In situ studies of structural stability and proton conductivity of titanate nanotubes
Tao
Gao
*,
Harald
Fjeld
,
Helmer
Fjellvåg
,
Truls
Norby
and
Poul
Norby
Centre for Materials Science and Nanotechnology, Department of Chemistry, University of Oslo, P.O. Box 1033, N-0315, Oslo, Norway. E-mail: tao.gao@kjemi.uio.no; Fax: +47-22855565; Tel: +47-22857428
First published on 12th February 2009
Abstract
Titanate nanotubes were prepared by hydrothermal treatment of anatase TiO2 with concentrated NaOH solution. In situsynchrotron X-ray diffraction studies revealed that the nanotubes are thermally unstable at temperatures above 360 °C and can transform directly to anatase via a dehydration and recrystallization process. This indicated that the titanate nanotubes possess an orthorhombic lepidocrocite (γ-FeOOH)-type layered structure. An aggregate of the as-prepared nanotubes showed an electric conductivity of about 1 × 10−6 S cm−1 at 50 °C in humid atmospheres. The conductivity depended on humidity of the atmosphere and decreased with increasing temperatures, suggesting that the proton conduction in the titanate nanotubes might correlate with the interlayer H3O+ ions.
Broader contextNanoscience and nanotechnology play important roles in addressing the energy problems facing humankind in the 21st century. Being TiO2-based compounds with ultrahigh surface area, titanate nanotubes have recently attracted great interest as promising candidate materials for energy conversion and storage. However, the applications are somewhat hindered by several fundamental issues related to crystal structures and structural stability of the nanotubes. Here, we report our findings concerning crystal structure, structural stability, and proton conduction of titanate nanotubes. We demonstrate that titanate nanotubes consist of lepidocrocite-type TiO6 octahedral layers with Ti site vacancies; the nanotubes are thermally unstable at temperatures above 360 °C and can transform directly into anatase. Moreover, it is found that the conductivity of titanate nanotubes is protonic and correlated with the interlayer H3O+ ions. An improved understanding on fundamental science and engineering of titanate nanotubes will contribute greatly to their practical applications. |
Introduction
The past two decades have shown that the exploration of material properties on the nanometer scale can lead to substantial new insights regarding fundamental issues as well as to novel technological perspectives.1 The enhanced surface-to-volume ratio and reduced scale of transport length for both mass and charge transport make nanomaterials ideal systems for sustainable and clean energy technologies.2 In this regard, fundamental science and engineering to address the synthesis, properties and applications of new, multifunctional nanomaterials are obviously important.3 Since the first report of titanate nanotubesvia a simple wet chemical method,4 this TiO2-based nanomaterial has triggered great excitement due to its interesting structural characteristics and potential applications in energy conversion and storage.5–8 Structurally, the titanate nanotubes can be described as multi-walled spiral nanoscrolls formed by rolling of two-dimensional (2D) TiO6 octahedral nanolayers,9 resulting in tubular structures with almost uniform inner diameters of ∼5 nm, outer diameters of ∼10 nm and lengths of several hundreds of nanometers. Water molecules and protons locate loosely in the interlayer space; the interlayer protons are mobile and exchangeable.9,10 These structural features make the titanate nanotubes promising candidate materials for photocatalysis, dye-sensitized solar cells, supercapacitors, and rechargeable lithium batteries.5–8However, the titanate nanotubes are unstable under some conditions. For example, they tend to transform into anatase and lose their tubular morphology in acidic solutions.11–13Annealing the nanotubes in air at high temperatures results in also the formation of anatase, although details of the transformation are still in debate.14–18 For example, Suzuki and Yoshikawa reported that the titanate nanotubes will transform into TiO2-(B) phase, a metastable polymorph of crystalline TiO2, under heating at ∼800 °C.14 In contrast, Zhang et al. found that, at annealing temperatures higher than 300 °C, the nanotubes lose interlayer hydroxyls and transform into anatase with the deterioration of the tubular morphology.15 Moreover, Wang et al. reported the formation of triclinic Ti5O9 from the titanate nanotubes under heating at 400 °C, apart from monoclinic H2Ti3O7 and anatase phases.17 Clearly, further investigations on crystal structure19 and structural stability of the titanate nanotubes are still necessary.
From a viewpoint of structure-property relationship, the recently reported proton conductivity20,21 of the titanate nanotubes and hence their potential as electrolytes in hydrogen fuel cells provides further incentive to investigate their structural stability. Thorne et al. reported a room-temperature protonic conductivity under nominally anhydrous conditions of ca. 5.5 × 10−6 S cm−1, decreasing with increasing temperatures.20 In contrast, Yamada et al. found that the proton conductivity increases with increasing temperature under humid conditions.21 It is obvious that the electrical conductivity issue of the titanate nanotubes should be evaluated by considering first their structural and compositional stability at elevated temperatures.
In this paper, structural stability and proton conductivity of titanate nanotubes are considered. In situsynchrotron X-ray diffraction (XRD) that enables time resolved studies of fast chemical reactions is employed to monitor the structural evolutions of the nanotubes during heating. In such a way, the formation of possible metastable intermediates14,17 will be identified. Moreover, the correlations between structural characteristics and proton conductivity of the titanate nanotubes are addressed.
Experimental
Hydrothermal synthesis of titanate nanotubes
Titanate nanotubes used in this work were synthesized by using the procedures developed by Kasuga et al.4 with a small modification. A small amount of anatase TiO2 powders (about 0.5 g) was added in 100 mL NaOH aqueous solution (10 M). After stirring at room temperature for 30 min, the resulting white suspension was charged into Teflon-lined autoclaves and heated to 140 °C for 24–72 h. After filtration, the resulting material was shaken in 200 mL HNO3 aqueous solution (0.2 M) for 3 days. The acid solution was renewed every 12 h to promote a complete ion exchange. Finally the solid product was filtered, washed with water, and dried at 70 °C overnight to give the as-prepared titanate nanotubes.In situ synchrotron X-ray diffraction
An in situ heating experiment was performed at the Swiss–Norwegian Beam Line BM01A, at the European Synchrotron Radiation Facility (ESRF) in Grenoble, France. A small amount of the as-prepared titanate nanotubes was loaded into a quartz glass capillary (inner diameter: 0.7 mm). Continuous synchrotron X-ray scanning was started at the same time when the sample was heated from room temperature to 600 °C at a rate of 5 °C min−1 under dry N2 flow. The XRD data were collected for scattering angles (2θ) of 1–40° with the X-ray wavelength of 0.07106 nm.In situ proton conductivity measurement
Methodology and equipment for the proton conductivity measurement are described elsewhere.22 About 300 mg of as-prepared titanate nanotubes was uniaxially cold-pressed to discs of 12–13 mm diameter and 2–3 mm thickness. Circular Al electrodes were pressed towards the specimen. The electrical characterization was performed in a ProboStat measurement cell (NorECs AS, Norway). The total a.c. conductivity at 11.3 kHz was measured for selected temperatures up to 250 °C in different atmospheres using a Hewlett-Packard HP4192 impedance spectrometer. At each temperature, the measurements were performed after several hours of equilibration.Characterization
The as-prepared titanate nanotubes were characterized also by X-ray powder diffraction (XRD, Siemens D5000 powder diffractometer with Cu Kα1 radiation), scanning electron microscopy (SEM, FEI Quanta 200F) and transmission electron microscopy (TEM, Philips CM30ST) equipped with an energy-dispersive X-ray spectrometer (EDX). Thermogravimetric analysis (TGA, Perkin Elmer TGA 7 system) of the as-prepared titanate nanotubes was carried out at a heating rate of 5 °C min−1 under N2 flow. Surface area and mesoporous structure of the nanotubes were characterized by nitrogen isothermal adsorption at 77 K using a BELSORP high precision adsorption measuring apparatus. Surface area of the nanotubes was determined from the Brunauer–Emmett–Teller equation and pore volume from the total amount adsorbed at relative pressures near unity. The pore size distribution was analyzed by using the Barrett–Joyner–Halenda method.Results and discussion
Structural features of titanate nanotubes
Fig. 1 shows typical home-lab XRD patterns collected from samples before and after acid washing. The product of hydrothermal reaction prior to acid washing is sodium titanate (Fig. 1a); a complete acid-exchange yields hydrogen titanate (Fig. 1b). As shown in Fig. 1, they are basically the same, implying their identical structural origin.23 The broad peak at 2θ = 10.23°, corresponding to a d-spacing of 0.86 nm, can be attributed to the interlayer diffraction of the as-prepared nanotubes.13,23,24 Note that there are only a few broad XRD peaks, any unequivocal indexing of the pattern to any known titanate polymorphs is thus impossible. Based on subsequent studies, the reflections in Fig. 1 are indexed according to an orthorhombic titanate H0.7Ti1.825![[rectangle open, vertical]](https://www.rsc.org/images/entities/char_25af.gif) 0.175O4·H2O (
0.175O4·H2O (![[rectangle open, vertical]](https://www.rsc.org/images/entities/char_25af.gif) : vacancy) with lattice parameters a = 0.3780 nm, b = 1.8687 nm, and c = 0.2978 nm.23 For example, the reflections at 2θ = 10.23°, 24.6°, 28.7°, 48.5°, and 62.8° can be indexed to (020), (110), (130), (200), and (002) reflections of the H0.7Ti1.825
: vacancy) with lattice parameters a = 0.3780 nm, b = 1.8687 nm, and c = 0.2978 nm.23 For example, the reflections at 2θ = 10.23°, 24.6°, 28.7°, 48.5°, and 62.8° can be indexed to (020), (110), (130), (200), and (002) reflections of the H0.7Ti1.825![[rectangle open, vertical]](https://www.rsc.org/images/entities/char_25af.gif) 0.175O4·H2O.
0.175O4·H2O.
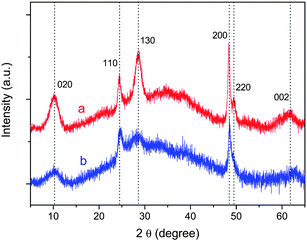 | ||
Fig. 1
Powder XRD patterns of the as-prepared nanotubes before (a) and after (b) acid washing (wavelength: 0.15406 nm). Indexes are given on the basis of an orthorhombic titanate H0.7Ti1.825![[rectangle open, vertical]](https://www.rsc.org/images/entities/char_25af.gif) 0.175O4·H2O ( 0.175O4·H2O (![[rectangle open, vertical]](https://www.rsc.org/images/entities/char_25af.gif) : vacancy). : vacancy). | ||
Morphology of the as-prepared titanate nanotubes was analyzed by SEM (Fig. 2). No morphological changes were noticed during the acidic washing process. As demonstrated by the SEM data, a mass production of the titanate nanotubes of high purity has been achieved via the wet chemical method.4EDX data (not shown here) reveal that the as-prepared titanate nanotubes are composed of only two elements, titanium and oxygen (note that hydrogen is not detectable by EDX), indicating that the ion-exchange reaction is complete and the nanotubes are pure hydrogen titanates.
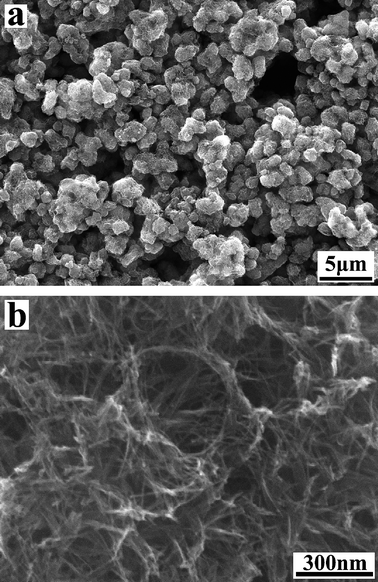 | ||
| Fig. 2 SEM images of as-prepared titanate nanotubes. | ||
TEM data (Fig. 3) reveal that the as-prepared titanate nanotubes have typical inner diameters of about 5 nm, outer diameters of about 11 nm, and lengths of up to several hundred nanometers. Electron diffraction (ED) analyses show poor crystallinity of individual tubes. A typical ED pattern taken from an area that contains many nanotubes is shown in the inset to Fig. 3a. Diffraction rings are observed as a result of the polycrystalline nature of the sample, with many nanotubes oriented along all directions. The strong diffraction rings with d-spacings of 0.366 and 0.187 nm correspond to (110) and (200), respectively, of the H0.7Ti1.825![[rectangle open, vertical]](https://www.rsc.org/images/entities/char_25af.gif) 0.175O4·H2O. Detailed TEM analyses (Fig. 3b) reveal that the number of layers on either side of the nanotubes is usually not equal, implying that the nanotubes might be formed by scrolling of sheet-like materials.9,13,19,23,24 The presence of a small amount (less than 5%) of sheet-like intermediates/by-products (as indicated by ↓ in Fig. 3b) seems in harmony with this assumption. The nanotubes are usually three to five layers in wall thickness with typical interlayer distance of about 0.8 nm (inset Fig. 3b), in agreement with the XRD data (Fig. 1b).
0.175O4·H2O. Detailed TEM analyses (Fig. 3b) reveal that the number of layers on either side of the nanotubes is usually not equal, implying that the nanotubes might be formed by scrolling of sheet-like materials.9,13,19,23,24 The presence of a small amount (less than 5%) of sheet-like intermediates/by-products (as indicated by ↓ in Fig. 3b) seems in harmony with this assumption. The nanotubes are usually three to five layers in wall thickness with typical interlayer distance of about 0.8 nm (inset Fig. 3b), in agreement with the XRD data (Fig. 1b).
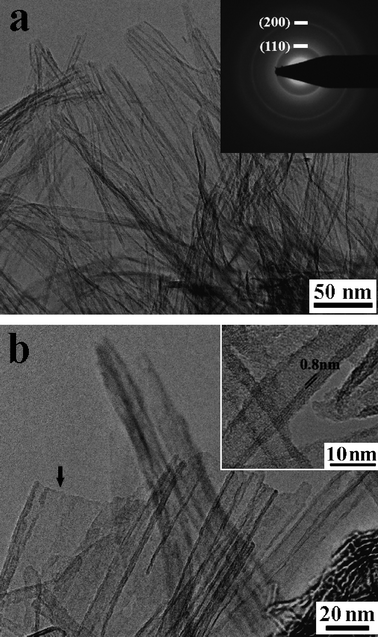 | ||
| Fig. 3 TEM images of as-prepared titanate nanotubes. Inset (a) shows a typical electron diffraction pattern of the nanotubes. Inset (b) is an enlargement showing an interlayer distance of about 0.8 nm. Arrow in panel (b) indicates the lamellar intermediates/by-products. | ||
The nanotubular structure of the as-prepared products was characterized further by nitrogen isothermal adsorption at 77 K. Typical isotherms for nitrogen adsorption and desorption of the titanate nanotubes are reported in Fig. 4. It can be seen that the nanotubes have type IV isotherms with an H3 hysteresis loop according to IUPAC classification,25 indicating that the products are mainly mesoporous. Moreover, the observed hysteresis loop approaches P/P0 = 1, revealing the presence of macropores (>50 nm) due to the aggregation of the nanotubes (see for example, Fig. 2). According to the Brunauer–Emmett–Teller equation, the surface area of the as-prepared titanate nanotubes is determined to be about 327 m2 g−1. The pore size distribution was analyzed by using the Barrett–Joyner–Halenda (BJH) method. The BJH desorption pore volume is about 1.97 cm3 g−1 with peak pore position around 1.64 nm. These data are in agreement with the published values of the titanate nanotubes.13,26
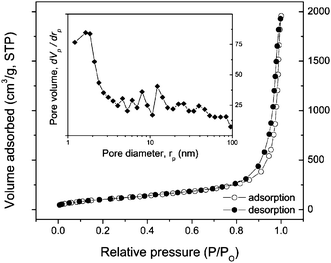 | ||
| Fig. 4 Isotherm of nitrogen adsorption and desorption of titanate nanotubes at 77 K. The inset shows a pore volume distribution. | ||
Structural stability of titanate nanotubes
The structural stability of the as-prepared nanotubes was studied first by thermogravimetric analysis (TGA). As shown in Fig. 5, a continuous weight loss occurs between 40 and 450 °C, with a change in rate at around 150 °C. The weight loss from 150 to 450 °C is about 4%. No further loss is observed up to 600 °C. The XRD pattern at 150 °C indicates that the crystalline integrity is degraded. Anatase forms at 450 °C. The TGA as well as the XRD data suggest that the titanate nanotubes first lose interlayer H2O, resulting in a dehydrated titanate phase, H0.7Ti1.825![[rectangle open, vertical]](https://www.rsc.org/images/entities/char_25af.gif) 0.175O4.27 Upon subsequent heating the nanotubular structure collapses under formation of anatase.
0.175O4.27 Upon subsequent heating the nanotubular structure collapses under formation of anatase.
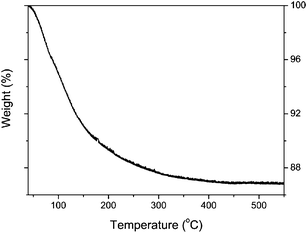 | ||
| Fig. 5 TGA data for as-prepared titanate nanotubes during heating in N2 flow at a rate of 5 °C min−1. | ||
Fig. 6 shows time-resolved synchrotron XRD patterns of titanate nanotubes during the in situ heating. It can be clearly seen that the nanotubes are thermally unstable upon increasing temperatures and can transform directly to anatase without formation of any intermediate phases. This observation is in agreement with the previous findings reported by Zhang et al.15 and Kim et al.16 It is interesting to point out that the orthorhombic titanates with lepidocrocite (γ-FeOOH)-type layered structure can transform directly into anatase upon heating. This is in contrast to the dehydration behavior of the monoclinic titanates, such as H2Ti3O7 or H2Ti4O9.24 Due to their stepped layered structures, the complete dehydration of these monoclinic compounds yields a novel polymorph of titania, TiO2-B, as intermediate, before they finally crystallize into anatase.28 The direct phase transformation of anatase observed here adds support to the view that the titanate nanotubes contain lepidocrocite-type TiO6 octahedral layers.10,13,19,23
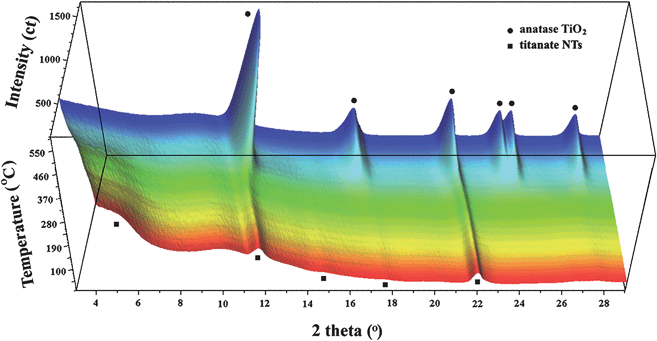 | ||
| Fig. 6 Three-dimensional representation of in situ synchrotron XRD data of titanate nanotubes upon heating (wavelength: 0.07106 nm). The broad bump at about 2θ = 10° is due to the quartz glass sample holder. | ||
Note that the lepidocrocite-type TiO6 octahedral layers of titanate nanotubes have both 4- and 2-coordinated O atoms; the latter being linked to interlayer H3O+ ions through hydrogen bonding.27 Upon dehydration, the nanotubes first lose interlayer water (H3O+ → H2O + H+) to form a dehydrated phase. The remaining H+ ions probably form hydroxyls by reacting with 2-coordinated O atoms. On further heating, dehydroxylation takes place (two H+ plus one 2-coordinated O atom), which triggers in turn the crystallization of a three-dimensional network structure, i.e. anatase. Here, the in situ synchrotron XRD study enables identifying the phase transition temperature, which is found to be about 360 °C (Fig. 7).
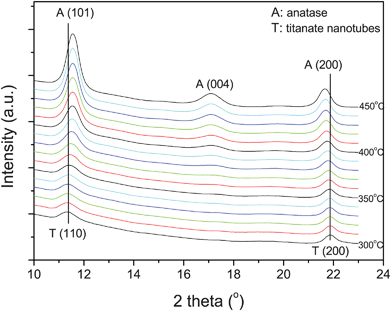 | ||
| Fig. 7 Selected synchrotron XRD patterns near the phase transformation for comparison. The patterns are shifted vertically for clarity (wavelength: 0.07106 nm). | ||
The mechanism of this direct transformation would appear to be as follows. Fig. 8 shows the similarity of the orthorhombic lepidocrocite-type TiO6 octahedral layers to that of the principle layers of tetragonal anatase.29,30 The former is compact and consists of two atomic planes of Ti and four atomic planes of O along the layer normal, as illustrated in Fig. 8a. The Ti atomic plane is sandwiched in between the O1 (i.e. 4-coordinated oxygen) and O2 (i.e. 2-coordinated oxygen) atomic planes, forming a corner- and edge-shared TiO6 octahedral sheet. Consequently, the orthorhombic lepidocrocite-type layer can be described as a combination of the two sets of corner-shared TiO6 octahedral sheets (as illustrated in green or yellow) with a glide plane: a mirror plane normal to the b-axis that is staggered by (0.5, 0.5) in the ac plane. Obviously, if one of the two sets of sheets is shifted with respect to each other by (0.5, 0) or (0, 0.5) in the ac plane, accompanying a change in lateral dimension (from 0.378 nm × 0.298 nm to 0.378 × 0.378 nm), one half of the anatase unit cell would be produced (Fig. 8b). Note that this transition should involve extensive atomic migration,29 which will be relaxed due to the presence of Ti site vacancies in the walls of the nanotubes. This is in agreement with its relatively low transition temperature, ∼360 °C. The presence of Ti site vacancies supports the viewpoint that the crystal structure of the titanate nanotubes can be described by the protonic titanate H0.7Ti1.825![[rectangle open, vertical]](https://www.rsc.org/images/entities/char_25af.gif) 0.175O4·H2O.10,19,23
0.175O4·H2O.10,19,23
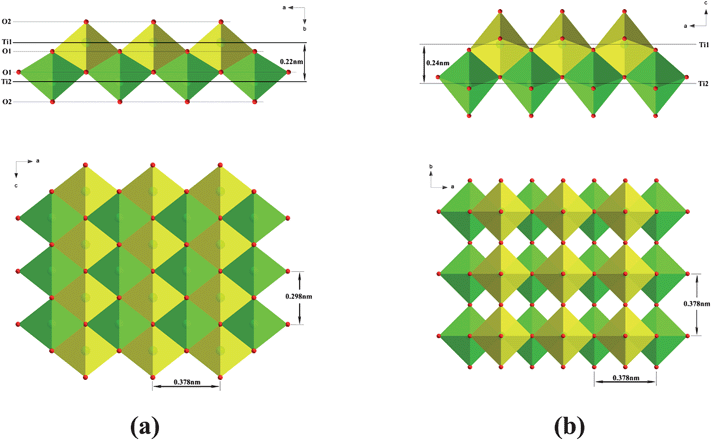 | ||
| Fig. 8 Comparison of (a) the lepidocrocite-type titanate layer and (b) the principle layer of anatase. The axis notation refers to the original orthorhombic layered titanate and tetragonal anatase. | ||
Proton conductivity of titanate nanotubes
The dehydration processes performed at elevated temperatures affect mainly the interlayer species such as H3O+ ions and/or OH−groups, and hence certainly also the proton conductivity of the titanate nanotubes. Fig. 9 presents electrical conductivity data for the nanotubes measured at a fixed frequency of 11.3 kHz, normally sufficient to exclude electrode impedances.22 Clearly, the conductivity of the titanate nanotubes decreases with increasing temperature, e.g., from 1.4 × 10−6 S cm−1 at 50 °C to 1.9 × 10−7 S cm−1 at 128 °C in oxygen (Fig. 8a). At 250 °C, the conductivity is too low to be measured with the equipment used. This variation is consistent with the findings of Thorne et al. (reporting 5.5 × 10−6 S cm−1 at 25 °C for anhydrous conditions);20 however, it is in contrast to the data reported by Yamada et al.,21 who claimed that the conductivity increases with increasing temperatures. It is also found that, the conductivity is correlated to water and it changes between dry and wet conditions. For example, the conductivity decreases by about a factor of two when the atmosphere is switched from wet to dry oxygen at 130 °C (Fig. 9b). Such behavior is typical of situations where the proton transport takes place as H3O+ ions, or by proton jumps between H2O molecules.31 In both cases the loss of water with increasing temperature will decrease the proton conductivity. This loss of water agrees with the structural studies (Figs. 5–7). It is worth pointing out that the poor stability at elevated temperatures might make the proposed use of titanate nanotubes as electrolytes in hydrogen fuel cells questionable. In contrast, the conductivity dependence on humidity of the atmosphere (Fig. 9b) may provide the means to monitor water at intermediate temperature conditions.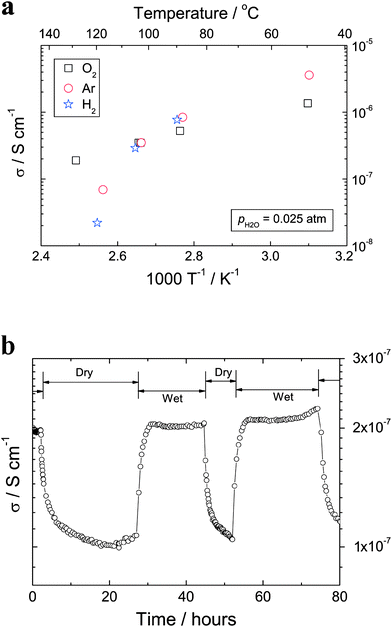 | ||
| Fig. 9 (a) AC (11.3 kHz) conductivities vs. inverse absolute temperature for titanate nanotubes measured in wet atmospheres (pH2O = 0.025 atm). (b) Conductivities of titanate nanotubes measured in wet (pH2O = 0.025 atm) and dry (pH2O = 5.0 × 10−5 atm) oxygen atmospheres at 130 °C. | ||
Conclusions
In summary, the structural stability and the proton conductivity of titanate nanotubes prepared via alkali treatment of TiO2 are discussed. In situsynchrotron XRD data demonstrate that the titanate nanotubes are unstable at temperatures above 360 °C and can transform directly into anatase. This revealed that the titanate nanotubes contain lepidocrocite-type TiO6 octahedral layers, and the presence of Ti site vacancies is prominent. The electrical characterization indicates that the conductivity of the titanate nanotubes is protonic and correlated with the interlayer H3O+ ions.Acknowledgements
The authors acknowledge the financial assistance from the Research Council of Norway through the NANOMAT program (163565-431). ESRF is gratefully acknowledged for beam time made available through proposal number CH-2140.References
- J. Maier, Nat. Mater., 2005, 4, 805 CrossRef.
- P. Balaya, Energy Environ. Sci., 2008, 1, 645 RSC.
- A. Manthiram, A. Vadivel Murugan, A. Sarkar and T. Muraliganth, Energy Environ. Sci., 2008, 1, 621 RSC.
- (a) T. Kasuga, M. Hiramatsu, A. Hoson, T. Sekino and K. Niihara, Adv. Mater., 1999, 11, 1307 CrossRef CAS; (b) T. Kasuga, M. Hiramatsu, A. Hoson, T. Sekino and K. Niihara, Langmuir, 1998, 14, 3160 CrossRef CAS.
- M. Adachi, Y. Murata, M. Harada and S. Yoshikawa, Chem. Lett., 2000, 942 CrossRef CAS.
- S. Liu and A. Chen, Langmuir, 2005, 21, 8409 CrossRef CAS.
- Y. G. Wang and X. G. Zhang, J. Electrochem. Soc., 2005, 152, A671 CrossRef CAS.
- H. Zhang, G. R. Li, L. P. An, T. Y. Yan, X. P. Gao and H. Y. Zhu, J. Phys. Chem. C, 2007, 111, 6143 CrossRef CAS.
- T. Gao, Q. L. Wu, H. Fjellvåg and P. Norby, J. Phys. Chem. C, 2008, 112, 8548 CrossRef CAS.
- R. Ma, T. Sasaki and Y. Bando, Chem. Commun., 2005, 948 RSC.
- D. V. Bavykin, J. M. Friedrich, A. A. Lapkin and F. C. Walsh, Chem. Mater., 2006, 18, 1124 CrossRef CAS.
- X. Sun and Y. Li, Chem.–Eur. J., 2003, 9, 2229 CrossRef CAS.
- (a) C. C. Tsai and H. Teng, Chem. Mater., 2006, 18, 367 CrossRef CAS; (b) J. N. Nian and H. Teng, J. Phys. Chem. B, 2006, 110, 4193 CrossRef CAS.
- Y. Suzuki and S. Yoshikawa, J. Mater. Res., 2004, 19, 982 CrossRef CAS.
- M. Zhang, Z. Jin, J. Zhang, X. Guo, J. Yang, W. Li, X. Wang and Z. Zhang, J. Mol. Catal. A: Chem., 2004, 217, 203 CrossRef CAS.
- G. S. Kim, S. G. Ansari, H. K. Seo, Y. S. Kim and H. S. Shin, J. Appl. Phys., 2007, 101 Search PubMed 024314.
- N. Wang, H. Lin, J. Li, X. Yang, B. Chi and C. Lin, J. Alloys Compd., 2006, 424, 311 CrossRef CAS.
- L. Zhang, H. Lin, N. Wang, C. Lin and J. Li, J. Alloys Compd., 2007, 431, 230 CrossRef CAS.
- T. Gao, H. Fjellvåg and P. Norby, Inorg. Chem., 2009, 48, 1423 CrossRef CAS.
- A. Thorne, A. Kruth, D. Tunstall, J. T. S. Irvine and W. Zhou, J. Phys. Chem. B, 2005, 109, 5439 CrossRef CAS.
- M. Yamada, M. Wei, I. Honma and H. Zhou, Electrochem. Commun., 2006, 8, 1549 CrossRef CAS.
- (a) T. Norby, Solid State Ionics, 1988, 28–30, 1586 CrossRef; (b) R. Haugsrud and T. Norby, Nat. Mater., 2006, 5, 193 CrossRef CAS.
- R. Ma, Y. Bando and T. Sasaki, Chem. Phys. Lett., 2003, 380, 577 CrossRef CAS.
- Q. Chen, G. H. Du, S. Zhang and L. M. Peng, Acta Cryst., 2002, B58, 587 CAS.
- K. S. W. Sing, D. H. Everett, R. A. W. Haul, L. Moscou, R. A. Pierotti, J. Rouquérol and T. Siemieniewska, Pure Appl. Chem., 1985, 57, 603 CrossRef CAS.
- D. V. Bavykin, V. N. Parmon, A. A. Lapkin and F. C. Walsh, J. Mater. Chem., 2004, 14, 3370 RSC.
- T. Gao, H. Fjellvåg and P. Norby, J. Phys. Chem. B, 2008, 112, 9400 CrossRef CAS.
- T. P. Feist and P. K. Davies, J. Solid State Chem., 1992, 101, 275 CrossRef.
- K. Fukuda, Y. Ebina, T. Shibata, T. Aizawa, I. Nakai and T. Sasaki, J. Am. Chem. Soc., 2007, 129, 202 CrossRef CAS.
- F. Alvarez-Ramirez and Y. Ruiz-Morales, Chem. Mater., 2007, 19, 2947 CrossRef CAS.
- (a) T. Norby, J. Chem. Eng. Jpn., 2007, 40, 1166 CrossRef; (b) K. D. Kreuer, Chem. Mater., 1996, 8, 610 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2009 |
