High efficient donor–acceptor ruthenium complex for dye-sensitized solar cell applications†
Jun-Ho
Yum
*a,
Il
Jung
a,
Chul
Baik
b,
Jaejung
Ko
b,
M. K.
Nazeeruddin
a and
Michael
Grätzel
a
aLaboratory for Photonics and Interfaces, Institute of Chemical Sciences and Engineering, School of Basic Sciences, École Polytechnique Fédérale de Lausanne, CH - 1015, Lausanne, Switzerland. E-mail: junho.yum@epfl.ch; Fax: +41 21 693 4111; Tel: +41 21 693 6124
bDepartment of New Material Chemistry, Korea University, Jochiwon, Chungnam 339-700, Republic of Korea
First published on 27th October 2008
Abstract
A highly efficient heteroleptic ruthenium (II) complex cis-di(thiocyanato)(4,4′-dicarboxylic acid-2,2′-bipyridine)(4,4′-di-(2-(4-ditolylamine phenyl)ethenyl)-2,2′-bipyridine) ruthenium (II) (IJ-1) was synthesized and characterized, which when anchored on nanocrystalline TiO2 films exhibited high power conversion efficiency, 10.3%, and incident photon to electron conversion efficiency, 87%.
Broader contextTo preserve the global environment, the development of clean renewable energy sources as an alternative to fossil fuels is paramount, and in this respect solar energy is considered as one of the best options. The conversion of solar energy to electricity using dye-sensitized solar cells represents one of the most promising methods for future large-scale power production because of its low-cost and high efficiency 10–11%. In dye-sensitized solar cells, the sensitizer is one of the key components which harvests solar radiation and converts it to electric current. We describe the development of a novel heteroleptic ruthenium (II) complex containing thiocyanate, with 4,4′-dimethylphenylaniline as donor and 4,4′-dicarboxylic acid as acceptor ligands, which yields 10.3% power conversion efficiency under one sun. |
Global environment concerns and the finite nature of fossil fuels have led to a growth of interest in the development of renewable energy. In this respect solar energy is one of the best candidates. The dye-sensitized solar cell (DSC) based on mesoporous nanocrystalline TiO2 films has attracted significant attention as a low-cost photovoltaic device.1 In these cells, the sensitizer is one of the key components for high power conversion efficiency, and the most efficient and successful sensitizers are ruthenium (II) complexes, which have yielded power conversion efficiency of 8–11%.2
The immobilized sensitizer forms a monomolecular film on the TiO2 surface, thereby facilitating charge transfer by electron injection. Efficient charge transfer occurs after anchoring the sensitizer through the carboxylate group onto the surface of the TiO2 semiconductor.3 The sensitizers with enhanced molar extinction coefficient allow a high light harvesting yield and a reduction in film thickness, and thus more efficient performance because of reduced transport losses in the nanoporous environment leading to increased open circuit potentials.4 In this regard, modifying the electron-donating group has been considered as one strategy.5 Thelakkat et al. introduced sensitizers with a secondary electron donor moiety such as triphenylamine (TPA) but, the power conversion efficiency did not exceed 10%.6 Here, we have further fine tuned TPA by substituting methyl groups to synthesize a novel bpy-donor antenna dye, namely cis-di(thiocyanato)(4,4′-dicarboxylic acid-2,2′-bipyridine)(4,4′-di-(2-(4-ditolylamine phenyl)ethenyl)-2,2′-bipyridine) ruthenium (II), (Fig. 1). Synthesis and analytical data are given in ESI.†
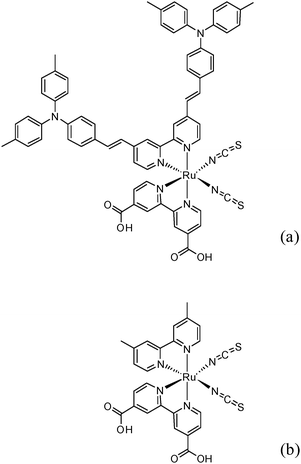 | ||
| Fig. 1 Molecular structure of IJ-1 (a) and N820 (b). | ||
The absorption spectrum of the IJ-1 sensitizer is dominated by metal to ligand charge transfer transitions (MLCT) in the visible region with the lowest allowed MLCT bands appearing at 432 and 536 nm. The molar extinction coefficients 43![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 and 19
400 and 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 060 M−1cm−1 of these bands are significantly higher than 11
060 M−1cm−1 of these bands are significantly higher than 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 M−1cm−1 of the similar heteroleptic complex N820 without TPA groups (Fig. 2). The bands in UV region at 210 and 308 nm are due to ligand π–π* charge transfer transitions. The sensitizer adsorbed on a 2.8 µm transparent TiO2 film shows features similar to those seen in the corresponding solution spectra but exhibits a slight red shift ∼5 nm due to the interaction of the anchoring groups to the surface.
600 M−1cm−1 of the similar heteroleptic complex N820 without TPA groups (Fig. 2). The bands in UV region at 210 and 308 nm are due to ligand π–π* charge transfer transitions. The sensitizer adsorbed on a 2.8 µm transparent TiO2 film shows features similar to those seen in the corresponding solution spectra but exhibits a slight red shift ∼5 nm due to the interaction of the anchoring groups to the surface.
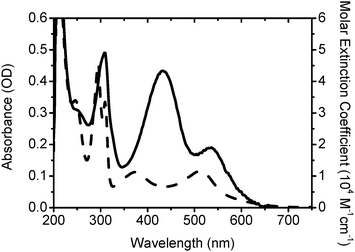 | ||
| Fig. 2 Absorption spectra of IJ-1 (solid) and N820 (dash) in ethanol with concentration 10−5 M. | ||
Cyclic voltammetry measurements were performed in dimethylformamide (DMF) solution using 0.1 M tetrabutyl ammonium tetrafluoroborate using ferrocene as the internal standard at 0.69 V vs.NHE. The oxidation and reduction potential of IJ-1 were obtained at E1/2 = 1.18, and −0.94 V vs.NHE, respectively. The oxidation potential is sufficiently more positive than the redox potential of an electrolyte leading to efficient dye regeneration. The optical transition E(0-0) energy of the IJ-1 sensitizer is at 1.96 eV, resulting an excited state oxidation potential of −0.78 V vs.NHE, which is sufficiently more negative than the TiO2 conduction band.
Dye sensitized photovoltaic devices were prepared according to the standard method reported earlier.7 The FTO glass substrates were immersed in 40 mM TiCl4aq. at 70 °C for 30 min and washed with water and ethanol. The 13 µm thick mesoporous nano-TiO2 films composed of 20 nm anatase TiO2 particles were coated on the FTO glass plates by repetitive screen printing. After drying the nanocrystalline TiO2 layer at 125 °C, a 4 µm thick second layer of 400 nm sized light scattering anatase particles (CCIC, HPW-400) was deposited by screen printing onto the transparent layer. The TiO2electrodes were gradually heated under an air flow at 325 °C for 5 min, at 375 °C for 5 min, at 450 °C for 15 min and 500 °C for 15 min. The TiO2electrodes were treated again by TiCl4 and sintered at 500 °C for 30 min. The TiO2electrodes were immersed into the IJ-1 solutions (0.3 mM in ethanol with 10 mM of chenodeoxycholic acid) and kept at room temperature for 20 h. The chenodeoxycholic acid is added to reduce aggregation of dye molecules leading to higher efficiency.8 A platinized FTO glass was used as counter electrode. Two different kinds of electrolytes are incorporated for DSCs. An electrolyte solution, A6141 is composed of 0.6 M M-methyl-N-butyl imidiazolium iodide, 0.03 M iodine, 0.1 M guanidinium thiocyanate (GuNCS) and 0.5 M tert-butylpyridine (TBP) in 15 : 85 (v/v) mixture of valeronitrile and acetonitrile). Another electrolyte, M1 consists of 0.6 M M-methyl-N-butyl imidiazolium iodide, 0.04 M iodine, 0.025 M LiI, 0.05 M GuNCS and 0.28 M TBP in 15/85 (v/v) mixture of valeronitrile and acetonitrile). The active area of DSCs was 0.2 cm2.
The photovoltaic performances (measured using 450 W xenon light source, under AM 1.5 conditions) of IJ-1 are shown in Fig. 3 and Table 1. The IJ-1 sensitized cells show high power conversion efficiency of about 10.3% with both of electrolytes under a standard global AM 1.5 solar condition. A6141 involving high concentration TBP and GuNCS and no LiI showed 17.6 mAcm−2 of short circuit current density (Jsc), 801 mV of open circuit voltage (Voc) and 73% of fill factor (FF). With M1, the Jsc increased to 19.2 from 17.3 mAcm−2 but Voc decreased to 748 mV without a big change of FF and power conversion efficiency. The photo-induced voltage (Voc) is determined by the difference between the quasi Fermi level of TiO2 and redox potential of the electrolyte and is able to be enhanced as a slow recombination process of injected electrons in TiO2 with oxidized species and a negative shift of band edge. TBP is known to increase Voc of DSC due to an enhanced electron lifetime and a negative shift of band edge.9 The GuNCS also increases Voc by an elongated electron lifetime even though the band edge is shifted downward.10 Hence, the higher Voc with A6141 is induced by incorporating TBP and GuNCS in an electrolyte. However, LiI in an electrolyte is known to decrease Voc, due to a positive shift of band edge, but increase electron transport property and act as the driving force of electron injection.11 The higher Jsc with LiI involved electrolyte, M1 is plausibly caused by the higher driving force for electron injection from IJ-1 to TiO2 as a result of the positively shifted band of edge of TiO2. Fig. 3 (insert) shows the incident monochromatic photon-to-current conversion efficiency (IPCE) of IJ-1 sensitized solar cell with M1, and it is evident that IJ-1 gives 87% of IPCE. The IPCE with A6141 is 81% lower than it is with M1 which is very consistent with photovoltaic data. Consequently, Ru(II) dye with a donor antenna unit, TPA attributes to the high molar extinction coefficient leading to highly efficient visible light harvesting capacity, which results in very high short-circuit current density (Table 1).
| Electrolyte | J sc/mA cm−2 | V oc/mV | FF | η (%) |
|---|---|---|---|---|
| A6141 | 17.6 | 801 | 0.73 | 10.3 |
| M1 | 19.2 | 748 | 0.72 | 10.3 |
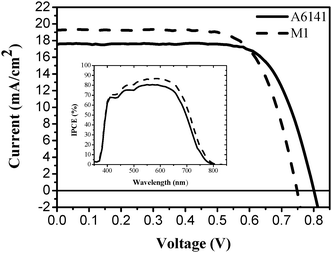 | ||
| Fig. 3 Photocurrent–voltage characteristics and incident photon-to-current conversion efficiency plotted as a function of excitation wavelength (insert) of IJ-1 sensitized solar cells with A6141 (solid) and M1 (dash) electrolytes under AM 1.5 full sunlight (100 mW cm−2). | ||
In conclusion, a novel heteroleptic ruthenium (II) complex with dimethylphenylaniline as the donor ligand has been synthesized and demonstrated. The ligand contributes to enhanced light harvesting yield leading to a high short-circuit current density and thus a highly efficient dye sensitized solar cell, 10.3%. Further optimization of the cell parameters and application to solid state solar cells is currently under study.
Acknowledgements
We thank Dr Robin Humphry-Baker, Dr Peter Péchy, Mr Pascal Comte and Dr Paul Liska for their kind assistance.References
- B. O'Regan and M. Grätzel, Nature, 1991, 353, 737 CrossRef CAS; R. F. Service, Science, 2003, 300, 1219 CrossRef CAS.
- M. K. Nazeeruddin, A. Kay, I. Rodicio, R. Humphry-baker, E. Muller, P. Liska, N. Vlachopoulos and M. Grätzel, J. Am. Chem. Soc., 1993, 115, 6382 CrossRef CAS; M. K. Nazeeruddin, S. M. Zakeeruddin, R. Humphry-Baker, M. Jirousek, P. Liska, N. Vlachopoulos, V. Shklover, C. H. Fischer and M. Grätzel, Inorg. Chem., 1999, 38, 6298 CrossRef CAS; M. K. Nazeeruddin, P. Péchy, T. Renouard, S. M. Zakeeruddin, R. Humphry-Baker, P. Comte, P. Liska, L. Cevey, E. Costa, V. Shklover, L. Spiccia, G. B. Deacon, C. A. Bignozzi and M. Grätzel, J. Am. Chem. Soc., 2001, 123, 1613 CrossRef CAS; C. Klein, M. K. Nazeeruddin, P. Liska, D. Di Censo, N. Hirata, E. Palomares, J. R. Durrant and M. Grätzel, Inorg. Chem., 2005, 44, 178 CrossRef CAS; Z. S. Wang, T. Yamaguchi, H. Sugihara and H. Arakawa, Langmuir, 2005, 21, 4272 CrossRef CAS; Y. Chiba, A. Islam, Y. Watanabe, R. Komiya, N. Koide and L. Y. Han, Jpn. J. Appl. Phys., Part 2, 2006, 45, L638 CrossRef CAS; D. B. Kuang, C. Klein, S. Ito, J. E. Moser, R. Humphry-Baker, N. Evans, F. Duriaux, C. Grätzel, S. M. Zakeeruddin and M. Grätzel, Adv. Mater., 2007, 19, 1133 CrossRef CAS; K. J. Jiang, J. B. Xia, N. Masaki, S. Noda and S. Yanagida, Inorg. Chim. Acta, 2008, 361, 783 CrossRef CAS.
- A. Fillinger and B. A. Parkinson, J. Electrochem. Soc., 1999, 146, 4559 CrossRef CAS.
- M. K. Nazeeruddin, Q. Wang, L. Cevey, V. Aranyos, P. Liska, E. Figgemeier, C. Klein, N. Hirata, S. Koops, S. A. Haque, J. R. Durrant, A. Hagfeldt, A. B. P. Lever and M. Grätzel, Inorg. Chem., 2006, 45, 787 CrossRef CAS.
- C. Y. Chen, S. J. Wu, C. G. Wu, J. G. Chen and K. C. Ho, Angew. Chem., Int. Ed., 2006, 45, 5822 CrossRef CAS; M. K. Nazeeruddin, T. Bessho, L. Cevey, S. Ito, C. Klein, F. De Angelis, S. Fantacci, P. Comte, P. Liska, H. Imai and M. Grätzel, J. Photochem. Photobiol., A, 2007, 185, 331 CrossRef CAS; F. Matar, T. H. Ghaddar, K. Walley, T. DosSantos, J. R. Durrant and B. O'Regan, J. Mater. Chem., 2008, 18, 4246 RSC.
- K. Peter and M. Thelakkat, Macromolecules, 2003, 36, 1779 CrossRef CAS; S. A. Haque, S. Handa, K. Peter, E. Palomares, M. Thelakkat and J. R. Durrant, Angew. Chem., Int. Ed., 2005, 44, 5740 CrossRef CAS.
- P. Wang, S. M. Zakeeruddin, J. E. Moser and M. Grätzel, J. Phys. Chem. B, 2003, 107, 13280 CrossRef CAS.
- A. Kay and M. Grätzel, J. Phys. Chem., 1993, 97, 6272 CrossRef; J. H. Yum, S. R. Jang, R. Humphry-Baker, M. Grätzel, J. J. Cid, T. Torres and M. K. Nazeeruddin, Langmuir, 2008, 24, 5636 CrossRef CAS.
- S. Y. Huang, G. Schlichthörl, A. J. Nozik, M. Grätzel and A. J. Frank, J. Phys. Chem. B, 1997, 101, 2576 CrossRef CAS; G. Schlichthörl, S. Y. Huang, J. Sprague and A. J. Frank, J. Phys. Chem. B, 1997, 101, 8141 CrossRef; G. Boschloo, L. Haggman and A. Hagfeldt, J. Phys. Chem. B, 2006, 110, 13144 CrossRef CAS.
- N. Kopidakis, N. R. Neale and A. J. Frank, J. Phys. Chem. B, 2006, 110, 12485 CrossRef CAS.
- Y. Liu, A. Hagfeldt, X. R. Xiao and S. E. Lindquist, Sol. Energy. Mater. Sol. Cells, 1998, 55, 267 CrossRef CAS; S. Pelet, J. E. Moser and M. Grätzel, J. Phys. Chem. B, 2000, 104, 1791 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures and synthesis of IJ-1. See DOI: 10.1039/b814863p |
| This journal is © The Royal Society of Chemistry 2009 |
