox and J. T. McDevitt, Lab Chip, 2005, 5, 261–269 RSC.
- B. H. Weigl, J. Gerdes, P. Tarr, P. Yager, L. Dillman, R. Peck, S. Ramachandran, M. Lemba, M. Kokoris, M. Nabavi, F. Battrell, D. Hoekstra, E. J. Klein and D. M. Denno, Proc. SPIE—Int. Soc. Opt. Eng., 2006, 6112, 1–11.
- A. D. Stroock, S. K. W. Dertinger, A. Ajdari, I. Mezic, H. A. Stone and G. M. Whitesides, Science, 2002, 295, 647–651 CrossRef CAS.
- P. Garstecki, J. F. M, M. A. Fischbach, S. K. Sia and G. M. Whitesides, Lab Chip, 2006, 6, 207–212 RSC.
- A. P. Sudarsan and V. M. Ugaz, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 7228–7233 CrossRef CAS.
- A. Bernard, B. Michel and E. Delamarche, Anal. Chem., 2001, 73, 8–12 CrossRef CAS.
- M. Wolf, D. Juncker, B. Michel, P. Hunziker and E. Delamarche, Biosens. Bioelectron., 2004, 19, 1193–1202 CrossRef CAS.
- D. B. Weibel, M. Kruithof, S. Potenta, S. K. Sia, A. Lee and G. M. Whitesides, Anal. Chem., 2005, 77, 4726–4733 CrossRef CAS.
- E. Garcia, J. R. Kirkham, A. V. Hatch, K. R. Hawkins and P. Yager, Lab Chip, 2003, 4, 78–82 Search PubMed.
- J. V. Zoval and M. J. Madou, Proc. IEEE, 2004, 92, 140–153 CrossRef CAS.
- M. J. Pugia, G. Blankenstein, R. P. Peters, J. A. Profitt, K. Kadel, T. Willms, R. Sommer, H. H. Kuo and L. S. Schulman, Clin. Chem., 2005, 51, 1923–1932 CrossRef CAS.
- N. Honda, U. Lindberg, P. Andersson, S. Hoffman and H. Takei, Clin. Chem., 2005, 51, 1955–1961 CrossRef CAS.
- S. A. Lange, G. Roth, S. Wittemann, T. Lacoste, A. Vetter, J. Grassle, S. Kopta, M. Kolleck, B. Breitinger, M. Wick, J. K. Horber, S. Dubel and A. Bernard, Angew. Chem., Int. Ed., 2005, 45, 270–273.
- F. S. Ligler and J. S. Erickson, Nature, 2006, 440, 159–160 CrossRef CAS.
- J. M. Nam, C. S. Thaxton and C. A. Mirkin, Science, 2003, 301, 1884–1886 CrossRef CAS.
- J. Schupbach, AIDS Rev., 2002, 4, 83–92 Search PubMed.
- J. Homola, S. S. Yee and G. Gauglitz, Sens. Actuators, B, 1999, 54, 3–15 CrossRef.
- T. M. Chinowsky, J. G. Quinn, D. U. Bartholomew, R. Kaiser and J. L. Elkind, Sens. Actuators, B, 2003, 91, 266–274 CrossRef.
- A. N. Naimushin, S. D. Soelberg, D. K. Nguyen, L. Dunlap, D. Bartholomew, J. Elkind, J. Melendez and C. E. Furlong, Biosens. Bioelectron., 2002, 17, 573–584 CrossRef CAS.
- T. Aytur, J. Foley, M. Anwar, B. Boser, E. Harris and R. Beatty, J. Immunol. Methods, 2006, 314(1–2), 21–29 CrossRef CAS.
- J. A. M. Vet, A. R. Majithia, S. A. E. Marras, S. Tyagi, S. Dube, B. J. Poiesz and F. R. Kramer, Proc. Natl. Acad. Sci. U. S. A., 1999, 96, 6394–6399 CrossRef CAS.
- D. Candotti, J. Temple, S. Owusu-Ofori and J. P. Allain, J. Virol. Methods, 2004, 118, 39–47 CrossRef CAS.
- C. Drosten, M. Panning, J. F. Drexler, F. Hansel, C. Pedroso, J. Yeats, L. K. de Souza Luna, M. Samuel, B. Liedigk, U. Lippert, M. Sturmer, H. W. Doerr, C. Brites and W. Preiser, Clin. Chem., 2006, 52, 1258–1266 CrossRef CAS.
- F. Rouet, D. K. Ekouevi, M. L. Chaix, M. Burgard, A. Inwoley, T. D. Tony, C. Danel, X. Anglaret, V. Leroy, P. Msellati, F. Dabis and C. Rouzioux, J. Clin. Microbiol., 2005, 43, 2709–2717 CrossRef CAS.
- J. Coloma and E. Harris, Br. Med. J., 2004, 329, 1160–1162 CrossRef.
- P. A. Auroux, Y. Koc, A. deMello, A. Manz and P. J. R. Day, Lab Chip, 2004, 4, 534–546 RSC.
- L. C. Waters, S. C. Jacobson, N. Kroutchinina, J. Khandurina, R. S. Foote and J. M. Ramsey, Anal. Chem., 1998, 70, 158–162 CrossRef CAS.
- J. W. Hong, V. Studer, G. Hang, W. F. Anderson and S. R. Quake, Nat. Biotechnol., 2004, 22, 435–439 CrossRef CAS.
- Y. Huang, E. L. Mather, J. L. Bell and M. Madou, Anal. Bioanal. Chem., 2002, 372, 49–65 CrossRef CAS.
- J. S. Marcus, W. F. Anderson and S. R. Quake, Anal. Chem., 2006, 78, 3084–3089 CrossRef CAS.
- R. H. Liu, J. N. Yang, R. Lenigk, J. Bonanno and P. Grodzinski, Anal. Chem., 2004, 76, 1824–1831 CrossRef CAS.
- M. A. Burns, B. N. Johnson, S. N. Brahmasandra, K. Handique, J. R. Webster, M. Krishnan, T. S. Sammarco, P. M. Man, D. Jones, D. Heldsinger, C. H. Mastrangelo and D. T. Burke, Science, 1998, 282, 484–487 CrossRef CAS.
- M. A. Northrup, B. Benett, D. Hadley, P. Landre, S. Lehew, J. Richards and P. Stratton, Anal. Chem., 1998, 70, 918–922 CrossRef CAS.
- J. Y. Lee, J. J. Kim and T. H. Park, Biotechnol. Bioprocess Eng., 2003, 8, 213–220 Search PubMed.
- J. S. Marcus, W. F. Anderson and S. R. Quake, Anal. Chem., 2006, 78, 956–958 CrossRef CAS.
- E. T. Lagally, J. R. Scherer, R. G. Blazej, N. M. Toriello, B. A. Diep, M. Ramchandani, G. F. Sensabaugh, L. W. Riley and R. A. Mathies, Anal. Chem., 2004, 76, 3162–3170 CrossRef CAS.
- J. N. Yang, Y. J. Liu, C. B. Rauch, R. L. Stevens, R. H. Liu, R. Lenigk and P. Grodzinski, Lab Chip, 2002, 2, 179–187 RSC.
- M. U. Kopp, A. J. Mello and A. Manz, Science, 1998, 280, 1046–1048 CrossRef CAS.
- Z. Chen, S. Qian, W. R. Abrams, D. Malamud and H. H. Bau, Anal. Chem., 2004, 76, 3707–3715 CrossRef CAS.
- M. Krishnan, V. M. Ugaz and M. A. Burns, Science, 2002, 298, 793–793 CrossRef.
- T. Tang, M. Y. Badal, G. Ocvirk, W. E. Lee, D. E. Bader, F. Bekkaoui and D. J. Harrison, Anal. Chem., 2002, 74, 725–733 CrossRef CAS.
- Y. Hataoka, L. Zhang, Y. Mori, N. Tomita, T. Notomi and Y. Baba, Anal. Chem., 2004, 76, 3689–3693 CrossRef CAS.
- A. Gulliksen, L. A. Solli, K. S. Drese, O. Sorensen, F. Karlsen, H. Rogne, E. Hovig and R. Sirevag, Lab Chip, 2005, 5, 416–420 RSC.
- M. Vincent, Y. Xu and H. M. Kong, EMBO Rep., 2004, 5, 795–800 Search PubMed.
- S. Tyagi and F. R. Kramer, Nat. Biotechnol., 1996, 14, 303–308 CrossRef CAS.
- S. Tyagi, S. A. E. Marras and F. R. Kramer, Nat. Biotechnol., 2000, 18, 1191–1196 CrossRef CAS.
- M. Culha, D. L. Stokes, G. D. Griffin and T. Vo-Dinh, Biosens. Bioelectron., 2004, 19, 1007–1012 CrossRef CAS.
- M. Culha, D. L. Stokes, G. D. Griffin and T. Vo-Dinh, J. Biomed. Opt., 2004, 9, 439–443 CrossRef CAS.
- T. G. Drummond, M. G. Hill and J. K. Barton, Nat. Biotechnol., 2003, 21, 1192–1199 CrossRef CAS.
- M. A. Dineva, D. Candotti, F. Fletcher-Brown, J. P. Allain and H. Lee, J. Clin. Microbiol., 2005, 43, 4015–4021 CrossRef CAS.
- D. Huh, W. Gu, Y. Kamotani, J. B. Grotberg and S. Takayama, Physiol. Meas., 2005, 26, R73–98 CrossRef.
- P. Mwaba, S. Cassol, R. Pilon, C. Chintu, M. Janes, A. Nunn and A. Zumla, Lancet, 2003, 362, 1459–1460 CrossRef CAS.
- A. Y. Fu, H. P. Chou, C. Spence, F. H. Arnold and S. R. Quake, Anal. Chem., 2002, 74, 2451–2457 CrossRef CAS.
- A. Goodey, J. J. Lavigne, S. M. Savoy, M. D. Rodriguez, T. Curey, A. Tsao, G. Simmons, J. Wright, S. J. Yoo, Y. Sohn, E. V. Anslyn, J. B. Shear, D. P. Neikirk and J. T. McDevitt, J. Am. Chem. Soc., 2001, 123, 2559–2570 CrossRef CAS.
- W. R. Rodriguez, N. Christodoulides, P. N. Floriano, S. Graham, S. Mohanty, M. Dixon, M. Hsiang, T. Peter, S. Zavahir, I. Thior, D. Romanovicz, B. Bernard, A. P. Goodey, B. D. Walker and J. T. McDevitt, PLoS Med., 2005, 2, e182 Search PubMed.
- M. Fryland, P. Chaillet, R. Zachariah, A. Barnaba, L. Bonte, R. Andereassen, S. Charrondière, R. Teck and O. Didakus, Trans. R. Soc. Trop. Med. Hyg., 2006, 100, 980–985 Search PubMed.
- I. V. Jani, G. Janossy, D. W. Brown and F. Mandy, Lancet Infect. Dis., 2002, 2, 243–250 Search PubMed.
- A. G. J. Tibbe, B. G. de Grooth, J. Greve, P. A. Liberti, G. J. Dolan and L. W. M. M. Terstappen, Cytometry, 2001, 43, 31–37 CrossRef CAS.
- N. Christodoulides, P. N. Floriano, S. A. Acosta, K. L. M. Ballard, S. E. Weigum, S. Mohanty, P. Dharshan, D. Romanovicz and J. T. McDevitt, Clin. Chem., 2005, 51, 2391–2395 CrossRef CAS.
- J. P. Shelby, J. White, K. Ganesan, P. K. Rathod and D. T. Chiu, Proc. Natl. Acad. Sci. U. S. A., 2003, 100, 14618–14622 CrossRef CAS.
- P. Gascoyne, J. Satayavivad and M. Ruchirawat, Acta Trop., 2004, 89, 357–369 CrossRef.
- F. K. Balagadde, L. You, C. L. Hansen, F. H. Arnold and S. R. Quake, Science, 2005, 309, 137–140 CrossRef.
- N. Futai, W. Gu, J. W. Song and S. Takayama, Lab Chip, 2006, 6, 149–154 RSC.
- S. Petronis, M. Stangegaard, C. B. Christensen and M. Dufva, Biotechniques, 2006, 40, 368–376 CrossRef CAS.
- X. Heng, D. Erickson, L. R. Baugh, Z. Yaqoob, P. W. Sternberg, D. Psaltis and C. Yang, Lab Chip, 2006, 6, 1274 RSC.
- M. H. Hazbon, N. Guarin, B. E. Ferro, A. L. Rodriguez, L. A. Labrada, R. Tovar, P. F. Riska and W. R. Jacobs, J. Clin. Microbiol., 2003, 41, 4865–4869 CrossRef CAS.
- G. T. A. Kovacs, Micromachined Transducers Sourcebook, McGraw-Hill, New York, 1998 Search PubMed.
- M. Madou, Fundamentals of Microfabrication, CRC Press, Boca Raton, FL, 1998 Search PubMed.
- I. H. A. Badr, R. D. Johnson, M. J. Madou and L. G. Bachas, Anal. Chem., 2002, 74, 5569–5575 CrossRef CAS.
- H. Hisamoto, M. Yasuoka and S. Terabe, Anal. Chim. Acta, 2006, 556, 164–170 CrossRef CAS.
- B. H. Lapizco-Encinas, B. A. Simmons, E. B. Cummings and Y. Fintschenko, Anal. Chem., 2004, 76, 1571–1579 CrossRef.
- P. S. Dittrich and A. Manz, Nat. Rev. Drug Discovery, 2006, 5, 210–218 CrossRef CAS.
- P. A. Singer and A. S. Daar, Science, 2001, 294, 87–89 CrossRef CAS.
- F. Salamanca-Buentello, D. L. Persad, E. B. Court, D. K. Martin, A. S. Daar and P. A. Singer, PLoS Med., 2005, 2, 383–386 Search PubMed.
- N. Tsapis, D. Bennett, B. Jackson, D. A. Weitz and D. A. Edwards, Proc. Natl. Acad. Sci. U. S. A., 2002, 99, 12001–12005 CrossRef CAS.
- D. A. LaVan, T. McGuire and R. Langer, Nat. Biotechnol., 2003, 21, 1184–1191 CrossRef.
- J. Z. Hilt and N. A. Peppas, Int. J. Pharm., 2005, 306, 15–23 CrossRef CAS.
- E. Rodgers, Diffusion of Innovations, the Free Press, New York, 4th edn, 1995 Search PubMed.
- P. Yager, T. Edwards, E. Fu, K. Helton, K. Nelson, M. R. Tam and B. H. Weigl, Nature, 2006, 442, 412–418 CrossRef CAS.
- Frost & Sullivan Report: United States In Vitro Cancer Diagnostics Market, Frost & Sullivan, March 1, 1999 Search PubMed.
- C. H. Ahn, J. W. Choi, G. Beaucage, J. H. Nevin, J. B. Lee, A. Puntambekar and J. Y. Lee, Proc. IEEE, 2004, 92, 154–173 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2007 |

