Template-free synthesis of closed-microporous hybrid and its application in quasi-solid-state dye-sensitized solar cells
Zhang
Lan
,
Jihuai
Wu
*,
Sancun
Hao
,
Jianming
Lin
,
Miaoliang
Huang
and
Yunfang
Huang
Institute of Materials Physical Chemistry, Huaqiao University, Quanzhou, 362021, P.R. China. E-mail: jhwu@hqu.edu.cn; Fax: +86 595-22693999; Tel: +86 595-22693899
First published on 25th February 2009
Abstract
A kind of closed-microporous hybrid with poly(acrylic acid)-poly(ethylene glycol)-polypyrrole (PAA-PEG-PPy) was synthesized template-free by in situ polymerization and micro-phase separation of PPy in PAA-PEG gel. This novel structure hybrid was used as a matrix in preparing polymer gel electrolytes for quasi-solid-state dye-sensitized solar cells (QS-DSSC). It shows high liquid electrolyte absorbency and salt tolerance, which are benefits for improving photovoltaic performance of QS-DSSC.
Broader contextIn the present study, a novel closed-microporous hybrid was synthesized template-free by in situ polymerization and micro-phase separation of polypyrrole in poly(acrylic acid)-poly(ethylene glycol) gel. The microporous hybrid can accommodate a larger amount of liquid electrolyte at high concentrations and does not produce the separating of ionic salt from the polymer gel electrolyte or shrinkage of the polymeric matrix. Based on the microporous hybrid, a polymer gel electrolyte with an ionic conductivity as a liquid electrolyte can be prepared. By using the polymer gel electrolyte to assemble quasi-solid-state dye-sensitized solar cells, the photovoltaic performance and long-term stability for this cell can be improved. The closed-microporous hybrid and its preparation method can be used in other functional materials and devices in energy and environmental science. |
Introduction
Since the prototype of dye-sensitized solar cells (DSSC) was reported in 1991 by O’Regan and Gratzel,1 it has aroused intensive interests over the past decades due to its low cost and simple preparation procedures.1,2 Based on liquid electrolytes, a light-to-electricity conversion efficiency of about 11 % of DSSC has been achieved.3 However, the potential problems caused by liquid electrolytes such as the leakage and the volatilization of the organic solvents, are considered as some of the critical factors limiting the long-term performance and practical use of DSSC. Thus, solid-state and quasi-solid-state electrolytes, such as organic hole conductors, inorganic p-type semiconductors and polymer electrolytes, have been proposed as alternatives to liquid electrolytes.4–7 Among them, polymer gel electrolytes are considered as one kind of the most prospective substitutes for liquid electrolytes to fabricate practical applicable quasi-solid-state dye-sensitized solar cells (QS-DSSC) due to their merits such as high ionic conductivity, good interfacial filling properties and relatively high long-term stability.8,9Polymer gel electrolytes have been actively developed as highly conductive electrolyte materials for lithium secondary batteries and fuel cells.10 The first reported QS-DSSC using polymer gel electrolytes was done by Cao et al.11 using a mixture of polyacrylonitrile, ethylene carbonate, acetonitrile and a specific concentration of NaI. The photovoltaic performance of this QS-DSSC is low in comparison with that of DSSC containing liquid electrolyte.11 The high photovoltaic performance and excellent stability of QS-DSSC was obtained by using a polymer gel electrolyte containing PVDF-HFP combined with methoxypropionitrile based liquid electrolyte.12 Recently, we have reported polymer gel electrolytes based on poly(ethylene glycol) (PEG)13 and a poly(acrylic acid)-poly(ethylene glycol) (PAA-PEG) hybrid14 for high photovoltaic performance and stable QS-DSSC. The highest light-to-electricity conversion efficiency of QS-DSSC containing polymer gel electrolytes is still among 6–7%,15 which is much lower than the highest 11% of DSSC containing liquid electrolyte due to the hindrance of polymeric thickener (in the physical crosslink gel) or polymeric networks (in the chemical crosslink gel) for ionic mobility. If the values of the liquid electrolyte absorbency and the absorbed ionic concentration can be increased in polymer gel electrolytes, the above negative influence of polymeric matrix will be counteracted. However, the high amount of absorbed liquid electrolyte will cause poor long-term stability of polymer gel electrolytes and the high ionic concentration will also cause phase separation or shrinkage of polymeric matrix from polymer gel electrolytes.9 So a novel structure of polymer matrix is needed to solve these problems. In the present study, a novel closed-microporous hybrid was synthesized template-free by in situ polymerization and micro-phase separation of polypyrrole (PPy) in poly(acrylic acid)-poly(ethylene glycol) (PAA-PEG) gel. This novel structure hybrid, used as a matrix in preparing polymer gel electrolyte, can solve the above mentioned problems and improve the photovoltaic performance and long-term stability of QS-DSSC.
Experimental
Our experiments were carried out as the following processes. Firstly, the PAA-PEG [PEG Mw = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, CH2OCH2/COOH (mol/mol) = 1/1] hybrid was synthesized using the same method as reported in our previous papers.14,16 Secondly, the PAA-PEG hybrid was soaked in the mixed solvents with pyrrole and N-methyl pyrrolidine (NMP) [C4H5N/COOH (mol/mol) = 4/10]. The sufficiently swollen hybrid was soaked in 0.5 M ammonium persulfate (initiator) aqueous solution to initiate polymerization of pyrrole. Thirdly, the obtained product was kept in a vacuum oven at 60 °C to remove any remaining water and NMP. Finally, the dried ternary hybrid was soaked in the liquid electrolyte containing suitable amounts of NaI, I2, 0.4 M pyridine, 30 vol. % NMP and 70 vol. % γ-butyrolactone (GBL) to form polymer gel electrolyte.16 Other details about fabrication and measurements of QS-DSSC and the polymer gel electrolytes are all the same as our previous papers except that the samples used for long-term stability measurements have not been sealed.14,16–18
000, CH2OCH2/COOH (mol/mol) = 1/1] hybrid was synthesized using the same method as reported in our previous papers.14,16 Secondly, the PAA-PEG hybrid was soaked in the mixed solvents with pyrrole and N-methyl pyrrolidine (NMP) [C4H5N/COOH (mol/mol) = 4/10]. The sufficiently swollen hybrid was soaked in 0.5 M ammonium persulfate (initiator) aqueous solution to initiate polymerization of pyrrole. Thirdly, the obtained product was kept in a vacuum oven at 60 °C to remove any remaining water and NMP. Finally, the dried ternary hybrid was soaked in the liquid electrolyte containing suitable amounts of NaI, I2, 0.4 M pyridine, 30 vol. % NMP and 70 vol. % γ-butyrolactone (GBL) to form polymer gel electrolyte.16 Other details about fabrication and measurements of QS-DSSC and the polymer gel electrolytes are all the same as our previous papers except that the samples used for long-term stability measurements have not been sealed.14,16–18
Results and discussions
As shown in Fig. 1, the morphology of the ternary hybrid is closed-microporous. The holes are independently distributed in the framework of the polymer gel electrolyte similar to the network structure in the PAA-PEG hybrid.14 The micro-phase separation mechanism can be used to illustrate the formation of the novel structure. The PAA-PEG gel containing pyrrole and NMP is homogenous before initiating the polymerization of pyrrole. When putting it in the ammonium persulfate aqueous solution, polymerization occurred and PPy is synthesized. It is known that PPy is quite difficult to dissolve in organic solvents, so it will separate from the homogenous gel. Because PPy is synthesized in the networks of the PAA-PEG hybrid, it forms interpenetrating networks among the three polymeric components, which can hinder the macro-phase separation of PPy from the ternary hybrid.9 Meanwhile, the hydrogen bonds can easily form among carbonyl groups, hydroxyl groups and nitrogen atoms in PPy. The above reasons cause the phase separation to occur only in the micro-size, which results in the novel closed-microporous structure.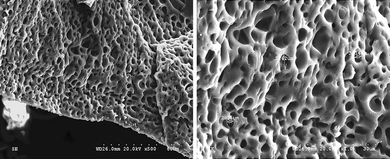 | ||
| Fig. 1 Scanning electron micrograph showing a cross-sectional view of the ternary hybrid. | ||
Fig. 2 shows the ATR-FTIR spectroscopy of the ternary hybrid and the PAA-PEG hybrid, the spectrum of the ternary hybrid contains both the main peaks in the PAA-PEG hybrid and the characteristic peaks in PPy.18 The bands at 1510 cm−1, 1310 cm−1 and 987 cm−1 are related to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching, N–H deformation and ring deformation in PPy, respectively.19 The maximum peaks of the carbonyl bands and the wagging motion of –OH groups lie at 1710 cm−1 and 1240 cm−1 in the PAA-PEG hybrid spectrum, which both shift to a little higher wavenumber (from 1710 cm−1 to 1720 cm−1, 1240 cm−1 to 1260 cm−1) in the ternary hybrid spectrum. Moreover, a split peak at 1640 cm−1 appears in the ternary hybrid spectrum. The data shows that the hydrogen bond interaction exists in the ternary polymeric components.20–22
C stretching, N–H deformation and ring deformation in PPy, respectively.19 The maximum peaks of the carbonyl bands and the wagging motion of –OH groups lie at 1710 cm−1 and 1240 cm−1 in the PAA-PEG hybrid spectrum, which both shift to a little higher wavenumber (from 1710 cm−1 to 1720 cm−1, 1240 cm−1 to 1260 cm−1) in the ternary hybrid spectrum. Moreover, a split peak at 1640 cm−1 appears in the ternary hybrid spectrum. The data shows that the hydrogen bond interaction exists in the ternary polymeric components.20–22
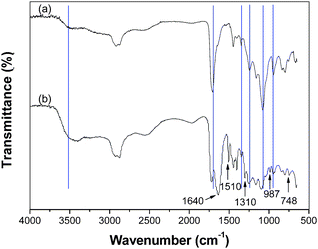 | ||
| Fig. 2 ATR-FTIR spectra of the PAA-PEG hybrid (a) and the ternary hybrid (b). | ||
The novel closed-microporous structure of the ternary hybrid shows good salt tolerance. As shown in Fig. 3, the maximum liquid electrolyte absorbency of the ternary hybrid is obtained when NaI concentration is 0.7 M in liquid electrolyte. However, for the PAA-PEG hybrid, the same NaI concentration causes the shrinkage of the gel to about 20 % of its initial data in pure mixed solvents. It is also seen that the closed-microporous structure of the ternary hybrid makes for obtaining high ionic conductivity of polymer gel electrolytes. For example, the maximum ionic conductivity of polymer gel electrolytes based on the PAA-PEG hybrid (PGEb) and the ternary hybrid (PGEa) is 3.53 mS cm−1 and 6.62 mS cm−1, respectively. The maximum ionic conductivity of PGEa of 6.62 mS cm−1 is obtained when NaI concentration is 1.0 M and the relative liquid electrolyte absorbency is 6.01 (g/g), equivalent to 83 wt % of the whole weight of PGEa. While for PGEb, the proportion is only 78 wt % and the ionic conductivity is about 2.94 mS cm−1 when NaI concentration is 0.9 M. The maximum liquid electrolyte absorbency of PGEb is 6.87 (g/g), equivalent to 85 wt % of the whole weight of PGEb, while its ionic conductivity is only 2.21 mS cm−1, 1/3 of the maximum ionic conductivity of PGEa. Obviously, the ionic concentration in liquid electrolyte determines the ionic conductivity of polymer gel electrolytes.
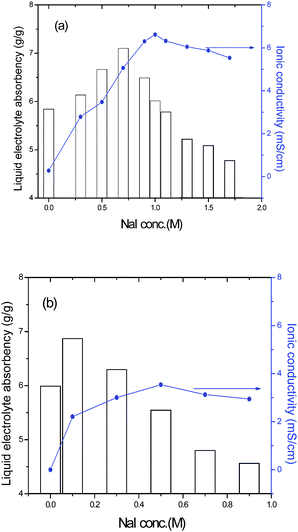 | ||
| Fig. 3 Liquid electrolyte absorbency of the ternary hybrid (a) and the PAA-PEG hybrid (b) in liquid electrolyte with a change in NaI concentration. | ||
The above mentioned data show that the novel closed-microporous structure of the ternary hybrid can absorb large amounts of liquid electrolyte at high ionic concentrations, which is the prerequisite for preparing high ionic conductivity of PGEa. While the case is quite different in PGEb with the networks structure of the PAA-PEG hybrid matrix. The high ionic concentration in liquid electrolyte causes the shrinkage of PGEb and results in a decrease of ionic conductivity.23,24 So there is a bottle-neck for traditional polymer gel electrolytes to increase ionic conductivity further.
The application of polymer gel electrolytes in QS-DSSC still needs another important component of I2 to generate I−/I3− redox couples. The optimized mole proportion of I−/I3− in liquid electrolyte is 10/1, while for gel electrolytes or other kinds of electrolytes, the case is quite different.25–27 So it is necessary to optimize I2 concentration in PGEa. As shown in Fig. 4, the ionic conductivity of PGEa and photovoltaic performance of QS-DSSC are changed with the increase of I2 concentration in liquid electrolyte. The ionic conductivity of PGEa increases gradually with the increase of I2 concentration from 0 M to 0.2 M. The open-circuit voltage of QS-DSSC changes little when I2 concentration changes from 0.05 M to 0.15 M, while the short-circuit current density increases largely from 7.78 mA cm−2 to 15.28 mA cm−2. For the further increase in I2 concentration, the above two key parameters both decrease due to the accelerated dark reaction caused by increased I3− concentration in PGEa reacting with photoinduced electrons. As shown in Fig. 4 (c), the onset of the dark current of QS-DSSC occurs at lower forward bias with the increase of I2 concentration, which demonstrates the accelerated dark reaction in QS-DSSC.
![Ionic conductivity (a) and photovoltaic performance (b) of QS-DSSC [(c) dark condition ] versus I2 concentration in PGEa containing 1.0 M NaI.](/image/article/2009/EE/b821908g/b821908g-f4.gif) | ||
| Fig. 4 Ionic conductivity (a) and photovoltaic performance (b) of QS-DSSC [(c) dark condition ] versus I2 concentration in PGEa containing 1.0 M NaI. | ||
A light-to-electricity conversion efficiency of about 6.30% of QS-DSSC is obtained when I2 concentration attains 0.15 M in PGEa containing 1.0 M NaI. The open-circuit voltage is 0.661 V, which is lower than that of QS-DSSC with PGEb or other polymer gel electrolytes.14,28 A reason is that the iodine-doped conducting polymer PPy in PGEa plays acts as a recombination center for photoinduced electrons and I3− reaction,29 meanwhile, high I3− concentrations in PGEa also accelerates the dark reaction, so the open-circuit voltage is low. However, the QS-DSSC shows high short-circuit current density. It should be owing partly to the high ionic conductivity of PGEa . The other more important reason is that PPy shows high catalytic function for transformation of I3− to I−, which has been demonstrated in our previous paper.30 PPy catalytic material in PGEa plays a role in shortening I3− diffusion distances to counter electrodes for regenerating I−, so I− is sufficient for regenerating oxide dyes. The utilization of sensitized dyes for producing photoinduced electrons can maintain high efficiency. After deducting the amount of consumed electrons in the dark reaction, the whole electrons collected on the conducting glass can still maintain high numbers, so the short-circuit current density shows a relatively high value.
For comparison, photovoltaic performance of DSSCs with different electrolytes including liquid electrolytes, PGEa, PGEb were measured and are shown in Fig. 5, the key parameters are listed in Table 1. The main reason for the lower light-to-electricity conversion efficiency of QS-DSSC containing PGEa than that of DSSC containing liquid electrolyte is its lower open-circuit voltage and fill factor. The fill factor is difficult to improve due to the intrinsic poorer filling property of polymer gel electrolytes than that of liquid electrolytes. The low open-circuit voltage of QS-DSSC containing PGEa is mainly caused by the fast dark reaction due to the negative influence of PPy and high I2 concentration, so if the dark reaction can be restrained, the light-to-electricity conversion efficiency of QS-DSSC may be further improved.
| Electrolyte | σ/mS cm−1 | Voc/V | Jsc/mA cm−2 | FF | η (%) |
|---|---|---|---|---|---|
| a Liquid: 0.5 M NaI, 0.05 M I2, 0.4 M pyridine in 30 vol. % NMP and 70 vol. % GBL mixed solvents. b PGEa, the ternary hybrid soaked in liquid electrolyte containing 1.0 M NaI, 0.15 M I2, 0.4 M pyridine, 30 vol. % NMP and 70 vol. % GBL. c PGEb, the PAA-PEG hybrid soaked in liquid electrolyte containing 0.5 M NaI, 0.05 M I2, 0.4 M pyridine in 30 vol. % NMP and 70 vol. % GBL mixed solvents. | |||||
| Liquid | 8.64 | 0.705 | 13.34 | 0.712 | 6.70 |
| PGEa | 8.16 | 0.661 | 15.28 | 0.624 | 6.30 |
| PGEb | 5.35 | 0.724 | 11.41 | 0.635 | 5.25 |
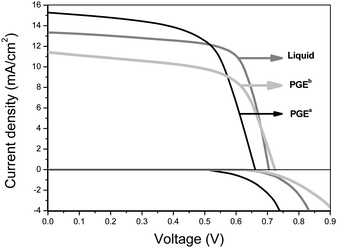 | ||
| Fig. 5 Photovoltage–current (light and dark conditions) curves of DSSCs with the different electrolytes listed in Table 1. | ||
Fig. 6 (b) shows another important parameter of QS-DSSC: long-term stability. Although PGEa contains a high amount of liquid electrolyte, QS-DSSC containing PGEa shows moderate long-term stability. It is seen that a thin hard layer on the surface of PGEa and PGEb is formed due to the shrinkage of surface polymer matrix by going with the volatilization of liquid electrolytes. This thin layer can hold back the continuous volatilization of liquid electrolyte in polymer gel electrolytes as shown in Fig. 6 (a), so QS-DSSCs show moderate long-term stability even without strictly sealing processes. It means that the novel structure of the ternary hybrid makes for high photovoltaic performance and high long-term stability of QS-DSSC.
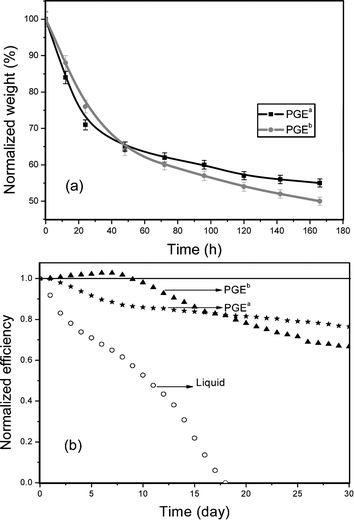 | ||
| Fig. 6 Normalized weight of PGEa and PGEb at 50 °C versus conservation time (a) and light-to-electricity conversion efficiencies of DSSCs versus conservation time in ambient environment (b). | ||
Conclusions
A kind of closed-microporous ternary hybrid with PAA-PEG-PPy was synthesized template-free by in situ polymerization and micro-phase separation of PPy in PAA-PEG gel. The novel structure ternary hybrid shows high liquid electrolyte absorbency in high ionic concentration liquid electrolyte, which solves the bottle-neck for further improving ionic conductivity existing in traditional polymer gel electrolytes. QS-DSSC containing the polymer gel electrolyte based on the ternary hybrid shows high photovoltaic performance due to high ionic conductivity of the polymer gel electrolyte and the catalytic function of PPy for transformation of I3− to I−. Although the polymer gel electrolyte based on the ternary hybrid contains large amounts of liquid component, it does not influence the long-term stability of QS-DSSC. The present findings should accelerate the practical applications of DSSC.Acknowledgements
The authors acknowledge the joint support of the National High Technology Research and Development Program of China (no. SQ2008AA03Z2470974), the National Natural Science Foundation of China (no. 50572030, 50842027), the Key Project of Chinese Ministry of Education (no. 206074) and Specialized Research Fund for the Doctoral Program of Chinese Higher Education (no. 20060385001).References
- B. O′ Regan and M. Gratzel, Nature, 1991, 353, 737 CrossRef CAS.
- M. Gratzel, Nature, 2001, 414, 338 CrossRef CAS.
- M. Gratzel, Prog. Photovoltaic: Res. Appl., 2006, 14, 429 Search PubMed.
- M. Biancardo, K. West and F. C. Krebs, J. Photochem. Photobiol., A, 2007, 187, 395 CrossRef CAS.
- U. Bach, D. Lupo and M. Gratzel, Nature, 1998, 395, 583 CrossRef.
- J. Kruger, R. Plass, M. Gratzel and H. J. Matthieu, Appl. Phys. Lett., 2002, 81, 367 CrossRef CAS.
- Z. H. Li, J. Jiang, G. Lei and D. S. Gao, Polym. Adv. Technol., 2006, 17, 604 CrossRef CAS.
- M. Biancardo, K. West and F. C. Krebs, Sol. Energy Mater. Sol. Cells, 2006, 90, 2575 CrossRef CAS.
- S. B. R. Murphy, R. F. T. Stepto (Ed.), Polymer Networks-Principles of Their Formation, Structure and Properties, Blackie Academic and Professional, London, 1998 Search PubMed.
- K. M. Abraham, in: B. Scrosati (Ed.), Application of Electroactive Polymer, Chapman & Hall, London, 1993 Search PubMed.
- F. Cao, G. Oskam and P. C. Searson, J. Phys. Chem. B, 1995, 99, 17071 Search PubMed.
- P. Wang, S. M. Zakeeruddin, J. E. Moser, M. K. Nazeeruddin, T. Sekiguchi and M. Gratzel, Nat. Mater., 2003, 2, 498 CAS.
- J. H. Wu, S. C. Hao, Z. Lan, J. M. Lin, M. L. Huang and Y. F. Huang, Adv. Funct. Mater., 2007, 17, 2645 CrossRef CAS.
- J. H. Wu, Z. Lan, J. M. Lin, M. L. Huang, S. Yin and T. Sato, Adv. Mater., 2007, 19, 4006 CrossRef CAS.
- R. Komiya, L. Han, R. Yamanaka, A. Islam and T. Mitate, J. Photochem. Photobiol., A, 2004, 164, 123 CrossRef CAS.
- J. H. Wu, Z. Lan, J. M. Lin, M. L. Huang and S. C. Hao, J. Power Sources, 2007, 164, 921 CrossRef.
- J. H. Wu, Z. Lan, J. M. Lin, M. L. Huang, S. C. Hao and L. Q. Fang, Electrochim. Acta, 2007, 52, 7128 CrossRef CAS.
- Z. Lan, J. H. Wu, J. M. Lin, M. L. Huang, S. Yin and T. Sato, Electrochim. Acta, 2007, 52, 6673 CrossRef CAS.
- R. V. Parthsarathy and C. R. Martin, Chem. Mater., 1994, 6, 1627 CrossRef.
- M. M. Coleman, J. F. Graf, P. C. Painter, Specific Interaction and the Miscibility of Polymer Blends, Technomic, London, 1991 Search PubMed.
- K. Halina and S. Aleksandra, J. Photochem. Photobiol., A, 2006, 180, 46 CrossRef.
- Y. Han and Y. Lu, Carbon, 2007, 45, 2394 CrossRef CAS.
- J. H. Wu, J. M. Lin and M. Zhou, Macromol. Rapid Commun., 2000, 21, 1032 CrossRef CAS.
- J. M. Lin, J. H. Wu, Z. F. Yang and M. L. Pu, Macromol. Rapid Commun., 2001, 22, 422 CrossRef CAS.
- R. Kawano and M. Watanbe, Chem. Commun., 2003, 330 RSC.
- W. Kubo, K. Murakoshi, T. Kitamura, S. Yoshida, M. Haruki, K. Hanabusa, H. Shirai and Y. J. Wada, J. Phys. Chem., 2001, 105, 12809 Search PubMed.
- R. Kawano and M. Watanabe, Chem. Commun., 2005, 2107 RSC.
- J. H. Wu, Z. Lan, D. B. Wang, S. C. Hao, J. M. Lin, Y. F. Huang, S. Yin and T. Sato, Electrochim. Acta, 2006, 51, 4243 CrossRef CAS.
- S. Handa, S. A. Haque and J. R. Durrant, Adv. Funct. Mater., 2007, 17, 2878 CrossRef CAS.
- J. H. Wu, Q. H. Li, L. Q. Fang, Z. Lan, P. J. Li, J. M. Lin and S. C. Hao, J. Power Sources, 2008, 181, 172 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2009 |
