A general nonaqueous route to crystalline alkaline earth aluminate nanostructures†
M.
Karmaoui
a,
M.-G.
Willinger
a,
L.
Mafra
a,
T.
Herntrich
a and
N.
Pinna
*ab
aDepartment of Chemistry, CICECO, University of Aveiro, 3810-193 Aveiro, Portugal. E-mail: pinna@ua.pt; pinna@snu.ac.kr
bWorld Class University (WCU) program of Chemical Convergence for Energy & Environment (C2E2), School of Chemical and Biological Engineering, College of Engineering, Seoul National University (SNU), Seoul 151-744, Korea
First published on 15th September 2009
Abstract
A nonaqueous route based on the solvothermal reaction of alkaline earth precursors with aluminium isopropoxide in benzyl alcohol is introduced. This simple process leads to crystalline complex nanostructures of alkaline earth aluminates, which, up to now, could only be obtained by solid state reaction at temperatures above 1100 °C or by sol –gel and further calcination at temperatures only slightly lower (∼800 °C). The approach appears to be rather general since under the same reaction conditions BaAl2O4, CaAl4O7, and SrAl4O7 could be obtained. The as-synthesized materials were characterized by X-ray diffraction, electron microscopy techniques, solid-state NMR and FT-IR spectroscopies. The reaction mechanism, which was studied as well, indicates the in-situ formation of benzoate species. These can preferentially bind to particular crystallographic facets of the aluminates via bridging bonds, thereby stabilizing the surfaces that give rise to the peculiar complex structure of the final material. In order to supplement the synthesis approach and to investigate the formation of impurity phases, pure aluminium oxide hybrid nanostructures were synthesized under similar conditions and fully characterized.
Introduction
Various soft chemistry approaches have been introduced in the past decades for the synthesis of metal oxide nanoparticles.1–5 They were reported to be extremely successful for the production of simple oxides (e.g. binary). Indeed, dozens of routes for the production of binary metal oxide nanoparticles such as titanium or zinc oxide can be found in the recent literature. In contrast, approaches leading to the formation of multi-metal and doped metal oxides by soft chemistry approaches are more rarely reported. This is mainly due to the fact that it is in general difficult to match the reactivity of the different metal precursors in solution, which is a prerequisite for the formation of multi-metal oxides.2 This is especially true for aqueous routes (e.g.sol –gel ) as the hydrolysis rates are generally fast and strongly depend on the metal center.6 Indeed, complex oxides are still almost exclusively produced by traditional solid state synthesis. In order to apply soft chemistry approaches for the synthesis of complex oxides, considerable effort was devoted on the modification of metal complexes in the aim of controlling and/or decreasing their reactivity.7 Nonaqueous sol –gel routes were introduced for the same purpose, and in fact, the reactivity of the metal oxide precursors is greatly decreased under water exclusion, thus making it easier to control the metal oxide formation.8,9 Nonaqueous routes were successfully applied for the synthesis of various metal oxide nanoparticles,2,10 hybrid materials11 and also for the growth of metal oxide thin films by atomic layer deposition.12 They gave access to various multi-metal oxides (c.f. Table 3 in ref. 2) because of the reasons explained above. Alkaline earth aluminates are an important class of phosphor materials when doped with lanthanide ions (cf. for example ref. 13–15). They can be obtained in the crystalline form by solid state reaction at temperatures above 1100 °C or by sol –gel and further calcination at temperatures only slightly lower (∼800 °C).14–19 Unfortunately, even though nonaqueous sol –gel routes proved to overcome the calcinations step in the synthesis of crystalline complex metal oxides,2 it is not yet possible to predict the reactivity of metal complexes in a particular solvent as the metal oxide formation is influenced by various additional effects such as intermediate products formed during the reaction, side reactions and catalytic effects of the metal centers, the metal oxide seeds and early formed nanoparticles.In the present manuscript we investigate the low temperature (275 °C) solvothermal synthesis of crystalline alkaline earth aluminate hierarchical nanostructures (BaAl2O4, CaAl4O7, and SrAl4O7), by the “benzyl alcohol route”. In order to supplement the synthesis approach and to investigate the formation of impurity phases, aluminium oxide hybrid nanostructures have been synthesized following the same approach.
Experimental section
Synthesis
The synthesis procedures were carried out in a glovebox (O2 and H2O < 1 ppm). In a typical synthesis of the alkaline earth aluminate nanostructures, metallic strontium (1.77 mmol), barium (1.63 mmol) or calcium methoxide (1.73 mmol) were added to 20 mL benzyl alcohol. Once the solution becomes clear (stirring at around 60 °C is needed to dissolve the metallic elements) aluminium isopropoxide (4.14, 3.80 and 4.03 mmol, respectively) was added. The reaction mixture was transferred into a stainless steel autoclave, and carefully sealed. The autoclave was taken out of the glovebox and heated in a furnace at 275 °C for 2 days. In a typical synthesis of the aluminium oxide lamellar hybrid, aluminium isopropoxide (500 mg) was added to 20 mL benzyl alcohol and reacted in a Teflon lined stainless steel autoclave at 180 °C. The resulting milky suspensions were centrifuged, the precipitates thoroughly washed with ethanol and dichloromethane, and subsequently dried in air at 60 °C. The same material was also obtained at higher reaction temperature.Characterization
The X-ray powder diffraction (XRD) data was collected on an X'Pert MPD Philips diffractometer (Cu Kα X-radiation at 40 kV and 50 mA). Scanning electron microscopy was carried out using a Hitachi SU-70 with a field emission gun. Transmission electron microscopy (TEM) was carried out on a Hitachi H-9000 microscope operating at 300 kV, high resolution microscopy was performed using a Jeol 2200FS microscope with a field emission gun. The microscope was operated at 200 kV. Samples were prepared by depositing a drop of a suspension of particles in ethanol on a copper grid coated with an amorphous carbon film.13C CPMAS and 27Al MAS NMR spectra were recorded on a Bruker AVANCE 400 (DSX) WB spectrometer (9.4 T), using a double-resonance 4 mm probe, with Larmor frequencies of 100.62 and 104.26 MHz for 13C and 27Al, respectively. The 27Al MAS NMR spectra were obtained with a small flip angle of ca. 10° (0.6 µs); recycle delay of 1 s; Number of scans = 64. 13C CPMAS spectrum was recorded using a RAMP-CP shape (100% to 50% amplitude); rf field strengths between 50 and 70 kHz for Hartman–Hahn condition calibration for 1H and 13C channels; number of scans = 2k; recycle delay of 5 s; contact time = 1.5 ms; 1H decoupling of 80 kHz during 13C signal acquisition. 13C and 27Al chemical shifts were externally referenced to TMS and Al(NO3)3 (aq), respectively.
Results and discussion
The XRD patterns (Fig. 1) of the as synthesized aluminates correspond to the BaAl2O4 (full line) (JCPDS [17-306]), CaAl4O7 (dashed line) Grossite (JCPDS [23-1037]) and SrAl4O7 (dash-dotted line) structures (JCPDS [25-1208]), respectively. The pattern of the CaAl4O7 shows also a series of reflections at low angle around 2θ ∼ 7, 14, and 21° (see also Fig. S1, ESI† ). They are attributed to a secondary phase which is constituted of very thin (sub-nanometer) aluminium oxide layers regularly separated by organic species. A similar phase is found when the reaction is performed without the presence of the alkaline earth precursors under otherwise similar conditions. Its corresponding XRD pattern (Fig. 2) is typical for a lamellar hybrid material. The pattern is very similar to the ones observed for the rare earth oxide hybrid materials synthesized in benzyl alcohol.20 The equally spaced low angle peaks are due to the lamellar periodicity. The reflection around 2θ ∼ 25° is due to the inorganic part. Because of the extremely small thickness of the aluminium oxide lamellae it is impossible to deduce its crystalline structure from XRD measurements.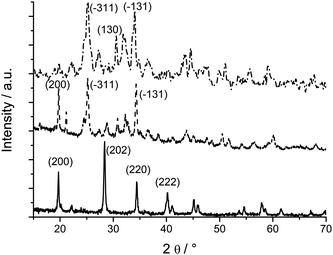 | ||
| Fig. 1 XRD powder patterns of the aluminate nanostructures: BaAl2O4 (full), CaAl4O7 (dashed) and SrAl4O7 (dash-dotted). Some of the most intense reflections are indicated. | ||
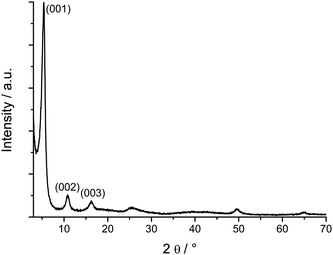 | ||
| Fig. 2 XRD powder patterns of the aluminium oxide hybrid nanostructure. The first three reflections of the lamellar structure are indicated. | ||
The synthesis of the alkaline earth aluminates leads to spherical particles as shown in the case of CaAl4O7 in Fig. 3a. Higher magnification reveals that the spherical particles consist of thin platelets or needles (Fig. 3). The nucleation of the particles obviously starts at the center of the spheres, where a high intergrowth of crystalline seeds can be observed (Fig. 3d,e). From there, thin platelets originate through branching off into consecutively thinner sheets. This leads to a further spreading up of the growth directions initially defined in the nuclei and results in dumbbell shaped particles (Fig. 3c). Finally, axial growth completely turns into radial growth and hence, to the formation of spherical particles. In some cases, the platelets building up the spheres are very narrow and are better described as rods or needles. Probably, it is the degree of intergrowth and the complexity of the nuclei that defines the branching behavior and hence, whether the aspect of the terminal branches is more platelet or needle like (Fig. 4). Interestingly, the aspect of the platelets within one particle is generally uniform and differently terminating spheres coexist within one sample. Such a growth pattern has also been found in fluorapatite, calcium carbonate, barium carbonate and barium sulfate, where the fractal branching of successive generations of rods or platelets follows a self-similar growth pattern (ref. 21,22 and references therein).
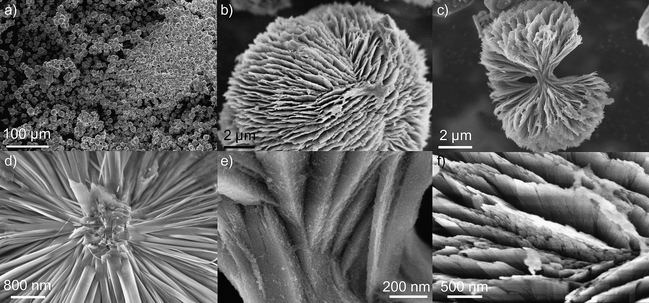 | ||
| Fig. 3 SEM images of CaAl4O7 particles. Overview image (a), single particles (b,c) and details (d,e: core region, f: surface). | ||
 | ||
| Fig. 4 Different surface morphologies of the formed spheres are coexisting. Here shown for the case of CaAl4O7. The same behavior was observed for SrAl4O7. Interestingly, within one sphere, the surface is always homogeneous. | ||
Images of the lattice fringes recorded by high resolution transmission electron microscopy from the outer region of the formed particles reveal the growth direction of the branches (Fig. 5). The CaAl4O7 platelets grow along the [001] direction with the platelets exposing their (100) planes. The growth pattern at the present synthesis conditions reflects some peculiarities of the crystal structure. In CaAl4O7, sheets of corner sharing AlO4 tetrahedra are extending in the y and z direction, while the Ca ions are embedded in between the sheets (Fig. 6a). Especially in the z-direction, a high interlinkage of AlO4 tetrahedra is found. In agreement with the TEM observations, this is the fast growing direction. On the other hand, there exist only a few bridges in the direction perpendicular to the sheets where also the Ca ions are located. These bridges are formed via oxygen atoms that belong to two adjacent AlO4 tetrahedra. Although these are the shortest Al–O bonds found in the structure, their areal density is much lower than in the other directions. TEM observations reveal that the [100] is the direction with the slowest growth and highest stability. It appears that benzoate ligands terminate the growth in the [100] direction by saturating the bridging O atoms (cf. discussion below). Due to the distribution of the Ca ions, the platelets have a polar surface. In that respect, the fractural branching of successive generations of rods or platelets and the self-similar growth pattern could be related to intrinsic electric fields, that influence the aggregate growth as previously suggested for similarly shaped mesocrystals.21,23,24
![(a) SEM image of a secondary morphology found in CaAl4O7, with more narrow and wavy brunches as shown by TEM (b) and HRTEM (c). (d) HRTEM of CaAl4O7 platelets, the growth direction is the [001] as demonstrated by the power spectrum (inset) of the region marked with a circle. The zone axis is [100].](/image/article/2009/NR/b9nr00164f/b9nr00164f-f5.gif) | ||
| Fig. 5 (a) SEM image of a secondary morphology found in CaAl4O7, with more narrow and wavy brunches as shown by TEM (b) and HRTEM (c). (d) HRTEM of CaAl4O7 platelets, the growth direction is the [001] as demonstrated by the power spectrum (inset) of the region marked with a circle. The zone axis is [100]. | ||
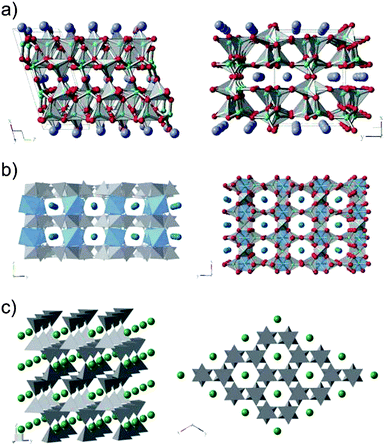 | ||
| Fig. 6 (a) Ball and stick model of the CaAl4O7 structure viewed from 010 and 001 direction, respectively. O atoms bridging layers of interlinked AlO4 tetrahedra along the 100 direction are located in the same plane as the Ca ions. (b) Model of the SrAl4O7 structure viewed from 100 and 001 direction, respectively. To reduce complexity, only the structural units and the Sr ions are shown on the left. Besides the AlO4 tetrahedra (drawn in grey) there exist AlO6 octahedra (drawn in light blue). (c) Arrangement of the atoms in BaAl2O4. As opposed to the CaAl4O7 and SrAl4O7, each tetrahedra is only connected to four neighbors by sharing a common oxygen atom. Due to the symmetry of the unit cell, the x and y directions are equivalent. O atoms are shown in red, Al in turquoise, Ca atoms in violet, Sr in blue and Ba in green. For clarity, atoms are grouped in structural units (i.e. tetrahedra and octahedra). | ||
Similar to the case of CaAl4O7, the SrAl4O7 also grows to spherical particles that consist of thin platelets originating from a nucleation point at the core of the particle through branching off into consecutively thinner sheets (Fig. 7a). In SrAl4O7, the platelets grow along the [100] direction. This is exemplified in Fig. 7b with a particle that is oriented in [001] direction (i.e. [001] is parallel to the electron beam). The formation of platelets and their preferred growth direction is again linked to the arrangement of structural units (Fig. 6b). Layers of highly interlinked AlO4 tetrahedra extend in the xy-plane and give rise to the enhanced growth speed in the x and y direction. In between the layers, one finds the Sr ions as well as the AlO6 octahedra that are acting as bridges. Under the present synthesis conditions, different possible terminations of the (001) plane further reduce the coupling between the layers. For example, benzoate species attached via bridged bonds to the aluminate structure might decrease the growth speed in [001] direction and stabilize the (001) surface, giving rise to the observed formation of platelets (cf. discussion below).
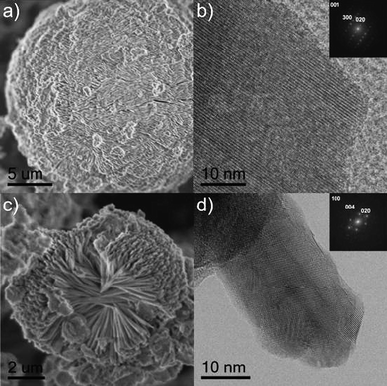 | ||
| Fig. 7 SEM and corresponding HRTEM images of SrAl4O7 (a,b) and BaAl2O4 (c,d). Insets in (b) and (d) show the power spectra of the respective HRTEM images. | ||
In BaAl2O4, platelets grow in the [001] direction as demonstrated by the particle shown in Fig. 7d. In contrast to the other two, the platelets are thicker, indicating that the difference in growth speed is smaller and that the stabilization of a specific surface is slightly less efficient. Further, the particles grow more irregular, revealing different facets. Indeed, the structure of the BaAl2O4 and the arrangement of structural units is slightly more isotropic as compared to the previous systems.
Elemental analysis reveals that the as-synthesized rare earth aluminates contain a relatively high amount of carbon (∼10–15% in weight, cf. ESI† ). FT-IR spectroscopy shows the presence of carboxylate species (Fig. SI-3, ESI† ). Indeed the frequency of the asymmetric stretching νa(CO2) is located around 1550 cm−1 and the symmetric one νs(CO2) at 1410 cm−1. The difference of frequency between these two vibrations gives valuable information on the bonding of the carboxylate species to the aluminate structure.25 Considering the positions observed, a difference in frequency of Δ ∼ 140 cm−1 can be estimated. This corresponds to a bridged kind of bonding similar to the one observed in yttrium and lanthanide hybrid materials synthesized in benzyl alcohol.20,26 The observation of the typical signature of carboxylate species speaks for the aptitude of the alkaline earth aluminate to promote the oxidation of benzyl alcohol to benzoate species. In order to prove this behavior the reaction mixture was analyzed by 1H and 13C NMR after removal of the aluminate nanostructures by centrifugation (cf. ESI† and Fig. SI-4 and SI-5).
In addition to benzyl alcohol, significant amounts of toluene and benzaldehyde with the ratio (1 : 0.33) and dibenzyl ether were found. The latter is the product of the ether elimination condensation step as already observed for various metal oxides synthesized in benzyl alcohol.2,27 The benzaldehyde and toluene are attributed to the formation of benzoate species via two successive reactions: (i) a disproportionation reaction of benzyl alcohol to toluene and benzaldehyde taking place at the surface of the freshly formed clusters (similar to Scheme 2 in ref. 26). And (ii) as benzaldehyde was found in lower proportion than toluene (0.33 : 1) and the nanocomposite contains benzoate molecules, the aluminate nanostructure is catalyzing a Cannizzaro-like reaction taking place at its surface leading to the formation of an aluminate-benzoate hybrid nanostructure under the elimination of toluene (similar to Scheme 3 in ref. 26). Such a reaction path was firstly reported for yttria26 and subsequently for many other lanthanide oxide hybrid materials synthesized in benzyl alcohol.11,20 Although it is not yet understood how the formation of the benzoate species influences the nanostructure of the aluminates, it can be assumed that they attach via bridged bonds to the aluminate structure. This results in a decrease of the growth speed and promotes the formation of thin platelets, which is in agreement with the observed morphology.
Aluminium oxide lamellar hybrid
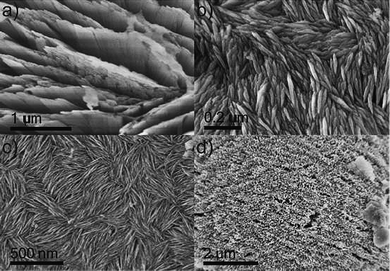
In all synthesized samples, a small amount of an impurity phase was observed. As discussed above, the XRD pattern of CaAl4O7 indicates the presence of a phase that is characterized by a regular arrangement of regions with different electron density. A hybrid lamellar material was also observed in SrAl4O7 and BaAl2O4 by TEM. However, the amount was too small to be detected by XRD. In order to assess the nature of the observed impurity phase and to get more insight on its formation, an additional sample was prepared without the presence of alkaline earth metal complexes under otherwise similar conditions (cf. also above).A typical SEM and TEM image of the impurities is shown in Fig. 8a,c. The aspect is identical to the images acquired for the pure aluminium oxide lamellar hybrid (Fig. 8b,d). The transmission electron microscopy image show that the material consists of equally spaced parallel lamellae with very different electron contrast. The dark layers indicate the presence of the strongest scatterers, whereas the organic material stays practically invisible between those layers.
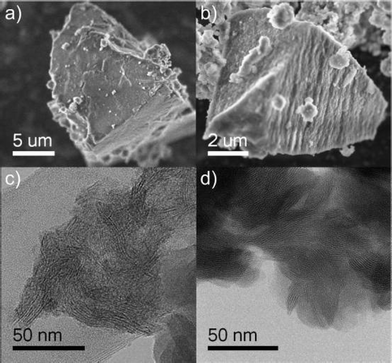 | ||
| Fig. 8 Left side: impurity phase present in all the alkaline earth aluminates discussed above, right side: the lamellar aluminium oxide hybrid material that was synthesized as a reference phase. Top row: SEM, bottom row: TEM. | ||
In order to get more insight on the structure of the lamellar aluminium oxide sample, solid state NMR studies were performed. 13C CPMAS NMR analysis (Fig. SI-6, ESI† ) shows three groups of resonances. The less shielded carbonyl resonances appearing at ca. 142 and 128 ppm are assigned to the aromatic carbons belonging to the phenyl ring. The resonance at 142 ppm corresponds to the aromatic C-1 and C-4 carbon environments while the one at 128 ppm corresponds to the remaining aromatic carbons. The presence of the 13C resonance at ca. 65 ppm, which is typical of –C–O– environments, and the complete absence of carbonyl (–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) resonances, confirm that organic residues, in the form of benzoyl species, are present in the hybrid structure. This is in contradiction with what is typically observed for rare earth based hybrid structures synthesized in benzyl alcohol11 and alkaline earth aluminates studied here. In these cases, benzoate species are observed and are made responsible for building up the ordered hybrid structure.11 In addition, 13C peaks resonating at ca. 26 and 22 ppm are characteristic of aliphatic carbon environments. They are due to organic moieties of the starting materials used in the synthesis or from the organic solvents used to wash the final solid.
O) resonances, confirm that organic residues, in the form of benzoyl species, are present in the hybrid structure. This is in contradiction with what is typically observed for rare earth based hybrid structures synthesized in benzyl alcohol11 and alkaline earth aluminates studied here. In these cases, benzoate species are observed and are made responsible for building up the ordered hybrid structure.11 In addition, 13C peaks resonating at ca. 26 and 22 ppm are characteristic of aliphatic carbon environments. They are due to organic moieties of the starting materials used in the synthesis or from the organic solvents used to wash the final solid.
The 27Al shift of the central transition of the 27Al MAS NMR spectrum provides useful information on the Al coordination number. In Al–O environments, the 27Al chemical shifts for 6-coordinated Al(VI) (AlO6) appear at about −10 to 15 ppm and are well separated from the 4-coordinated Al sites (AlO4) appearing at 50 to 80 ppm. The 27Al central transition resonating at ca. 6 ppm therefore proves, unequivocally, the presence of octahedral Al environments in the layers (Fig. SI-7, ESI† ). The characteristic tail observed toward lower 27Al chemical shifts indicates a distribution in Al(VI) quadrupolar coupling constants and chemical shifts.
Compared to previously reported rare earth oxide lamellar hybrids and to the aluminate nanostructures introduced in this work, the lamellar aluminium oxide hybrid presents a less rigid structure and lower long-range order. This behavior may be explained by the different coordination strength of the organic molecules forming the hybrid structure (i.e.benzoate in the case of the previously reported rare earth oxide lamellar hybrids versus the benzoyl that is formed in the present synthesis from the benzyl alcohol).
Conclusions
In this article a novel nonaqueous route to crystalline alkaline earth aluminate nanostructures based on the reaction of alkaline earth metal oxide precursors and aluminium isopropoxide in benzyl alcohol was introduced. The one pot reaction takes place under solvothermal conditions at relatively low temperature (275 °C). The fact that the reactivity of metal complexes can be easier matched under nonaqueous sol –gel conditions makes it possible to synthesize such an important family of materials. Up to now, this was only possible via traditional solid state routes or sol –gel followed by a high temperature annealing process. The as synthesized aluminates show a complex nanostructure that is characterized by a self-similar growth pattern. The well calibrated spheres of a few µm in size consist of bundles of thin platelets or needles. The formation of similar nanostructures has been explained on the basis of fractal growth that is directed by dipolar electric fields. The role of the benzoate species formed during the course of the reaction could then be attributed to the stabilization of polar surfaces. As proven by FT-IR, these ligands are bound via bridged chelating bonds to the surface of the aluminate structures.Finally, this report brings further confirmation on the fact that the “benzyl alcohol route” is the most general, versatile and widely applicable approach for the synthesis of crystalline metal oxide nanostructures in solution.
Acknowledgements
This work was partially supported by the WCU (World Class University) program through the Korea Science and Engineering Foundation funded by the Ministry of Education, Science and Technology (400-2008-0230).References
- J. Park, J. Joo, Soon G. Kwon, Y. Jang and T. Hyeon, Angew. Chem., Int. Ed., 2007, 46, 4630–4660 CrossRef CAS.
- N. Pinna and M. Niederberger, Angew. Chem., Int. Ed., 2008, 47, 5292–5304 CrossRef CAS.
- Y. W. Jun, J. S. Choi and J. Cheon, Angew. Chem., Int. Ed., 2006, 45, 3414–3439 CrossRef CAS.
- M. Niederberger, Acc. Chem. Res., 2007, 40, 793–800 CrossRef CAS.
- B. L. Cushing, V. L. Kolesnichenko and C. J. O'Connor, Chem. Rev., 2004, 104, 3893–3946 CrossRef CAS.
- J. Livage, M. Henry and C. Sanchez, Prog. Solid State Chem., 1988, 18, 259–341 CrossRef CAS.
- C. Sanchez, J. Livage, M. Henry and F. Babonneau, J. Non-Cryst. Solids, 1988, 100, 65–76 CrossRef CAS.
- A. Vioux, Chem. Mater., 1997, 9, 2292–2299 CrossRef.
- M. Niederberger and G. Garnweitner, Chem.–Eur. J., 2006, 12, 7282–7302 CrossRef CAS.
- P. H. Mutin and A. Vioux, Chem. Mater., 2009, 21, 582–596 CrossRef CAS.
- N. Pinna, J. Mater. Chem., 2007, 17, 2769–2774 RSC.
- G. Clavel, E. Rauwel, M.-G. Willinger and N. Pinna, J. Mater. Chem., 2009, 19, 454–462 RSC.
- F. Clabau, X. Rocquefelte, S. Jobic, P. Deniard, M. H. Whangbo, A. Garcia and T. Le Mercier, Chem. Mater., 2005, 17, 3904–3912 CrossRef CAS.
- Y. H. Lin, Z. T. Zhang, F. Zhang, Z. L. Tang and Q. M. Chen, Mater. Chem. Phys., 2000, 65, 103–106 CrossRef CAS.
- S. H. M. Poort, W. P. Blokpoel and G. Blasse, Chem. Mater., 1995, 7, 1547–1551 CrossRef CAS.
- P. Escribano, M. Marchal, M. L. Sanjuan, P. Alonso-Gutierrez, B. Julian and E. Cordoncillo, J. Solid State Chem., 2005, 178, 1978–1987 CrossRef CAS.
- Y. Liu and C. N. Xu, J. Phys. Chem. B, 2003, 107, 3991–3995 CrossRef CAS.
- C. A. Zhang, L. Wang, L. P. Cui and Y. F. Zhu, J. Cryst. Growth, 2003, 255, 317–323 CrossRef CAS.
- C. Ye, Y. Bando, G. Shen and D. Golberg, Angew. Chem., Int. Ed., 2006, 45, 4922–4926 CrossRef CAS.
- M. Karmaoui, R. A. Sá Ferreira, A. T. Mane, L. D. Carlos and N. Pinna, Chem. Mater., 2006, 18, 4493–4499 CrossRef CAS.
- S. Busch, H. Dolhaine, A. DuChesne, S. Heinz, O. Hochrein, F. Laeri, O. Podebrad, U. Vietze, T. Weiland and R. Kniep, Eur. J. Inorg. Chem., 1999, 1643 CrossRef.
- H. Cölfen and M. Antonietti, Angew. Chem., Int. Ed., 2005, 44, 5576–5591 CrossRef.
- Z. Li, Y. Khimyak and A. Taubert, Materials, 2008, 1, 3–24 Search PubMed.
- M. Niederberger and H. Cölfen, Phys. Chem. Chem. Phys., 2006, 8, 3271–3287 RSC.
- G. B. Deacon and R. J. Phillips, Coord. Chem. Rev., 1980, 33, 227 CrossRef CAS.
- N. Pinna, G. Garnweitner, P. Beato, M. Niederberger and M. Antonietti, Small, 2005, 1, 112–121 CrossRef CAS.
- N. Pinna, G. Garnweitner, M. Antonietti and M. Niederberger, Adv. Mater., 2004, 16, 2196–2200 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Further spectra and elemental analysis data. See DOI: 10.1039/b9nr00164f |
| This journal is © The Royal Society of Chemistry 2009 |
