Controlling the electrodeposition of mesoporous metals for nanoplasmonics†
Rong
Zhu
,
Martyn
McLachlan
,
Steve
Reyntjens‡
,
Farid
Tariq
,
Mary P.
Ryan
* and
David W.
McComb
*
Department of Materials, Imperial College London, London, SW7 2AZ, UK
First published on 13th November 2009
Abstract
We have investigated the mechanism for formation of large-scale ordered structures of copper and silver by direct electrodeposition through colloidal crystal templates. It is demonstrated that the morphology is controlled by the diffusion of reactant species (metal cations and oxygen molecules) from the bulk solution to the electrode surface. The Cu system can be tuned to fill or not to fill the original drying cracks in the template in order to produce isolated or interconnected domains. This has great potential for the formation of individual or interconnected antennae in nanoplasmonic structures.
Metallic structures with extended ordered arrays of pores are of great interest in the rapidly developing field of nanoplasmonics, in particular their ability to act as light concentrators in the visible and near-infrared regime through excitation of localised surface plasmons (LSP) is attracting much research effort.1–7 In the literature there are examples showing how such structures can be grown by using arrays of self-assembled polystyrene spheres as a template. The formation of such structures from monolayers of spheres using electrodeposition has been pioneered by Bartlett et al.8–11 using gold, platinum, palladium and silver, and there are a few reports of small areas of multilayer metallic opals. However, in multilayer templates drying cracks between colloidal crystal domains are unavoidable. Intuitively one might predict that if a “cracked” template is filled by electrodeposition then the cracks would be preferentially filled with dense metal. This was observed by Wijnhoven et al.12 who made macroporous gold with domains extending over tens of sphere diameters with the order maintained from the original template. In the cracks between the domains, dense gold grows in forms of large flakes. However, in the case of zinc and copper, as observed by Jaurez et al.13 and Kulinowski et al.,14 growth occurred only in the template and the cracks appear to have remained unfilled. Although this result is clearly unexpected it was not explicitly commented upon in either of the publications. To the best of our knowledge, researchers appear to have focussed on obtaining smooth macroporous domains but the question as to why cracks may or may not be filled during electrodeposition has not been addressed. This paper addresses two issues: firstly the formation of multilayer macroporous silver and copper films over large lengthscales by electroplating is reported and the optical properties of the films are investigated. Secondly we discuss the growth mechanism and in particular show that it is possible to control the electrodeposition of dense metal in the cracks between the domains – in other words it is possible to choose, in some cases, whether “to fill or not to fill” the cracks. In the field of nanoplasmonics there is enormous interest in the creation of nano-antennae and other structures that can be used to manipulate light for applications ranging from optical sensing to enhanced absorption in thin film solar cells. The presence or absence of dense non-porous barriers to light propagation will have a critical influence on the efficacy of such structures.
Electroplating is a readily scalable, simple, cheap and low-temperature process. More importantly since the metal grows from the electrical contact at the bottom of the colloidal template outwards to the exposed surface it fills the entire voids of the template, and shrinkage is negligible when the colloidal template is removed. This is in contrast to the more widely used sol–gel methods where shrinkage can be as large as 40%. Moreover, electroplating allows precise control over the mass and composition of the deposits via the plating parameters e.g. solution composition, applied potential or current density.
Generally metals are quite lossy at optical frequencies due to the imaginary part of the dielectric constant, which could destroy the photonic band gap of periodic metallic structures.15 However, noble metals such as gold, silver and copper have been shown to have negligible absorption at frequencies in the visible and near infrared, with silver performing the best. For example, silver and copper are capable of exhibiting large effective refractive indices at frequencies near the plasmon resonance frequency at about 4 eV and 2 eV, respectively.16 However, to date researchers have put most efforts into the preparation of macroporous gold8–12,17,18 or platinum9–11 films which can be replaced by macroporous silver or copper in most envisaged applications at a much lower cost (and arguably with much better efficiency).
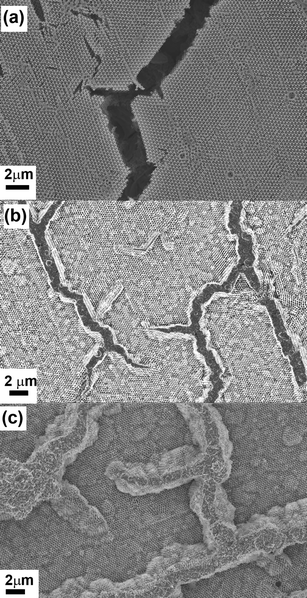
In Fig. 1FESEM images of the original polystyrene (PS) template and the inverse Cu and Ag films are shown. The procedure of colloidal crystal templating is described in detail elsewhere.19 In brief: monodisperse polystyrene (PS) spheres are assembled into a colloidal crystal on a substrate by evaporation induced self-assembly;20 the free spaces between the spheres are subsequently infiltrated with the metal by electrodeposition; the colloidal particles are selectively removed by dissolution in toluene, leaving behind a periodic arrangement of spherical voids embedded in a solid macroporous matrix.
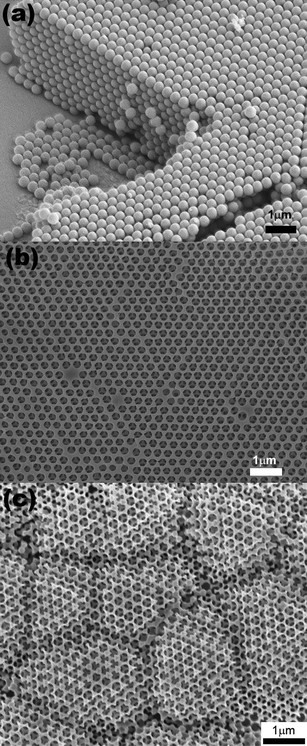 | ||
| Fig. 1 FESEM images of, (a) a cleaved colloidal crystal template formed from polystyrene spheres, and the top surfaces of an inverse (b) Cu film and (c) Ag film. | ||
Non-additive and cyanide-free electrolytes were employed to avoid organic contamination and toxic pollution. Copper was plated from either a simple solution of 0.30 M copper sulfate (CuSO4·5H2O) and 0.05 M hydrochloric acid (HCl, 37%); or from a solution of complex copper ions containing 0.10 M copper sulfate, 0.20 M glycerol, 0.60 M sodium hydroxide, and 1.0 M sodium sulfate. Silver was plated from a solution of 0.10 M silver nitrate (AgNO3, 0.10 M sodium chloride (NaCl), and 0.25 M sodium thiosulfate (Na2S2O3, 99%). All chemicals were purchased from Aldrich in the highest available purity and were used without further purification. Electroplating was carried out with a conventional three-electrode set-up: a saturated mercury sulfate electrode (MSE) as the reference electrode, a platinum wire as the auxiliary electrode, and the as-prepared colloidal template/ITO the working electrode. The templates were masked, leaving a circular area of 1 cm in diameter exposed to the electrolyte. Plating was performed galvanostatically at a current density of −2mA cm−2. After electrodepostion, the PS spheres were removed by dissolution in toluene to reveal the inverse macroporous structures.
The colloidal crystals and the inverse structures appear opalescent under white light illumination, and the colour is uniform over the whole area of the film. The PS templates are robust and adhere well to the glass substrates. There was no evidence for re-suspension of the PS particles when placed in contact with the electroplating solution. The periodicity of the inverse films determines their performance in applications. However since they are the inverse replicas of the colloidal crystals, the crystalline quality of the parent structure is a key factor. A typical surface view of the close-packed colloidal crystal in shown in Fig. 1(a). The Cu inverse film [Fig. 1(b)] appears to be an almost perfect replica of the original template. As well as maintaining the long-range periodicity, it is noted that the surface of the Cu film is as flat as the original template. In contrast, the Ag inverse film [Fig. 1(c)] appears at first to have nucleated as a number of grains rather than maintaining the domain size of the original template. Closer examination however, reveals that this is not the case: in fact the Ag film is perfectly ordered and exhibits the same long-range periodicity of the original template and the Cu film. However, the Ag film is not flat. The top surface has roughened and exhibits surface steps that are predominantly aligned along the close-packed surface directions i.e. <110>. The effect is to create a rippled or wavy surface that has a wavelength of a few microns.
The three-dimensional periodicity was further evaluated by optical spectroscopy . Angle-resolved reflectance spectra exhibit peaks corresponding to multiple Bragg reflections arising from the ordered structures. When the wavelength of incident light satisfies the Bragg condition, it is diffracted away from the propagation axis, leading to a peak in the reflectance spectrum. (Figs. S1 and S2 of the ESI† ) An approximate calculation for the sphere sizes of the periodic structures can be obtained by combining Bragg's law and Snell's law, demonstrating the position of the peak is determined by the sphere diameter, D, and the effective refractive index, neff. From the optical measurements the diameter of the colloidal particles is calculated as 344 nm and the void sizes are 336 nm and 333 nm for copper and silver respectively. This indicates that volume shrinkages are not more than 4%. In contrast, metals grown from sol–gel or nanoparticleinfiltration have shown shrinkages of up to 40%.21,22 Since the void sizes are approximately the same as that of the original polystyrene spheres, the shifts of the reflectance spectra are due to the material properties: i.e. the significant peak-shift to shorter wavelength is actually due to decreases of the effective refractive index. (Table S1 of the ESI† )
The cracks in the original PS template are the inevitable result of the strain developed during the drying process. The cracks are aligned along the <110> directions of the colloidal crystal separating the template into domains 10–50 μm in width and >500 μm in length [Fig. 2(a)]. In the inverse structures, macroporous domains of both Cu and Ag extend over similar sizes of the original polystyrene domains, and this indicates that the electroplating does not disrupt the long-range order of the structure. In general, one would expect the pre-existing cracks to be preferential plating positions, since transport to these sites is more facile than in between the polystyrene spheres. However, a surprising difference was observed between the macroporous Ag and Cu. Ag plated directly into both the interstitial spaces between the colloidal spheres and all the drying cracks between the domains [Fig. 2(b)], while Cu formed isolated domains located exclusively along the crack direction, with the cracks remaining unfilled [Fig. 2(c)].
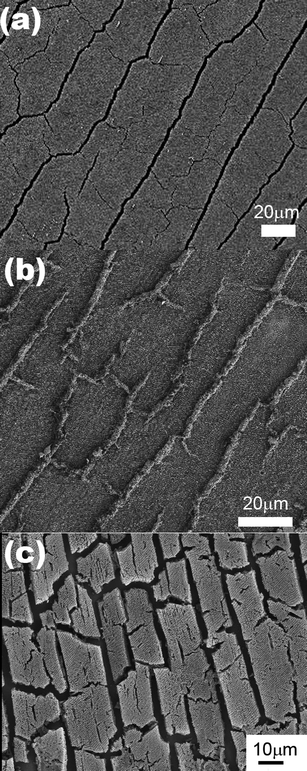 | ||
| Fig. 2 (a) SEM image of the original colloidal crystal template showing the drying cracks that form along the growth direction of the film. SEM images of the inverse (b) Ag and (c) Cu films. The drying cracks have been filled by electrodeposition in the Ag film and remain empty in the case of the Cu film. | ||
Plan view and cross-sectional images recorded in a focused ion beam (FIB) instrument provide further insight in to the contrast between the Cu and Ag inverse structures. The plan view image of the Cu sample clearly demonstrates that the top surface is very flat with no surface steps apparent [Fig. 3(a)]. The cracks in the original template are clearly visible and have not been filled during the electrodeposition. This is further confirmed by the cross-section image obtained by milling a trench in the structure [Fig. 3(b)]. At the edges of the cracks electrodeposition has proceeded beyond the template structure resulting in flat, non-porous Cu in these regions. The plan view image of the Ag surface is very different – it appears that domains have roughened significantly with surface steps clearly visible [Fig. 3(c)]. In the plan view and cross-sectional images it is clear that the cracks are filled with dense, polycrystalline Ag and moreover appear to have been “pushed up” above the height of the original template surface [Fig. 3(d)]. Finally it appears that large voids have been formed at the interface between the inverse Ag film and the substrate. These voids are not present in the original template and by recording images of sequential slices in the FIB it can be seen that the pores are indeed closed voids rather than channels or continuous porous networks (Fig. S3 of the ESI† ). Using sequential images to reconstruct the three-dimensional structure of the voids (shown in colour in Fig. 4) illustrates their spatial relationship to one of the cross-sectional images. Critically this shows that there is a dense layer of Ag formed at the interface with the ITO-coated glass and that the voids are formed within the colloidal crystal, rather than at this interface.
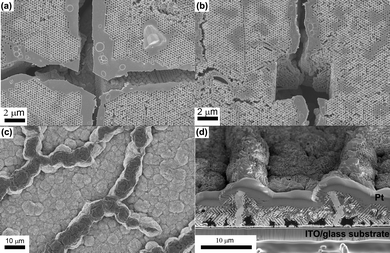 | ||
| Fig. 3 (a) Plan view and (b) cross-sectional images of the Cu film. It is clear that the cracks in the template have not been filled with Cu. (c) Plan view and (b) cross-sectional images of the Ag film showing that the cracks have been filled with electrodeposited Ag. Dark patches of contrast near the substrate in (d) are due to pores/voids that have formed as a result of electrodeposition of Ag. | ||
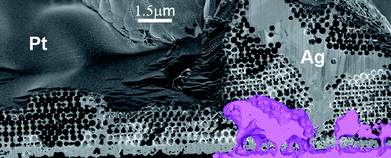 | ||
| Fig. 4 SEM image of the cross-section of the Ag film. Continuous polycrystalline Ag can be seen in the drying crack of the template. A 3D reconstruction of one of the voids observed in Fig. 3(d) is shown in colour. The reconstruction illustrates that these are actually closed voids located between a thin Ag layer on the ITO and the porous Ag structure. | ||
As it could have a significant influence on the use of such structures for nanoplasmonics it is important to explain why cracks in the original template are filled in the case of Ag electrodeposition and not in the case of Cu. During the electrodeposition of Cu, several reactions can occur:
| Cu2+(aq) + 2e− → Cu(s) | (1.1) |
| Cu(s) + ½O2(g) → CuO(s) | (1.2) |
| 2Cu(s) + ½O2(g) → Cu2O(s) | (1.3) |
At the start of the experiment, Cu2+(aq) ions are reduced to Cu(s) (eqn 1.1) and this process is mainly controlled by diffusion of Cu2+ from the solution to the surface of the electrode. When oxygen exists in the solution, at low oxygen pressure, the copper oxidation rate is controlled by the transport of dissolved oxygen to the surface of working electrode.20 Hence the rate of both Cu depositionandoxidation would be expected to be higher in the cracked areas where diffusion is more rapid. Critically the supply of oxygen to the different regions will result in preferential oxidation along the crack. However, since the solution pH is low (pH∼1.5), CuO is not stable.23 Furthermore, the presence of chloride ions is known to destabilise both the oxide and the metal as shown in eqns (2.1) and 2.2, respectively.
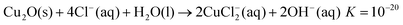 | (2.1) |
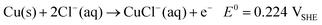 | (2.2) |
As a result, any Cu metal plated in the drying cracks will be oxidised and subsequently dissolved.24,25 Indeed it is well-known that the rate of re-dissolution of protectiveCu2O is much higher than that observed in chloride free solutions26 Under the galvanostatic conditions used the potential was ∼ −100 mVSHE which is close to the equilibrium potential for the oxidation reaction as shown both by thermodynamic calculations and experimental polarisation data.21,23,27 Standard thermodynamic analysis of Pourbaix diagrams (based on the Nernst equation) indicates that each of these reactions are possible in the potential–pH window for film deposition.23 It was not possible to determine if there was any galvanic coupling between the template and the cracked areas due to the differential aeration, but this might be expected to accelerate any dissolution behaviour. Since these films were deposited galvanostatically and so the deposition rate is fixed; we used a current density aimed to produce good replicas. There may be a theoretical current density where the deposition rate is higher than the dissolution rate but in our experiments higher current densities lead to non-uniform filling of the pores and were not pursued.
To prove the hypothesis that oxygen diffusion is critical to the copper dissolution the experiments were repeated in oxygen-free solutions. This was achieved in two independent experiments. In the first experiment, argon was used to purge oxygen from the solution. In the second experiment, copper was deposited from a solution that was free of both oxygen and chloride ions. This was possible by using a copper–glycerol solution in which the Cu forms two complexes Cu(glyc) and [Cu(glyc)2]2− which are reduced to form metallic Cu as follows:
| Cu(glyc)(aq) + 2H2O(l) + 2e− → Cu(s) + C3H5(OH)5(l) + 2OH−(aq) | (3.1) |
| [Cu(glyc)2]2−(aq)+ 4H2O(l) + 2e− → Cu(s) + 2C3H5(OH)5(l) + 4OH−(aq) | (3.2) |
The results presented in Fig. 5 clearly demonstrate that the empty cracks present in inverse Cu films prepared from an acidic CuSO4 solution [Fig. 5(a)] are filled in both the argon-purged [Fig. 5(b)] and the complex ion [Fig. 5(c)] experiment. These results fully support the hypothesis that oxygen is critical to the dissolution behaviour. It also appears that while the cracks are filled to the original top surface in the case of the argon-purged experiment, in the complex ion experiment, which is free of both oxygen and chloride ions, the cracks are overfilled and pushed upwards beyond the original top surface in the same manner as was observed with Ag electrodeposition. Coupled with the filling of the cracks, it clear that surface roughness of the inverse Cu structure has increased. In the complex ion electrodeposition experiment the final surface [Fig. 5(c)] is remarkably similar to that of the inverse Ag structure [Fig. 3(c)]. Thus, it is clear that the roughness of the surface, i.e. the formation of surface steps, is linked directly to whether or not the cracks have been filled with dense polycrystalline metal.
 | ||
| Fig. 5 Plan view images showing (a) the empty cracks present in inverse Cu films prepared from an acidic CuSO4 solution, (b) cracks filled with Cu in the argon-purged electrodeposition experiment, (c) cracks “over-filled” with Cu in the complex ion experiment. | ||
It is well-known that growth stresses develop during electrochemical deposition of metallic thin films and that these relate to the coalescence of nucleating centres resulting in tensional strains in the metal lattice. In particular Ag has been found to exhibit very large growth stress.28 If one considers the evolution of such stress during film growth in constrained versus isolated domains, the difference in film morphology becomes clear. During formation of the inverse opal it is energetically favourable for it to relax laterally as a result of the tensional stresses; however if the cracks are filled then the inverse domains are constrained. Ultimately, the growth stresses must then be relieved by other mechanisms such as the creation of surface steps and also by the formation of voids at the interface between the inverse structure and the substrate. This also results in the observed shrinkage in pore diameter for the metallic films compared to the original template.
We have demonstrated the formation of large-scale ordered structures of copper and silver by direct electrodepostion through a colloidal crystal template. Our films show ordered structures formed over domains that are about 10–50 μm wide with a length several times the width. Imaging and optical characterization has confirmed their three-dimensional periodicity and indicated the inverse structures have stop-band properties in the visible wavelength range. The growth behaviour of the electroplated Cu and Ag films has been investigated and it has been demonstrated that the morphology is controlled by the diffusion of metal ions from the solution to the electrode surface. The Cu system can be tuned to fill or not to fill the original drying cracks in the template in order to produce isolated or interconnected domains. This is potentially useful for the formation of individual or interconnected antennae in nanoplasmonic structures.
Acknowledgements
We would like to express our gratitude to Hutchison-EPSRC Dorothy Hodgkin postgraduate awards for financial support.Reference List
- S. A. Maier Plasmonics – Fundamentals and Applications, Springer, New York, 1st edn, 2007 Search PubMed.
- P. V. Braun and P. Wiltzius, Curr. Opin. Colloid Interface Sci., 2002, 7(1–2), 116–123 CrossRef CAS.
- K. Kneipp, H. Kneipp, I. Itzkan, R. R. Dasari and M. S. Feld, J. Phys.: Condens. Matter, 2002, 14, R597–R624 CrossRef CAS.
- S. M. Nie and S. R. Emory, Science, 1997, 275, 1102–1106 CrossRef CAS.
- K. Kneipp, Y. Wang, H. Kneipp, L. T. Perelman, I. Itzkan, R. R. Dasari and M. S. Feld, Phys. Rev. Lett., 1997, 78, 1667–1670 CrossRef CAS.
- T. Sannomiya and C. Hafner, Nano Lett., 2008, 8, 3450–3455 CrossRef CAS.
- M. Moskovits and D. H. Jeong, Chem. Phys. Lett., 2004, 397, 91–95 Search PubMed.
- M. E. Abdelsalam, P. N. Bartlett, J. J. Baumberg, S. Cintra, T. A. Kelf and A. E. Russell, Electrochem. Commun., 2005, 7(7), 740–744 CrossRef CAS.
- P. N. Bartlett, J. J. Baumberg, P. R. Birkin, M. A. Ghanem and M. C. Netti, Chem. Mater., 2002, 14(5), 2199–2208 CrossRef CAS.
- P. N. Bartlett, J. J. Baumberg, S. Coyle and M. E. Abdelsalam, Faraday Discuss., 2004, 125, 117–132 RSC.
- P. N. Bartlett, P. R. Birkin and M. A. Ghanem, Chem. Commun., 2000,(17), 1671–1672 Search PubMed.
- J. E. G. J. Wijnhoven, S. J. M. Zevenhuizen, M. A. Hendriks, D. Vanmaekelbergh, J. J. Kelly and W. L. Vos, Adv. Mater., 2000, 12(12), 888–890 CrossRef CAS.
- B. H. Juárez, C. López and C. Alonso, J. Phys. Chem. B, 2004, 108(43), 16708–16712 CrossRef CAS.
- K. M. Kulinowski, P. Jiang., H. Vaswani and V. L. Colvin, Adv. Mater., 2000, 12(11), 833–838 CrossRef CAS.
- X. Y. Ao and S. L. He, Opt. Lett., 2004, 29(21), 2542–2544 Search PubMed.
- J. H. Weaver, H. P. R. Frederikse in Optical Properties of Selected Elements – CRC Handbook of Chemistry and Physics, ed. D. R. Lide, CRC Press, Boca Raton, Florida, 87th edn, 2006. Search PubMed.
- S. Cintra, M. E. Abdelsalam, P. N. Bartlett, J. J. Baumberg, T. A. Kelf, Y. Sugawara and A. E. Russell, Faraday Discuss., 2006, 132, 191–199 RSC.
- P. M. Tessier, O. D. Velev, A. T. Kalambur, J. F. Rabolt, A. M. Lenhoff and E. W. Kaler, J. Am. Chem. Soc., 2000, 122(39), 9554–9555 CrossRef CAS.
- P. V. Braun and P. Wiltzius, Nature, 1999, 402(6762), 603–604 CrossRef CAS.
- P. Jiang, J. F. Bertone, K. S. Hwang and V. L. Colvin, Chem. Mater., 1999, 11(8), 2132–2140 CrossRef CAS.
- B. T. Holland, C. F. Blanford and A. Stein, Science, 1998, 281(5376), 538–540 CrossRef CAS.
- J. E. G. J. Wijnhoven and W. L. Vos, Science, 1998, 281(5378), 802–804 CrossRef CAS.
- M. Pourbaix in Atlas of Electrochemical Equilibria in Aqueous Solutions, National Association of Corrosion Engineers, Houston, 1974, pp. 384–392 Search PubMed.
- M. V. Vazquez, S. R. Desanchez, E. J. Calvo and D. J. Schiffrin, J. Electroanal. Chem., 1994, 374, 179–187 CrossRef CAS.
- F. King, C. D. Litke and Y. Tang, J. Electroanal. Chem., 1995, 384, 105–113 CrossRef.
- G. Kear, B. D. Barker and F. C. Walsh, Corros. Sci., 2004, 46, 109 CrossRef CAS.
- K. L. Stewart and A. A. Gewirth, Langmuir, 2007, 23, 9911–9918 CrossRef CAS.
- O. E. Kongstein, U. Bertocci and G. R. Stafford, J. Electrochem. Soc., 2005, 152(3), C116–C123 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Figs. S1 and S2, Table S1 and supplementary movie. See DOI: 10.1039/b9nr00213h |
| ‡ FEI Company, 5600 KA, Eindhoven, Netherlands |
| This journal is © The Royal Society of Chemistry 2009 |
