Natural dye senstizers for photoelectrochemical cells
Giuseppe
Calogero
a,
Gaetano
Di Marco
a,
Stefano
Caramori
*b,
Silvia
Cazzanti
b,
Roberto
Argazzi
c and
Carlo Alberto
Bignozzi
*b
aCNR, Istituto per i Processi Chimico-Fisici, Sede di Messina, Salita Sperone, C.da Papardo, I-98158, Faro Superiore Messina, Italy
bDipartimento di Chimica Università di Ferrara, Via L.Borsari 46, 44100, Ferrara, Italy
cIstituto per la Sintesi Organica e la Fotoreattività (ISOF-CNR) c/o Dipartimento di Chimica, Università di Ferrara, Via L.Borsari 46, 44100, Ferrara, Italy
First published on 27th August 2009
Abstract
In nature, fruit, vegetable, leaves, flowers and algae contain several dyes which can be easily extracted and employed in dye sensitized photoelectrochemical cells. In this contribution, the most significant advances made in the search for efficient and convenient natural sensitizers are reported through meaningful examples and case studies.
To date, selected chlorophyll derivatives, raw anthocyanine and betalain extracts are the most successful natural sensitizers, resulting in the generation of monochromatic photon to current conversion yields exceeding 60%. Maximum overall conversion efficiencies above 2% under simulated sunlight have been achieved, which is comparable to that of natural photosynthesis. Finding appropriate additives for improving VOC without causing dye degradation might result in a further enhancement of cell performance, making the practical application of such systems more suitable to economically viable solar energy devices for our society.
 Stefano Caramori | Stefano Caramori was born in Copparo on October 19th 1977. He has been working in Bignozzi's group since his graduation in November 2001. In March 2005, he obtained a PhD in Chemical Sciences from the University of Ferrara. His scientific activity focuses mainly on the electrochemical and spectroscopic study of semiconductor/electrolyte interfaces. He has authored and co-authored 20 papers in international journals and has authored 5 patents for nanostructured materials, solar energy conversion and electrochemical biosensors. |
 Carlo Alberto Bignozzi | Carlo Alberto Bignozzi is professor of Inorganic Chemistry at the University of Ferrara since 2000 and Chairman of the Department of Chemistry. He was Associate Professor of Inorganic Chemistry (1985–2000) and Assistant Professor (1977–1984). He has been a member of the International Organizing Committee of the Solar Energy Conference (1995–2000) and visiting professor in several European, Japanese, South and North American Universities. His research investigates photoinduced energy and electron transfer processes in inorganic coordination compounds, in extended solids and in functional nanomaterials. Practical applications of this research include solar energy conversion, chemical sensing, photocatalysis, design of nanomaterials for environmental decontamination, photochromic and electrochromic devices. He is the author of over 150 papers and review articles on international journals and of 16 patents. |
Broader contextDye sensitized solar cells rely on photoactive dyes chemically adsorbed at the surface of a wide band gap nanocrystalline semiconductor. The photosensitizer is the core of such devices, since, following photon absorption, it gives rise to a photoinduced charge transfer into the empty band of the semiconductor, realizing the charge separation. Therefore, unlike conventional solar devices based on p-n junction, charge separation takes place directly at the semiconductor/electrolyte interface without need of exciton transport and limitations arising from the built-in-potential. Moreover, these cells can be made transparent and can be used in a broad architectural context, being potentially integrable in photovoltaic windows and façades. To date, the most effective and stable dyes are based on ruthenium and osmium metal-organic complexes, which, however, require multistep synthetic procedures and careful chromatographic purification. As a consequence, there is an inherent practical and fundamental interest in exploring and exploiting photoactive dyes directly extracted from natural pigments. By using selected natural dyes, overall conversion efficiencies comparable to that of natural photosynthesis (2%) have already been achieved. Thus, as the search for efficient natural pigments progresses, economically viable solar energy devices for our society become closer. |
Dye sensitized solar cells (DSSCs) have attracted considerable interest as a low cost photovoltaic technology for building integration and indoor application.1–4Sensitization of wide band gap semiconductor electrodes with dyes absorbing visible light has been a topic of continuous interest since its theoretical and experimental foundation by Gerischer, Tributsch and Calvin in the early '70s.5,6 Although quantum efficiency as high as 0.125 electrons per absorbed photon were achieved using ZnO photosensitized by chlorophyll derivatives, the photocurrents delivered by the early devices were extremely small (few µA/cm2, under monochromatic illumination of unspecified intensity) due to the limited light harvesting efficiency of a dye monolayer on a semiconductor monocrystal. The advent of porous films consisting of nanometer sized semiconductor nanoparticles (TiO2,2ZnO,7,8 SnO2,9,10 Nb2O511) (Fig. 1) allowed for a dramatic enhancement of the effective surface area, making light absorption efficient by a single dye monolayer adsorbed on each particle.
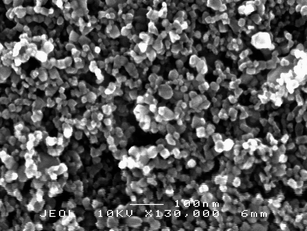 | ||
| Fig. 1 SEM micrograph of a TiO2 film obtained by a sol gel procedure in acidic conditions (HNO3). Magnification: 130000 X. | ||
The elementary events at the basis of semiconductor sensitization can be schematized as follows:
| S + hν → S* | (1) |
| S* + TiO2 → S+ + e−(TiO2) | (2) |
| S+ + I− → S + I• | (3) |
A schematic picture of the processes, which are at the basis of the functioning of sandwich type solar devices is shown in Fig. 2 together with an example of a DSSC device currently under advanced development.
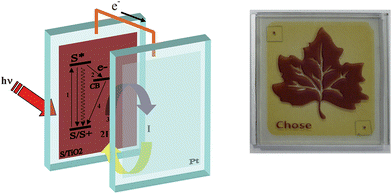 | ||
| Fig. 2 Schematic representation of the working principle and assembly of a sandwich type cell (left). Example of advanced DSSC currently under development (Polo Solare Organico Regione Lazio). | ||
In (1) the sensitizer (S), upon photon absorption, is promoted to an electronically excited state (S*). If this state lies energetically above the conduction band of the semiconductor, electron injection to the empty states of the semiconductor can occur on a very fast time scale (2), successfully competing with the other excited state deactivation pathways (radiative and non radiative processes).
The oxidized sensitizer (S+) is then regenerated by a redox electrolyte (usually the I−/I3− couple) according to process (3), in order to prevent electron recapture as the following process
| S+ + e−(TiO2) → S | (4) |
The I−/I3− system, dissolved in organic solvents, has been almost universally used as a charge carrier in dye sensitized photoelectrochemical cells, since oxidation of iodide by the photooxidized dye is usually fast and effectively competes with (4). Recently much attention has also been devoted to the development of alternative redox couples based on non corrosive transition metal complexes.12
Generally, synthetic organic dyes13–15 and transition metal coordination compounds (mostly ruthenium16,17 and osmium18–20 polypyridil complexes) have been used as effective sensitizers, since they couple broad and intense charge transfer bands to favourable ground and excited state energetics for the electron transfer reactions (2) and (3). However, the preparation routes for metal complexes are often based on multi step procedures involving tedious and expensive chromatographic purification procedures. Thus, for both practical and fundamental reasons, some groups have investigated the possibility of achieving solar energy conversion exploiting nanocrystalline titania sensitization with natural pigments.
In nature, fruit, vegetable, leaves, flowers and algae contain several dyes which can be easily extracted and employed in dye sensitized solar cells. For example, Tennakone et al. investigated the use of tannins and related phenolic substances extracted from black tea, nuts and pomegranate, as well anthocyanins from flowers and leaves.21 Dai, Rabani,22,23 Murakami Iha,24 Zhang,25 Calogero26 and Bignozzi27 investigated anthocyanins and betalain pigments extracted from a variety of vegetable species typical of mediterranean, tropical and sub-tropical areas. Graetzel and Kay were probably the first to elucidate the photoelectrochemical behaviour of natural chlorophylls28 as well as anthocyanin dyes from California blackberries.29
In this contribution we will report, through meaningful examples and case studies, the most significant advances made through efficient and convenient natural sensitizers.
1. Chlorophyll derivatives
Chlorophylls are the key components of natural photosynthetic systems in green plants, bacteria and algae. In photosynthetic reaction centers, bacteriochlorophylls are organized in a special pair which, upon photoexcitation initiates the charge separation events leading to the fabrication of NADPH and ultimately of ATP, thus converting solar energy into chemical energy for the living organism. It is worthwhile to note that the special pair is excited by energy transfer from antenna systems made of different types of porphyrin and carotenoid arrays, abundantly distributed in grana structures, which effectively harvest sunlight and funnel the electronic excitation energy to the reaction centers.The porphyrin macrocycle is the core of chlorophyll structures. The nature of the frontier orbital in porphyrin macrocycles has been an active area of research for computational and spectroscopic investigations. Ab initio methods (restricted Hartree Fock, local density functional theory) have shown that free base, substituted and metal (Mg(II), Cu(II), Zn(II), Ni(II)) containing porphyirins generally possess two a1u and a2u orbitals as the HOMO and HOMO-1.30 Typical electron density distribution in these orbitals are shown in Fig. 3. The electron density in a1u type orbital is essentially localized on the Cα and Cβ atoms of the pyrrole rings, while in the a2u orbital is mainly found on the pyrrole nitrogens and on the meso carbons.
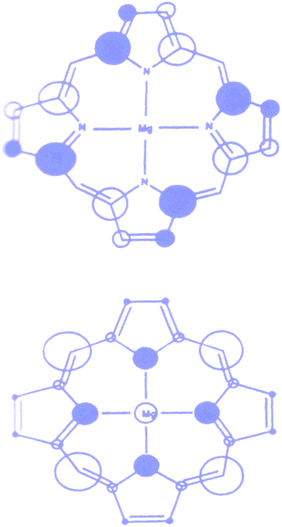 | ||
| Fig. 3 Highest occupied molecular orbitals in typical metal (Mg) porphyrin complex. The size of the circles are proportional to the square of the orbital coefficients of each atom. | ||
As for the highest occupied orbitals, the lowest lying unoccupied orbitals are also similar in metal free and metal containing porphyrins: there are two nearly degenerate eg(π*) orbitals essentially centred on the porphyrin macrocycle. The persistence of a1u(π) and a2u(π) orbitals in diverse metal porphyrins can account for the facile formation of a π radical cation that plays an important role in the biochemical function of porphyrins. For example, the π radical cation formation is central in the photooxidation of chlorophyll which is essentially a Mg containing porphyrin. The nature of the frontier orbitals accounts also for the similar features in the absorption spectra of porphyrin derivatives. These show two characteristic π–π* absorption regions, the weaker Q band at lower visible wavelengths (550–700 nm) and the intense soret band in the near UV region (400–450 nm).
One of the most successful applications of natural chlorophyll derivatives to TiO2sensitization dates back to 1993 by Graetzel et al.Chlorophyll a (Fig. 4, (1)) was either used as pure commercial product or extracted with methanol from spinach leaves and partially purified by precipitation in dioxane/water. Treatment of a diethylether solution with dilute HCl gave the magnesium free pheophytin (Fig. 4, (2)), whereas the acid hydrolysis of the phytyl ester bonds gave pheophorbides (a) (Fig. 4, (3)) and (b) (not shown) which could be separated by extraction in diethylether at their respective HCl numbers.
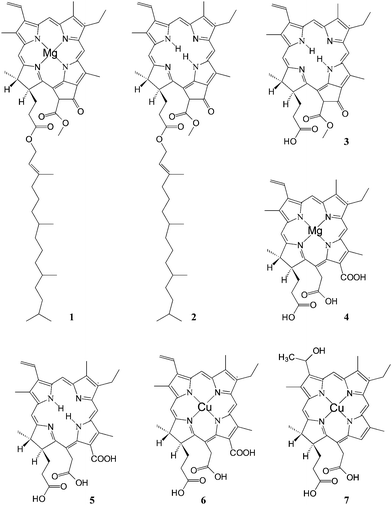 | ||
| Fig. 4 Structure of natural chlorophyll derivatives: (1) chlorophyll a; (2) pheophytin; (3) pheophorbide a; (4) Mg chlorin e6; (5) chlorin e6; (6) Cu chlorine e6; (7) Cu-2-α-oxymesochlorin. | ||
Alkaline hydrolysis of chlorophyll not only saponifies the phytil ester but also opens the cyclopentanone ring with the formation of two additional carboxylic groups leading to chlorophylline (Fig. 4, (4)), the Mg complex of chlorin e6. Copper chlorophyllin is produced on a large scale as a water soluble dye for application in the food and cosmetic industries. The introduction of copper in the chlorin ring (Fig. 4, (5)) improves the photostability of the dye by reducing the excited state lifetime. However, commercial chlorophyllin contains several degradation products, due to decarboxylation side reactions which lead to the absence of the carboxylic group conjugated to the π electron system. Thus, to avoid decarboxylation, the carboxylic acid groups of chlorin have been protected as trimethyl esters by treatment with NaOCH3 before the introduction of copper by addition of a saturated solution of cupric acetate in acetic acid. Saponification was carried out afterwards, leading to a good quality Cu chlorin dye (Fig. 4, (6)).
Chlorophyll a (1) does not adsorb efficiently on TiO2 from most polar organic solvents, due to the weak interaction of the ester keto and carbonyl groups with the oxide surface. However the adsorption is carried out quite efficiently (A670 = 0.3) from diethyl ether or hexane. The absorption spectrum on TiO2 film is broadened and red shifted compared to the absorption spectrum in solution, probably due to the interaction with the surface and dye aggregation. The photocurrent efficiency (number of electron per incident photon (IPCE)) is low, with an IPCE670 = 3.5% in the presence of pyridine, which acting as an axial Mg ligand, reduces dye aggregation and excited state quenching. Pheophytin (2), the free base of chlorophyll, shows a similarly poor photosensitization behaviour.
The carboxylic group of pheophorbide (3) allows for a much stronger adsorption onto TiO2, resulting in an optical density of the electrode in the Qy band (corresponding to the lowest singlet excited state) at 675 nm, of the order of 0.7. Despite this, the IPCE675 was only 25%, far behind the unit quantum efficiency for natural photosynthesis. Under the hypothesis that the propionic acid side chain might act as an insulating barrier for charge injection into the conduction band of TiO2, chlorophyll derivatives with a carboxylic acid conjugated to the π electron system of the tetrapyrrole macrocycle were investigated (4–6). The free base chlorin (5) and Cu chlorin (6) resulted in good efficiencies in the whole visible region, with an IPCE up to 70% for (6) at 650 nm (Fig. 5).
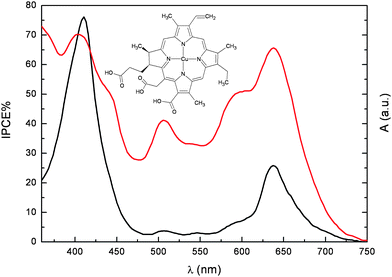 | ||
Fig. 5 Photoaction spectrum (IPCEvs wavelength) of (6) adsorbed onto 12 µm thick TiO2 photoelectrodes ( ). Absorption spectrum in ethanol + 20 mM deoxycholic acid ( ). Absorption spectrum in ethanol + 20 mM deoxycholic acid ( ). ). | ||
However, the presence of the conjugated carboxylic anchoring group could not be considered entirely responsible for the good quantum efficiencies, since, for example, Cu-2-α-oxymesochlorin (7), obtained as a major product from saponification of raw chlorophyll in the presence of Cu2+, gave very similar IPCE (up to 70% at 630 nm). Based on this evidence, conjugation of the attaching group with the porphyrin ring does not seem strictly necessary for efficient injection.
Both the photocurrent and the photovoltage generated with chlorophyll derivatives could be significantly improved by addition of relatively high concentration (20–100 mM) of cholanic acids to the ethanolic dye solutions. This family of bile acids is characterized by a steroid skeleton with a carboxylic acid side chain and a variable number of OH groups on one side of the steroid backbone. Due to these groups, cholanic acids bind to the TiO2 surface, and act as spacers preventing dye aggregation and avoiding excited state self quenching. Adsorption of these acidic species also cause a positive shift of the conduction band edge of TiO2, resulting in a larger driving force for photoinjection from the excited dye. The effect is most prominent with Cu-2-α-oxymesochlorin (7) which has an excited state oxidation potential barely more negative (−0.5 V vsSCE) than the conduction band edge of TiO2. Indeed, the IPCE630 generated by (7) increases from 25% to 68% in the presence of 20 mM chenodeoxycholic acid.
Detailed investigations based on sub-nanosecond laser spectroscopy31 have demonstrated that non fluorescent chlorophyll derivatives like Cu chlorins have smaller charge injection rate constants (kinj = 3 108 s−1) than dyes injecting from singlet states like the free base chlorin e6 (kinj = 2.2 109 s−1), however the longer lived triplet state of Cu chlorins still allows for an high electron injection quantum yield. Moreover competitive excited state quenching due to exciton migration and annihilation is reduced with metal porphyrins undergoing efficient intersystem crossing, since long range energy transfer (Förster type) is spin forbidden. Interestingly photon to current conversion yields have been reached in the red part of the spectrum, with overall efficiencies of 2.6% (JSC = 9.4 mA/cm2, VOC = 530 mV for (7)) under full sunlight (0.1 W/cm2).
Following the first efforts at the EPFL, other researchers have pursued efficient TiO2sensitization exploiting natural pyrrole macrocycles. Interestingly, Amao et al.32 have used Zn chlorine e6 (Fig. 6a) to spontaneously form side-to-side π aggregates (J-aggregates) on the TiO2 surface. The formation of aggregates results in a considerable red shift of the electronic spectrum of the sensitizer which shows an absorption maximum at 800 nm. Although the absolute values of the IPCE were rather low (<10%), an IPCE % of 7% at 800 nm was achieved, indicating that, through careful tuning of the electronic properties of the sensitizer, a photoelectrochemical device based on natural dyes can absorb and convert near IR photons into electrons.
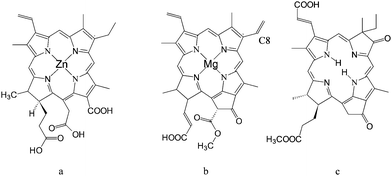 | ||
| Fig. 6 Structure of: Zn chlorin e6 (a); chlorophyll c2 (Chl–c2) (b); synthetic oxo-bacteriochlorin B1. | ||
Wang et al.33 achieved global power conversion efficiencies as high as 4.6% under simulated sunlight, by employing purified chlorophylls extracted from Wakame brown seaweed (Undaria pinnatifida), of which the most effective was chlorophyll c2, probably due to the presence of the vinyl group in the C8 position (see Fig. 6b) which, acting as an electron donor, increases the electronic density of the chlorophyill ring. This results in a slight red shift of the absorption spectrum of the dye and, more importantly, in an improvement of the electron donating capabilities of its excited state, thanks to a less positive ground state oxidation potential (1.06 V vsNHE with respect to 1.13 V vsNHE measured for the saturated Chl–c2 with the ethyl group in the C8 position).
By using TiO2 photoelectrodes equipped with a light scattering layer, Chl–c2 generated IPCE % ranging from 80 to 60% in the 380–700 nm range, and correspondingly displayed a relevant performance under AM 1.5 conditions, with JSC ≈ 14 mA/cm2 and Voc = 0.58 V.
These performances have been very recently surpassed by an entirely synthetic bacteriochlorin sensitizer34 capable of a panchromatic absorption, up to 800 nm. Although, strictly speaking, this latter dye is not natural, its design was clearly inspired by nature, since its structure (Fig. 6c) closely resembles previously reported chlorophyll derivatives.
Starting from 800 nm, oxo-bacteriochlorin B1 achieved an IPCE % of 70% in the whole visible region and a global efficiency of 6.6%, which to date, is the highest reported solar to electrical power conversion for chlorin sensitizers.
2. Anthocyanin dyes
Flavonoids are sugar bound polyphenols found in all land plants. A class of flavonoids called anthocyanins are responsible for the red and purple coloration of many fruits and flowers. An ample repertoire of colors in the red blue range are available to anthocyanins as a result of their complexation with other polyphenols, pectins and metal ions.35 Proposed biological roles of anthocyanins include insect attraction, photoprotection,35antioxidant activity36 and photosynthesis enhancement, but the full scope of their abilities has yet to be fully understood.In acidic solution anthocyanine dyes appear red, due to an intense band centred at ca. 520 nm (Fig. 7).
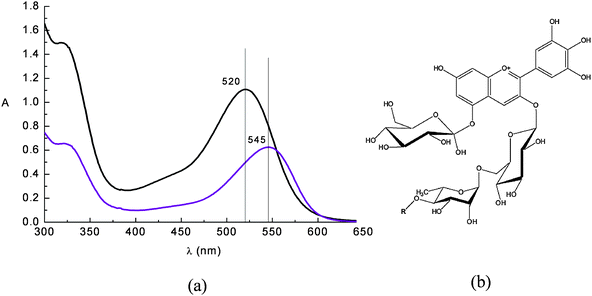 | ||
Fig. 7 (a) Absorption spectrum of a raw eggplant extract in water solution at pH = 1 ( ) and in ethanol solution ( ) and in ethanol solution ( ). Nasunin (b) is the main pigment contained in eggplant peels. ). Nasunin (b) is the main pigment contained in eggplant peels. | ||
The visible band is assigned to a π–π* charge transfer transition which results in a shift of the electronic charge density from the chromenium portion to the catechol end of the anthocyanin molecule. Hence the LUMO presents increased charge density in close proximity to the titanium binding site (Fig. 8) allowing for good electronic coupling for charge injection. The absorption band of anthocyanins is pH and solvent sensitive, showing the red flavylium form in acidic solution and the purple deprotonated quinonoidal form as pH increases. The visible absorption band also shifts to lower wavelengths upon coordination to metal ions (Fig. 8a,b).
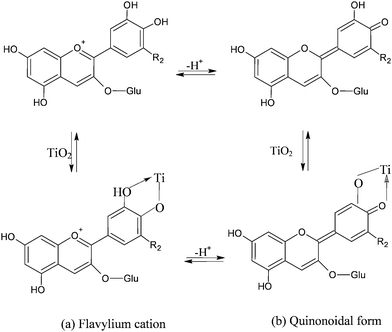 | ||
| Fig. 8 Equilibrium between flavylium and quinonoidal form in cyanidine-3-glucoside in solution and in presence of TiO2. Upon adsorption onto TiO2 the equilibrium between the forms is believed to shift towards the quinonoidal form, consistent with the purple coloration of the electrodes. | ||
Adsorption of cyanine onto TiO2 is a quick reaction that leads to the formation of a strong complex via displacement of an OH− from the surface and formation of H2O with a proton donated by the cyanin. The complexation geometry, mononuclear bidentate, has been deduced from comparisons with anthocyanines which do not bind metal ions,37 from Al(III) complexation studies38 and from investigations involving cathecol adsorption on metal oxides.39–41 Adsorption from raw acidic aqueous dye extracts in the presence of 0.1 M HCl was found to give more intensely coloured photoanodes (OD > 2.5–3 for selected eggplant and red grapes extracts) (Fig. 9b) than those obtained from ethanolic solutions (Fig. 9a). This fact is most probably due to the predominance of the flavylium form (protonated) which, initially, is capable of more effective coordination to Ti4+ sites.
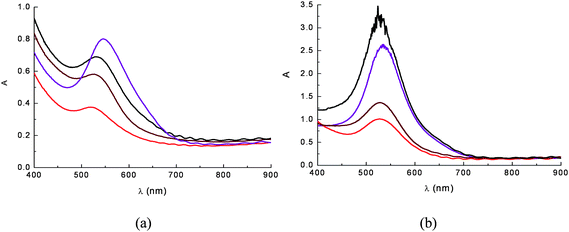 | ||
Fig. 9
Absorption spectra on TiO2 film of anthocyanine extracts from ethanolic (a) and 0.1 M HCl aqueous solutions (b). The dye sources were eggplant peels ( ), red radicchio ( ), red radicchio ( ), Nero d'Avola grapes ( ), Nero d'Avola grapes ( ) and Giacchè grapes ( ) and Giacchè grapes ( ). Nero d'Avola and Giacchè are typical red wines from Sicily. ). Nero d'Avola and Giacchè are typical red wines from Sicily. | ||
Nanosecond differential absorption spectra of different anthocyanine dye extracts upon 532 nm Nd:YAG excitation (Fig. 10), reveal common spectral features: positive absorption in the 400–480 nm and 580–700 nm regions accompanied by intense ground state bleaching, peaking at 550–570 nm, depending on the dye. These spectral signatures are consistently different from those observed for the dye adsorbed on TiO2. The transient absorption spectra of the adsorbed dyes lack the positive absorption in the 580–700 nm region and show intense bleaching red shifted with respect to the one observed for the dye solution and which mirrors the ground state absorption onto TiO2. These spectroscopic differences are most probably due to the fast (instrument response limited) electron injection to the conduction band of TiO2, leaving the radical cation of the quinonoidal species on the semiconductor surface.
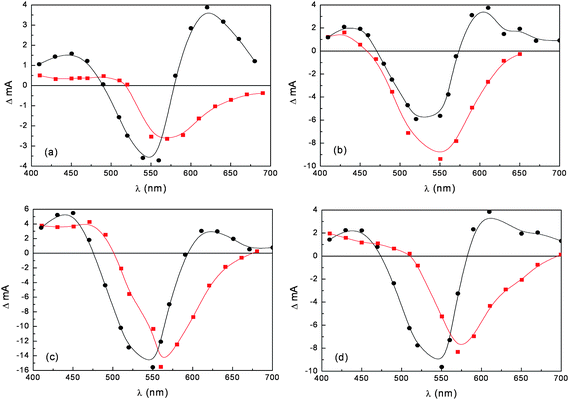 | ||
Fig. 10 Nanosecond transient absorption spectra in ethanol solution (●) and on TiO2 film ( ) of anthocyanines extracted from (a) eggplant; (b) red radicchio; (c) Nero d'Avola and (d) Giacchè. ) of anthocyanines extracted from (a) eggplant; (b) red radicchio; (c) Nero d'Avola and (d) Giacchè. | ||
The existence of ultrafast charge injection processes involving blackberry anthocyanines has been demonstrated by Cherepy et al.29 who observed, by femtosecond laser spectroscopy, a pulse width limited (<100 fs) absorption rise, due to electrons injected into the d band of the semiconductor. This behaviour is consistent with the strong electronic coupling occurring through catechol moieties which results in a charge transfer interaction between the cyanine and Ti(IV).
Despite very fast charge injection, the IPCE % generated by blackberry anthocyanins was quite low (max. 20%) for reasons possibly related to dye aggregation, electron recombination with the oxidized sensitizer and electron recapture by I3− which is dependent upon dye structure and surface coverage.
However it must be considered also that the source and the extraction conditions of natural dyes are important for achieving efficient sensitization. In fact, different vegetable species can develop peculiar pools of antioxidant compounds that protect fruits and leaves from specific environmental and soil conditions. The search for effective dye sources has led to the discovery of efficient sensitization from raw extracts of Jaboticaba and Calafate, native of Brazil,24,42 from pomegranate23 and also from specific varieties of sicilian orange and eggplant.26 The eggplant (Solanum melongena) of the family of Solanaceae, native of India, has been cultivated in Sicily since ancient times. It was introduced by the Arabs and is well adapted to the Sicilian climate. The extract of eggplant peels contains mainly nasunin, a mixture of cis-trans isomers of delphinidine. Maximum IPCE% values of 65% were obtained with transparent TiO2 photoanodes sensitized with eggplant extracts in 0.1 M HCl using a compact TiO2 blocking underlayer to suppress electron back recombination with the oxidized electrolyte (I3−). Interestingly IPCE % (45 and 40% respectively) was also achieved with red radicchio (Cychorium intybus sativa) and Giacchè red grapes (a cultivar of Vitis vinifera containing a particularly high concentration of flavonoids) (Fig. 11).
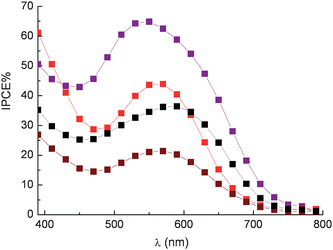 | ||
Fig. 11 Photoaction spectra recorded on sandwich type DSSCs sensitized with eggplant extract ( ), red radicchio ( ), red radicchio ( ), Nero d'Avola grapes ( ), Nero d'Avola grapes ( ) and Giacchè grapes ( ) and Giacchè grapes ( ) using LiI/I2 0.5/0.05 M in acetonitrile as electron mediator. ) using LiI/I2 0.5/0.05 M in acetonitrile as electron mediator. | ||
The effect of the blocking underlayer (Fig. 12) in enhancing cell performance agrees with the recent findings of Burke et. al.43 in which it is pointed out that, compared to bulkier Ru(II) complexes, smaller and flat organic molecules like the sensitizers reported here may not be able to insulate well the underlying conductive oxide (fluorine tin oxide) from the oxidized electrolyte, resulting in an increased dark current. For this reason, interface optimization is required to exploit the full potentialities of such dyes. In general, natural anthocyanine dyes suffer from low VOC (350 mV) which is at best 200 mV lower than that of an equivalent cell sensitized with Ru(II) polypyridine complexes. This can be due both to possible efficient electron/dye cation recombination pathways and to the acidic dye adsorption environment. On the other hand the addition of 4-tert-butylpyridine, an additive known to increase the photovoltage of the cell, had to be avoided since it caused dye desorption
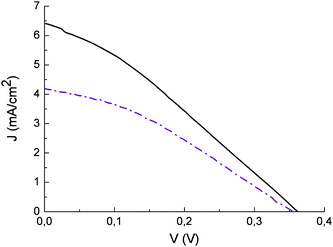 | ||
Fig. 12 Current potential curves of photoelectrochemical cells sensitized with eggplant extract with blocking underlayer (![[thick line, graph caption]](https://www.rsc.org/images/entities/char_e117.gif) ) and without blocking underlayer ( ) and without blocking underlayer ( ). Incident irradiance ≈ 0.1 W/cm2. LiI/I2 0.5/0.05 in acetonitrile as electron transfer mediator. ). Incident irradiance ≈ 0.1 W/cm2. LiI/I2 0.5/0.05 in acetonitrile as electron transfer mediator. | ||
The importance of a blocking layer in controlling recombination with the electrolyte has been pointed out, but, in the case of natural dyes, recombination with the oxidized dye can also be important. Compared to ruthenium sensitizers, in which the hole is confined into a metal centred d orbital relatively decoupled from the semiconductor surface, electron recapture by the cation is expected to be faster because the hole is located in closer proximity to the nanoparticle surface. Nanosecond laser spectroscopy carried out on nasunine sensitized thin films (Fig. 13a), using an excitation energy of 0.35 mJ/cm2/pulse, evidenced a relatively fast electron/dye cation recombination process following multiexponetial kinetics due to the distribution of surface states from which recombination usually occurs. In the presence of 0.5 M Li+ an apparent time constant corresponding to 2/3 of the initial signal amplitude (τ2/3) of 174 ns was found. In the presence of 0.15 M iodide the lifetime of the photoxidized anthocyanin was substantially decreased (56 ns) due to the interception of the oxidized dye by I− and recovery was >90% complete within 0.65 µs from the laser pulse. As a comparison, in analogous conditions, the [Ru(H2DCB)(NCS)2] (N3) sensitizer showed τ2/3 = 500 ns in the presence of 0.1 M LiClO4 which was reduced to 56 ns in the presence of 0.1 M I−, with dye regeneration almost totally complete within 200 ns from the laser pulse (Fig. 13b). Thus, the kinetic competition between recombination and regeneration seems to be much more critical in the case of anthocyanine dyes with respect to the best Ru(II) sensitizers, particularly under intense irradiation which leads to electron accumulation within the mesoporous TiO2 film and to the activation of a larger number of electron/hole recombination pathways as higher lying TiO2 states are progressively filled.
![Differential absorption changes on transparent TiO2 sensitized with: (a) eggplant extract (nasunine) in the presence of 0.1 M Li+ (black) and 0.15 M LiI (red) and (b) [Ru(H2DCB)(NCS)2] in the presence of 0.1 M Li+ (black) and 0.1 M LiI (red). H2DCB = 2,2′bipyridine-4,4′-dicarboxylic acid. Kinetic traces are observed at 470 nm for nasunine and at 480 nm for N3.](/image/article/2009/EE/b913248c/b913248c-f13.gif) | ||
| Fig. 13 Differential absorption changes on transparent TiO2 sensitized with: (a) eggplant extract (nasunine) in the presence of 0.1 M Li+ (black) and 0.15 M LiI (red) and (b) [Ru(H2DCB)(NCS)2] in the presence of 0.1 M Li+ (black) and 0.1 M LiI (red). H2DCB = 2,2′bipyridine-4,4′-dicarboxylic acid. Kinetic traces are observed at 470 nm for nasunine and at 480 nm for N3. | ||
In the case of anthocyanine dyes a fast redox couple like I−/I2 seems to be necessary in order to compete effectively with electron recombination and to obtain reasonably high photon to electron conversion efficiencies. In fact the employment of a kinetically slower redox couple, like certain Co2+ complexes12 results in an evident drop in IPCE (IPCEmax = 14% for eggplant extract) (Fig. 14), ostensibly due to an inefficient dye cation reduction, evidenced by the increased lifetime in dye cation recovery (τ2/3 = 120 ns for nasunine in the presence of 0.15 M Co(II)).
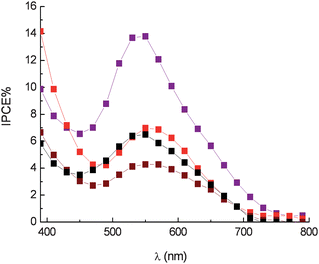 | ||
Fig. 14 Photoaction spectra in the presence of Co(DTB)32+ 0.15 M and Li+ 0.5 M. Eggplant extract ( ), red radicchio ( ), red radicchio ( ), Nero d'Avola ( ), Nero d'Avola ( ) and Giacchè ( ) and Giacchè ( ). DTB = 4,4′-di-tert-butyl-2,2′bipyridyl. ). DTB = 4,4′-di-tert-butyl-2,2′bipyridyl. | ||
3. Betalain dyes
Betalain pigments represent an additional class of organic natural dyes of potential interest. These pigments are present in Caryophyllales plants, have high molar extinction coefficients in the visible region and pH dependent redox properties. The pigments are present in the different parts of the plant including flowers petals, fruits, leaves, stems and roots. The results discussed here relate to a series of experiments carried out on raw extracts of red turnip (Beta vulgaris rubra, Kogel), wild purple sicilian prickly pear (Opuntia engelmannii var. Lindhemeir), sicilian indian fig (Opuntia ficus indica, [L] Mill.) and bougainvillea flowers. The red beet originally grew wild in the Mediterranean area, particularly in regions that have cold nights during the spring season. Its ball-shaped red roots contain a high concentration of betalain pigments. The prickly pear is a member of the Cactaceae family, originating from Mexico and widely distributed in much of Latin America, South Africa and in the Mediterranean area. Bougainvillea plants are often found growing in mild climates and have typically small flowers enclosed by large, brilliant red or purple bracts (modified leaves). The prevailing pigment coloration of the cited plants varies form orange to red, due to the combination of two main dyes: betanine (red-purple) and betaxanthins (yellow-orange) whose schematic structures are reported in Fig. 15. As shown in the figure, both dyes contain carboxylic functions which allow a strong TiO2 surface binding.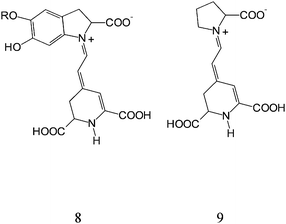 | ||
| Fig. 15 General structure of betalains: betanin (8) and betaxanthin (9). | ||
Betalain extracts from red turnip in 0.1 M HCl solution display an intense absorption band centered at 530 nm and a shoulder at 480 nm due to the mixed contributions of the yellow-orange betaxanthins (480 nm) and of the red-purple betanins (540 nm) (Fig. 16)
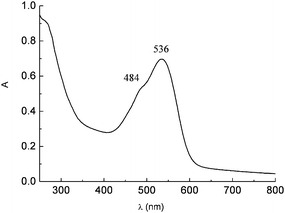 | ||
| Fig. 16 UV-Vis spectrum of raw red turnip extracts in 0.1 M HCl solution showing the betaxanthin (9) (484 nm) and betanin (8) (536 nm) visible absorption. | ||
DFT and TDDFT calculations carried out on betanin,44 have shown that the HOMO is mainly localized on the benzene ring. During the optical transition the benzene ring loses electron density while the dihydropyridine (DHP) ring gains charge, indicative of partial excited state electron transfer (Fig. 17). Thus the main visible absorption band of betalains is essentially a charge transfer π–π* transition. Since the DHP unit is assumed to be attached at the TiO2 surface via the carboxylic groups, the excited state should be electronically coupled with the acceptor states of TiO2, leading to an effective charge injection. Moreover, the predicted excited state oxidation potential, of the order of −1.3 V vsNHE in good agreement with experimental values, is consistently more negative than the conduction band edge of TiO2 (0.5 V vsNHE), ensuring an ample driving force for the photoinduced electron transfer.
![HOMO (a) and LUMO (b) for betanidin+ (c) where R1 = R2 = H. From [ref. 44].](/image/article/2009/EE/b913248c/b913248c-f17.gif) | ||
| Fig. 17 HOMO (a) and LUMO (b) for betanidin+ (c) where R1 = R2 = H. From [ref. 44]. | ||
Upon adsorption on the TiO2electrodes of both Beta vulgaris rubra and Opuntia engelmannii extracts, the visible absorption band shifts to higher energy, showing a broad maximum around 470–450 nm. This is most probably determined by a preferential binding of betaxanthin (9). The acidic environment is essential for obtaining electrodes characterized by high optical densities, capable of almost complete absorption of visible photons in the 400–600 nm range. The reason is ostensibly related to protonation of betalainic carboxylic groups which are otherwise unable, in their anionic form (they are probably dissociated under plant physiologic conditions), to interact strongly with the oxide surface. Using transparent TiO2 photoanodes equipped with a compact TiO2 underlayer, betalains from red turnip displayed promising photoelectrochemical performances showing, in the presence of an electrolyte composed of 0.6 M 1-propyl-3-methyl-imidazolium iodide (PMII), 0.1 M LiI and 0.2 M I2 in methoxypropionitrile (MPN) JSC = 9.5 mA/cm2 and VOC = 0.48 V and a global efficiency of 1.75%, to our knowledge among the highest so far reported for natural raw dyes (Fig. 18 red line).
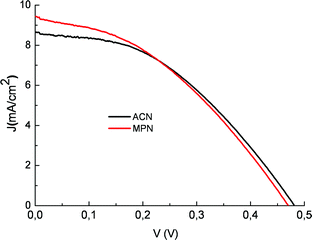 | ||
Fig. 18 J–V curve of a photoelectrochemical cell sensitized with a natural red turnip extract in the presence of 0.5/0.05 M LiI/I2 in acetonitrile ( ) and of 0.6 M propyl-methyl-imidazolium iodide (PMII), 0.1 M LiI and 0.2 M I2 in methoxypropionitrile ( ) and of 0.6 M propyl-methyl-imidazolium iodide (PMII), 0.1 M LiI and 0.2 M I2 in methoxypropionitrile ( ). ). | ||
The performance of Sicilian prickly pear dyes (Opuntia engelmannii) were also very close to the red turnip ones, exhibiting a short circuit photocurrent close to 8 mA/cm2 which could be brought to ∼10 mA/cm2 with the use of a scattering TiO2 overlayer. On the contrary, Bougainvillea and Opuntia ficus indica were a poorer source of betalain dyes. Cells fabricated with such raw extracts achieved only modest power conversion efficiencies, with maximum photocurrents slightly higher than 2 mA/cm2, due to a modest light harvesting capability (Amax = 0.3–0.5).
As previously observed with the anthocyanine family, in this case natural dyes are also limited by low VOC, probably for reasons analogous to those previously outlined. However, in this case the addition of 4-tert-butylpyridine did not cause dye desorption, but was equally unsuccessful in enhancing cell performance. Considering the red turnip sensitized solar cell, a modest gain in photovoltage and in Fill Factor (0.53 V and 0.53, respectively) corresponded to a dramatic drop in photocurrent (1.53 mA/cm2). This phenomenon, which has been independently verified by two laboratories, is probably determined by a dramatic enhancement of the betaxanthin reducing activity under basic conditions45 leading to dye degradation due to side reactions with other chemical species like oxidized electrolyte (I3− or I2), dissolved oxygen and other minor impurities. This effect was not documented by Zhang et al.25 who used purified betanine that is indeed quite insensitive to pH changes. Nevertheless purified betanine extract resulted in a rather poor sensitizer, despite an extended spectral absorption at lower energy. The reason for this is not completely clear, but it has been pointed out that often raw natural dye mixtures exhibit better performance than commercial or purified analogues.43 This fact may be related to the presence in the natural extract of ancillary molecules (i.e. alcohols, organic acids, etc.) which act as coadsorbates, suppressing recombination with the electrolyte, reducing dye aggregation and favouring injection.
The photoaction spectra of red turnip and prickly pear extracts show a maximum IPCE of 65% in the 450–470 nm region (Fig. 19).
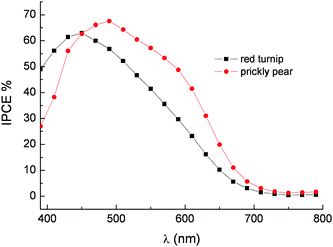 | ||
Fig. 19 Photoaction spectra on transparent TiO2 of betalains from red turnip (■) and wild prickly pear ( ). LiI/I2 0.5 M/0.05 M in ACN was used as a redox electrolyte. Cells equipped with blocking underlayer. ). LiI/I2 0.5 M/0.05 M in ACN was used as a redox electrolyte. Cells equipped with blocking underlayer. | ||
Since photon absorption in the 400–600 nm interval is almost complete, conversion efficiencies inferior to 80–85% (a value limited by the transmittance of the conductive glass) can only be determined by injection efficiency and/or by electron collection efficiency smaller than unity. Thus the IPCE is essentially determined by the ϕinjη product where ϕinj is the charge injection efficiency and η is the electron collection efficiency. The negative excited state oxidation potential of the order of −1.3 V vsSCE and the good electronic coupling with the d band of the TiO2 should result in ‘activationless’ injection into the conduction band of TiO2. Based on these indications, it is unlikely that charge injection is the IPCE limiting process. On the other hand recombination losses can reduce the η factor. The ground state oxidation potential of 0.7/0.75 V vsSCE, estimated by cyclic voltammetry both at a glassy carbon electrode and at an FTO electrode functionalized with the same dye extract used for DSSC fabrication, should ensure good dye regeneration rate by iodide, whose E1/2 lies at ∼0.4 V vsSCE. However electron recapture by betalain dye cation is expected to be faster compared to Ru(II) sensitizers, as already pointed out when considering the photoelectrochemical behavior of cyanine dyes. Other losses may arise from recombination involving I3− and electrons trapped in TiO2nanoparticles. This process is strongly dependent upon specific factors like surface coverage, dye structure and specific interactions between I3− and the organic dye. To date, detailed investigation into the electronic recapture by I3− on betalain sensitized substrates has yet to be reported.
Conclusions
For both practical and fundamental reasons, the possibility of generating high photoconversion efficiencies exploiting nanocrystalline titania photoanodes sensitized with natural pigments has been investigated. To date, selected chlorophyll derivatives, raw anthocyanine and betalain extracts are the most successful natural sensitizers, resulting in the generation of monochromatic photon to current conversion yields exceeding 60%. Maximum overall conversion efficiencies under simulated sunlight comparable to that of natural photosynthesis (2%) have been achieved. Finding appropriate additives for improving VOC without causing dye degradation might result in further enhancement of cell performance, making the practical application of such systems more suitable to achieving economically viable solar energy devices for our society.Acknowledgements
Funding from Polo Solare Organico Regione Lazio (CHOSE) is gratefully acknowledged.References
- P. Liska, N. Vlachoupoulos, M. K. Nazeeruddin, P. Comte and M. Graetzel, J. Am. Chem. Soc., 1988, 110, 3686–3687 CrossRef CAS.
- B. O'Regan and M. Graetzel, Nature, 1991, 353, 737 CrossRef CAS.
- M. Graetzel, Inorg. Chem., 2005, 44, 6841 CrossRef.
- S. Maldonado; G. A. Fitch; N. S. Lewis. In Series on Photoconversion of Solar Energy: Nanostructured and Photoelectric Chemical System for Solar Photon Conversion; Archer, M. D., Nozik, A., Eds.; Imperial College Press: London, 2007; Vol. III, p 537 Search PubMed.
- H. Gerischer, Photochem. Photobiol., 1972, 16, 243 CAS.
- H. Tributsch and M. Calvin, Photochem. Photobiol., 1971, 14, 95 CrossRef CAS.
- K. Keis, L. Vayssieres, S. E. Lindquist and A. Hagfedlt, Nanostruct. Mater., 1999, 12, 487 CrossRef.
- A. B. F. Martinson, S. M. Goes, F. Fabregat-Santiago, J. Bisquert, M. J. Pellin and T. J. Hupp, J. Phys. Chem. A, 2009, 113, 4015 CrossRef CAS.
- C. Bauer, G. Boschloo and A. Hagfedlt, Int. J. Photoenergy, 2002, 4, 17 CrossRef CAS.
- S. Chappel, S.-G. Chen and A. Zaban, Langmuir, 2002, 18, 3336 CrossRef CAS.
- K. Sayama, H. Sugihara and H. Arakawa, Chem. Mater., 1998, 10, 3825 CrossRef CAS.
- S. A. Sapp, C. M. Elliott, C. Contado, S. Caramori and C. A. Bignozzi, J. Am. Chem. Soc., 2002, 124, 11215–11222 CrossRef CAS.
- J. H. Yum, P. Walter, S. Huber, D. Rentsch, T. Geiger, F. Neusch, F. DeAngelis, M. Graetzel and M. K. Nazeeruddin, J. Am. Chem. Soc., 2007, 129, 10320–10321 CrossRef CAS.
- W. M. Campbell, K. W. Jolley, P. Wagner, K. Wagner, P. J. Walsh, K. C. Gordon, L. Schmidt-Mende, M. K. Nazeeruddin, Q. Wang, M. Graetzel and D. L. Officer, J. Phys. Chem. C, 2007, 111, 11760–11762 CrossRef CAS.
- H. Tian, X. Yang and A. Hagfeldt, Energy Environ. Sci., 2009, 2, 674 RSC.
- M. K. Nazeeruddin, A. Kay, I. Rodicio, R. Humphry-Baker, E. Mueller, P. Liska, N. Vlachopoulos and M. Graetzel, J. Am. Chem. Soc., 1993, 115, 6382–6390 CrossRef CAS.
- M. K. Nazeeruddin, P. Pechy, P. Liska, T. Renouard, S. M. Zakeeruddin, R. Humphry-Baker, P. Comte, L. Cevey, E. Costa, V. Shklover, L. Spiccia, G. B. Deacon, C. A. Bignozzi and M. Graetzel, J. Am. Chem. Soc., 2001, 123, 1613–1624 CrossRef CAS.
- D. Kuciauskas, M. S. Freund, H. B. Gray, R. J. Winkler and N. S. Lewis, J. Phys. Chem. B, 2001, 105, 392 CrossRef CAS.
- R. Argazzi, G. Larramona, C. Contado and C. A. Bignozzi, J. Photochem. Photobiol., A, 2004, 164, 15–21 CrossRef CAS.
- S. Altobello, R. Argazzi, S. Caramori, C. Contado, S. Da Fre, P. Rubino, C. Chone, G. Larramona and C. A. Bignozzi, J. Am. Chem. Soc., 2005, 127, 15342–15343 CrossRef CAS.
- K. Tennakone, G. R. Kumara, I. R. Kottegoda and K. Wijayantha, Semicond. Sci. Technol., 1997, 12, 128 CrossRef CAS.
- Q. Dai and J. Rabani, Chem. Commun., 2001, 2142 RSC.
- Q. Dai and J. Rabani, J. Photochem. Photobiol., A, 2002, 148, 17 CrossRef CAS.
- A. Sarto Polo and N. Y. Murakami Iha, Sol. Energy Mater. Sol. Cells, 2006, 90, 1936 CrossRef.
- D. Zhang, S. M. Lanier, J. A. Downing, J. L. Avent, J. Lum and J. L. McHale, J. Photochem. Photobiol., A, 2008, 195, 72–80 CrossRef CAS.
- G. Calogero and G. Di Marco, Sol. Energy Mater. Sol. Cells, 2008, 92, 1341–1346 CrossRef CAS.
- G. Calogero; S. Cazzanti; S. Caramori; G. Di Marco; R. Argazzi; A. Di Carlo; C. A. Bignozzi. J.Photochem.Photobiol. A: Chemistry, submitted Search PubMed.
- A. Kay and M. Graetzel, J. Phys. Chem., 1993, 97, 6272 CrossRef.
- N. J. Cherepy, G. P. Smestad, M. Graetzel and G. J. Zhang, J. Phys. Chem. B, 1997, 101, 9342 CrossRef CAS.
- G. Lowe. In Inorganic Electronic Structure and Spectroscopy: Electronic Structure of Heme Sites; Solomon, E. I., Lever, A. B. P., Eds.; J.Wiley &Sons: New YorkVol. 2, p 451 Search PubMed.
- A. Kay, R. Humphry Baker and M. Graetzel, J. Phys. Chem., 1994, 98, 952 CrossRef CAS.
- Y. Amao and Y. Yamada, Langmuir, 2005, 21, 3008–3012 CrossRef CAS.
- X.-F. Wang, C.-H. Zhan, T. Maoka, Y. Wada and Y. Koyama, Chem. Phys. Lett., 2007, 447, 79–85 CrossRef CAS.
- X.-F. Wang, O. Kitao, H. Zhou, H. Tamiaki and S.-I. Sasaki, J. Phys. Chem. C, 2009, 113, 7954–7961 CrossRef CAS.
- H. D. Martin, Chimia, 1995, 49, 45 CAS.
- H. Yamasaki, H. Uefuji and Y. Sakihama, Arch. Biochem. Biophys., 1996, 332, 183 CrossRef CAS.
- J. B. Harborne. Comparative Biochemistry of the Flavonoids; Academic Press: London, 1967 Search PubMed.
- O. Dangles, M. Elhabiri and R. Brouillard, J.Chem.Soc., Perkin Trans., 1994, 2, 2587 Search PubMed.
- J. A. Moser, S. Punchiewa, P. P. Infelta and M. Graetzel, Langmuir, 1991, 7, 3012 CrossRef CAS.
- P. A. Connor, K. D. Dobson and A. J. McQuillan, Langmuir, 1995, 11, 4193 CrossRef CAS.
- Y. Xu, W. K. Chen, S.-H. Liu, M.-J. Cao and J. Q. Li, Chem. Phys., 2007, 331, 275 CrossRef CAS.
- A. Sarto Polo, M. K. Itokazu and N. Y. Murakami Iha, Coord. Chem. Rev., 2004, 248, 1343–1361 CrossRef CAS.
- A. Burke, S. Ito, H. Snaith, U. Bach, J. Kwiatkowski and M. Graetzel, Nano Lett., 2008, 8, 977 CrossRef CAS.
- C. Quin and A. E. Clark, Chem. Phys. Lett., 2007, 438, 26–30 CrossRef.
- M. A. Pedreno and J. Escribano, J. Sci. Food Agric., 2001, 81, 627–631 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2009 |
